Abstract
Objective
Preclinical evidence indicates that rapid changes in levels of allopregnanolone, the predominant metabolite of progesterone, confer dramatic behavioral changes and may trigger postpartum depression (PPD) in some women. Considering the pathophysiology of PPD (i.e., triggered by reproductive steroids), the need for fast‐acting, efficacious treatments and the negative consequences of untreated PPD, there is an increasing focus on developing PPD therapies. Brexanolone (USAN; formerly SAGE‐547 Injection), a proprietary injectable allopregnanolone formulation, was evaluated as a treatment for severe PPD in a proof‐of‐concept, open‐label study.
Methods
Four women with severe PPD, defined as a baseline 17‐item Hamilton Rating Scale for Depression (HAMD) score of ≥20, received brexanolone, titrated to a dose reflecting third‐trimester allopregnanolone levels. After a 36‐hour maintenance infusion, tapering occurred over 12 hours. Primary outcomes were measures of safety. Secondary outcomes were assessments of efficacy, including HAMD.
Results
All enrolled patients completed the study. Fourteen adverse events were reported, of which none was severe. Starting at the first measure after infusion initiation and continuing through Hour 84, mean HAMD total scores were reduced to levels consistent with remission of symptoms. All other efficacy assessments showed similar improvements.
Conclusions
Brexanolone was well tolerated and demonstrated activity in severe PPD. Larger, double‐blind trials are needed for further evaluation.
Keywords: GABAA receptor, neuroactive steroid, positive allosteric modulation, postpartum depression, psychiatric disorder, brexanolone
1. INTRODUCTION
Postpartum depression (PPD) is common in the perinatal period and remains underdiagnosed and often untreated (Earls, 2010). Major or minor depression is reported by up to 19.2% of mothers in the first three postpartum months (Gavin et al., 2005), and PPD can lead to significant morbidity and mortality for mother and infant (Beck, 1995; Fihrer, Mcmahon, & Taylor, 2009; Howard, Flach, Mehay, Sharp, & Tylee, 2011; O'connor, Monk, & Burke, 2016). Hormonal changes during pregnancy and postpartum are thought to play a role in the aetiology of PPD (Bloch et al., 2000; Schiller, Meltzer‐Brody, & Rubinow, 2015). The clinical presentation of PPD has distinguishing characteristics in comparison to depression outside of the perinatal period (Bernstein et al., 2008; Hendrick, Altshuler, Strouse, & Grosser, 2000). Symptoms of severe anxiety and rumination can manifest as the primary clinical concern and significantly impact functioning (Abramowitz et al., 2010; Bernstein et al., 2008).
Levels of allopregnanolone, the major metabolite of progesterone, increase commensurate with the rise in progesterone during pregnancy, with highest physiologic levels in the third trimester (Luisi et al., 2000), and then subsequently decline precipitously after childbirth, when progesterone levels decrease (Nappi et al., 2001; Paoletti et al., 2006). Allopregnanolone is a neuroactive steroid that has been shown in animal models to modulate neuronal excitability through direct action on synaptic and extrasynaptic GABAA receptors (Belelli et al., 2009; Lambert, Belelli, Harney, Peters, & Frenguelli, 2001). It appears to play a significant role in affective disturbances that occur with changes in reproductive endocrine function, such as during the postpartum period (Amin et al., 2006; Schiller, Meltzer‐Brody, & Rubinow, 2014a; Schiller, Schmidt, & Rubinow, 2014b). Failure of GABAA receptors to adapt to abrupt changes in allopregnanolone levels at parturition may play a part in triggering PPD (Maguire & Mody, 2008). Allopregnanolone has been implicated in preclinical models of anxiolysis and mood improvement (Schiller et al., 2014b; Schule, Nothdurfter, & Rupprecht, 2014), with evidence that elevated allopregnanolone levels may protect against depressed mood during pregnancy (Hellgren, Åkerud, Skalkidou, Bäckström, & Sundström‐Poromaa, 2014). Preclinical data suggest anxiogenic effects of low allopregnanolone concentrations (Andréen et al., 2009). On the basis of preclinical models, we hypothesize that the abrupt postpartum decline in allopregnanolone may be associated with symptoms of PPD. This supports the rationale for examining the effects of treatment of PPD patients with therapeutic doses of allopregnanolone equivalent to third trimester levels.
Although selective serotonin reuptake inhibitors (SSRIs) are commonly used as first‐line PPD treatment, there is limited evidence for their use in the postpartum period specifically, and the proportion of PPD patients treated successfully with SSRIs has a wide range (43%–88%; Austin, Middleton, Reilly, & Highet, 2013; De Crescenzo, Perelli, Armando, & Vicari, 2014; Kim, Epperson, Weiss, & Wisner, 2014; Misri, Swift, Abizadeh, & Shankar, 2016). Considering the pathophysiology of depression in the perinatal period and the negative consequences of untreated PPD, development of efficacious new treatments with more targeted mechanisms of action is warranted. Brexanolone (USAN; formerly SAGE‐547 Injection), a proprietary, aqueous formulation of allopregnanolone in sulfobutylether‐β‐cyclodextrin, was evaluated in an open‐label, proof‐of‐concept study for treatment of severe PPD, with the primary objective of evaluating safety and tolerability. Secondary and exploratory objectives included assessment of the effects of brexanolone on depression and anxiety symptoms.
2. METHODS
2.1. Design and patients
This open‐label, proof‐of‐concept study enrolled healthy females 18–45 years old admitted to the University of North Carolina at Chapel Hill Perinatal Psychiatry Inpatient Unit (PPIU) for a major depressive episode beginning no earlier than the third trimester and no later than 12 weeks following delivery. Admission to the PPIU occurred 14 days to 20 weeks postpartum. At the screening visit, a score of ≥20 on the 17‐item Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960) was required for inclusion. If patients were taking antidepressants for longer than 2 weeks on a stable dose, they were allowed to continue their medications during the brexanolone dosing period. Two patients were taking sertraline (50 and 100 mg) during brexanolone administration. Three of the women had prior PPD episodes, and two also experienced prior major depressive episodes outside of the peripartum period. All women participated in the standard PPIU program, which included psychotherapy and has been previously described (Kimmel et al., 2016; Meltzer‐Brody et al., 2014). Permanent weaning from breastfeeding prior to Visit 1 was required. Written informed consent was obtained prior to screening, and the study was approved by the UNC institutional review board. The first patient was enrolled January 9, 2015; last patient follow‐up was June 8, 2015.
2.2. Dosing and administration
Brexanolone dosing was chosen to target allopregnanolone exposure in the third trimester (~150 nM, Luisi et al., 2000). Study timeline, dosing schedule, and dosing regimen are presented in Figure 1 and Table 1. After the first and second patient completed dosing and Hour 84 assessments, safety data were reviewed before enrolling the next patient.
Figure 1.
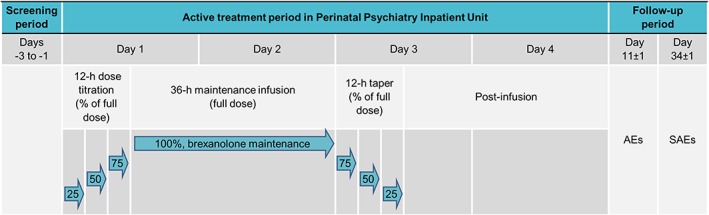
Overview of trial design and dosing. Dosing began in the morning of Day 1 with a 12‐hour titration period in which brexanolone was infused at 25%, 50%, then 75% of the maintenance dose for 4 hours at each level. Maintenance dosing began at Hour 12 and continued for 36 hours to achieve a target steady‐state plasma concentration of ~150 nM. At Hour 48, the dose was then tapered over the next 12 hours to 75%, 50%, and then 25% of the maintenance dose to allow physiologic adjustment to decreasing allopregnanolone levels. AE = adverse event; SAE = serious adverse event
Table 1.
Brexanolone dosing regimen
| Study day (D) | Hour | Type and duration of Brexanolone infusion (h) | Description |
|---|---|---|---|
| Titration infusion | |||
| D1 | H1–H4 | 4 | 21.5 μg·kg−1·hr−1 (25% of the maintenance rate) |
| H5–H8 | 4 | 43 μg·kg−1·hr−1 (50% of the maintenance rate) | |
| H9–H12 | 4 | 64.5 μg·kg−1·hr−1 (75% of the maintenance rate) | |
| Maintenance infusion | |||
| D1–D3 | H13–H48 | 36 | 86 μg·kg−1·hr−1 |
| Taper infusions | |||
| D3 | H49–H52 | 4 | 64.5 μg·kg−1·hr−1 (75% of the maintenance rate) |
| H53–H56 | 4 | 43 μg·kg−1·hr−1 (50% of the maintenance rate) | |
| H57–H60 | 4 | 21.5 μg·kg·hr−1 (75% of the maintenance rate) |
2.3. Procedures
Prior to Day 1 dosing, vital signs, HAMD, Edinburgh Postnatal Depression Scale (EPDS), Generalized Anxiety Disorder 7‐item Scale (GAD‐7), Patient Health Questionnaire (PHQ‐9), Stanford Sleepiness Scale, and Columbia–Suicide Severity Rating Scale were administered (Cox, Holden, & Sagovsky, 1987; Hoddes, Zarcone, Smythe, Phillips, & Dement, 1973; Kroenke, Spitzer, & Williams, 2001; Posner et al., 2011; Spitzer, Kroenke, Williams, & Löwe, 2006). Clinical Global Impression‐Improvement (CGI‐I) was first assessed at 12 hours postinfusion initiation. All assessments were repeated at Hours 24, 36, 48, 60, and 84 (HAMD also at Hour 12). All evaluators received HAMD training and were experienced in the evaluation of patients. Adverse events (AEs) were monitored throughout treatment and follow‐up. Blood samples for pharmacokinetic (PK) analysis were obtained preinfusion and periodically through Hour 72 (Supplemental Methods).
2.4. Analysis
Descriptive statistics were calculated and summarized for all endpoints. Baseline values were defined as the last value prior to study drug infusion. Primary outcomes were safety and tolerability measures, including treatment‐emergent AEs and changes from baseline in physical and psychiatric evaluations. Secondary outcomes were assessments of efficacy. The key efficacy measure was change from baseline in HAMD total score. A paired t test was performed to evaluate whether the change in HAMD from baseline to the end of infusion (Hour 60) was nonzero. Exploratory endpoints included EPDS, GAD‐7, PHQ‐9, and Stanford Sleepiness Scale scores (Supplemental Methods).
3. RESULTS
3.1. Patients
Four of six screened patients (age 27–42 years) met inclusion criteria, enrolled, and completed the study. Based on the observed outcomes in the four enrolled patients, the trial was stopped before reaching the protocol criterion of 10 evaluable patients. Three enrolled patients had a prior history of PPD, and three were taking concomitant psychotropic medications. Mean HAMD total score at baseline was 26.5 ± 4.1 (range 22–32), indicating severe depression (Zimmerman, Martinez, Young, Chelminski, & Dalrymple, 2013). Preinfusion, three patients reported nonspecific thoughts of suicide ideation (one reported an actual suicide attempt during her lifetime).
3.2. Safety, PK, and efficacy
AEs were reported by all four patients (14 total; 10 considered treatment related). Sedation was reported by two patients, and all other events were reported by one patient each (infusion site discomfort, infusion site erythema, infusion site pain, rash, thyroid stimulating hormone increase, dizziness, flushing, and oropharyngeal pain). All AEs were of mild or moderate severity and self‐limited. No serious AEs were reported. No patients were discontinued because of an AE. Three patients had AE‐related dosage adjustments (Supplemental Results and Table S1). There were no clinically important changes from baseline in physical exam, electrocardiogram, laboratory parameters, or vital signs. There were no reports of suicidal behaviour or ideation postbaseline through Hour 84. PK data are summarized in Table S2 and Figure S1.
Mean HAMD, EPDS, GAD‐7, and PHQ‐9 scores decreased at the first assessment after infusion initiation and remained low through the end of infusion (Hour 60) and the last time point assessed (Hour 84; Figure 2). Mean HAMD total score was 1.8 ± 1.5 (p = .001 vs. baseline) at Hour 60 and 5.3 ± 2.9 at Hour 84 (81% decrease from baseline at Hour 84). HAMD total scores for all patients were ≤7 at all assessments from Hour 24 onward, indicating symptom remission (Zimmerman et al., 2013; Figure S2). Mean CGI‐I score was 1.8 ± 0.5 at first assessment (Hour 12) and remained <2 at every subsequent assessment.
Figure 2.
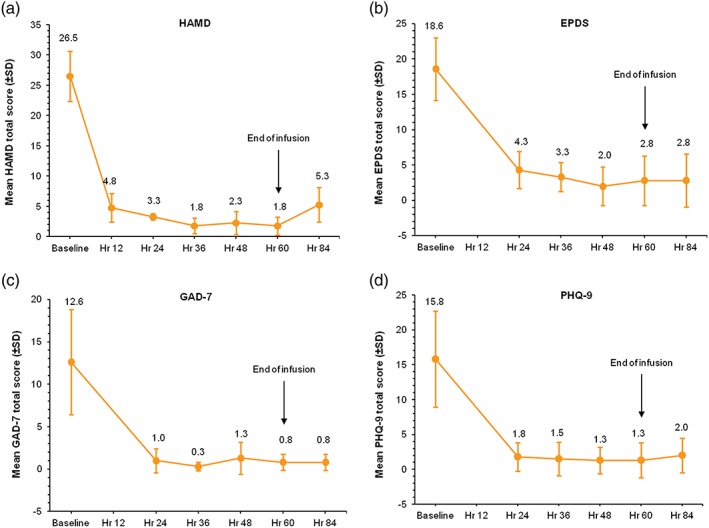
Mean total scores for efficacy assessments (N = 4). Mean change from baseline values for HAMD, EPDS, GAD‐7, and PHQ‐9 total scores at each time point assessed. For HAMD, total score ≤ 7 was considered a measure of symptom remission. Planned assessment at end of infusion (Hour 60) revealed a significant decrease versus baseline (p = .001). HAMD = Hamilton Rating Scale for Depression; EPDS = Edinburgh Postnatal Depression Scale; GAD‐7 = Generalized Anxiety Disorder 7‐item Scale; PHQ‐9 = Patient Health Questionnaire
4. DISCUSSION
In this small proof‐of‐concept study, brexanolone appeared well tolerated in four patients with severe PPD. Three patients had precautionary dosage adjustments due to mild or moderate AEs but completed the trial, and no serious AEs were reported. Assessments of depression (HAMD and EPDS) and anxiety (GAD‐7) demonstrated rapid symptom reduction that was maintained through the last assessment.
This is the first evaluation of allopregnanolone as a PPD treatment. Current standard of care for severe PPD often comprises pharmacologic treatment in conjunction with psychotherapy and other nonpharmacological interventions. Among pharmacological approaches, SSRIs tend to be preferred despite varying response rates (Austin et al., 2013; Kim et al., 2014; Misri et al., 2016), suggesting a need for more targeted PPD therapies. In this study, patients achieved rapid reduction of symptoms based on HAMD and CGI‐I scores, with clinically relevant improvements from baseline beginning at the first assessment post infusion initiation (Hour 12) and continuing through Hour 84, an important finding given that SSRIs can require more time to have a therapeutic effect (De Crescenzo et al., 2014). Notably, because established psychotropic medications were continued during the trial in 3 out of the 4 patients, we cannot exclude that this effect may not be only due to brexanolone administration. An additional limitation is the lack of external raters.
Because severe maternal PPD has far‐reaching impact on the mother, infant, and family, we hypothesize that a pharmacotherapy with potentially fewer AEs and greater efficacy due a targeted mechanism of action is desirable. Further, recent reviews of the treatment of PPD with SSRI antidepressants have highlighted the lack of data or small number of studies available for analysis (Molyneaux, Howard, Mcgeown, Karia, & Trevillion, 2014), indicating a need for further investigations and additional pharmacological PPD therapies. The potential for brexanolone to be broadly applicable to depression beyond PPD is unclear and has not been evaluated.
5. CONCLUSION
Though limitations include its open‐label design and relatively short treatment and assessment period, this small exploratory study suggests that brexanolone, and by extension positive allosteric GABAA receptor modulation with neuroactive steroids, may be an important therapeutic modality in PPD. The activity signal supports a role for allopregnanolone in the pathophysiology of PPD and warranted early study discontinuation in order to accelerate implementation of a larger placebo‐controlled, double‐blind trial to validate and extend these findings.
CONFLICT OF INTEREST
SJK and HC are employees of Sage Therapeutics, Inc. and have a patent pending for brexanolone for neuropsychiatric conditions. JD and EH are employees of Sage Therapeutics, Inc. SR received fees from Sage Therapeutics, Inc. for statistical consulting services. SM‐B and DR's institution received consultation fees for time spent developing the protocol for this study. DR received consultation fees from Sage Therapeutics, Inc. for study design. SM‐B did not personally receive any compensation from Sage Therapeutics, Inc. SM‐B and DR's institution has received a research grant from Johnson & Johnson, unrelated to this study. The employees of Sage Therapeutics, Inc. listed above hold shares or stock options in the company.
CONTRIBUTORS
SJK, HC, JD, DR, and SM‐B designed the study. SM‐B was the principal investigator and was involved in recruitment and data collection. SJK performed the literature search and designed the figures. SR and EH analysed the data. All authors were involved in the interpretation of the data. SJK, JD, SR, EH (PK sections), DR, SK, JD, and SM‐B drafted the manuscript. All authors provided a critical review and final approval of the manuscript.
ACKNOWLEDGEMENTS
Sage Therapeutics, Inc. funded the trial. Editorial assistance was provided by Boston Strategic Partners, Inc., and statistical analysis was provided by SR, with support from Sage Therapeutics, Inc. The active pharmaceutical ingredient for this study was contributed under agreement with the Regents of the University of California and the University of California Davis.
Kanes SJ, Colquhoun H, Doherty J, et al. Open‐label, proof‐of‐concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol Clin Exp. 2017;32:e2576 https://doi.org/10.1002/hup.2576
REFERENCES
- Abramowitz, J. S. , Meltzer‐Brody, S. , Leserman, J. , Killenberg, S. , Rinaldi, K. , Mahaffey, B. L. , & Pedersen, C. (2010). Obsessional thoughts and compulsive behaviors in a sample of women with postpartum mood symptoms. Archives of Women's Mental Health, 13, 523–530. [DOI] [PubMed] [Google Scholar]
- Amin, Z. , Mason, G. F. , Cavus, I. , Krystal, J. H. , Rothman, D. L. , & Epperson, C. N. (2006). The interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacology Biochemistry and Behavior, 84, 635–643. [DOI] [PubMed] [Google Scholar]
- Andréen, L. , Nyberg, S. , Turkmen, S. , Van Wingen, G. , Fernández, G. , & Bäckström, T. (2009). Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology, 34, 1121–1132. [DOI] [PubMed] [Google Scholar]
- Austin, M. P. V. , Middleton, P. , Reilly, N. M. , & Highet, N. J. (2013). Detection and management of mood disorders in the maternity setting: The Australian clinical practice guidelines. Women and Birth. Australian College of Midwives, 26, 2–9. [DOI] [PubMed] [Google Scholar]
- Beck, C. T. (1995). The effects of postpartum depression on maternal–infant interaction: A meta‐analysis. Nursing Research, 44, 298–304. [PubMed] [Google Scholar]
- Belelli, D. , Harrison, N. L. , Maguire, J. , Macdonald, R. L. , Walker, M. C. , & Cope, D. W. (2009). Extrasynaptic GABAA receptors: Form, pharmacology, and function. The Journal of Neuroscience, 29, 12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, I. H. , Rush, A. J. , Yonkers, K. , Carmody, T. J. , Woo, A. , Mcconnell, K. , & Trivedi, M. H. (2008). Symptom features of postpartum depression: Are they distinct? Depression and Anxiety, 25, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch, M. , Schmidt, P. J. , Danaceau, M. , Murphy, J. , Nieman, L. , & Rubinow, D. R. (2000). Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry, 157, 924–930. [DOI] [PubMed] [Google Scholar]
- Cox, J. L. , Holden, J. M. , & Sagovsky, R. (1987). Detection of postnatal depression: Development of the 10‐item Edinburgh Postnatal Depression scale. British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- De Crescenzo, F. , Perelli, F. , Armando, A. , & Vicari, S. (2014). Selective serotonin reuptake inhibitors (SSRIs) for post‐partum depression (PPD): A systematic review of randomized clinical trials. Journal of Affective Disorders, 152‐154, 39–44. [DOI] [PubMed] [Google Scholar]
- Earls, M. F. (2010). Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics, 126, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Fihrer, I. , Mcmahon, C. A. , & Taylor, A. J. (2009). The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. Journal of Affective Disorders, 119, 116–123. [DOI] [PubMed] [Google Scholar]
- Gavin, N. I. , Gaynes, B. N. , Lohr, K. N. , Meltzer‐Brody, S. , Gartlehner, G. , & Swinson, T. (2005). Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology, 106, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren, C. , Åkerud, H. , Skalkidou, A. , Bäckström, T. , & Sundström‐Poromaa, I. (2014). Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology, 69, 147–153. [DOI] [PubMed] [Google Scholar]
- Hendrick, V. , Altshuler, L. , Strouse, T. , & Grosser, S. (2000). Postpartum and nonpostpartum depression: Differences in presentation and response to pharmacologic treatment. Depression and Anxiety, 11, 66–72. [DOI] [PubMed] [Google Scholar]
- Hoddes, E. , Zarcone, V. , Smythe, H. , Phillips, R. , & Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology, 10, 431–436. [DOI] [PubMed] [Google Scholar]
- Howard, L. M. , Flach, C. , Mehay, A. , Sharp, D. , & Tylee, A. (2011). The prevalence of suicidal ideation identified by the Edinburgh Postnatal Depression Scale in postpartum women in primary care: Findings from the RESPOND trial. BMC Pregnancy and Childbirth, 11, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. R. , Epperson, C. N. , Weiss, A. R. , & Wisner, K. L. (2014). Pharmacotherapy of postpartum depression: An update. Expert Opinion on Pharmacotherapy, 15, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel, M. C. , Lara‐Cinisomo, S. , Melvin, K. , Di Florio, A. , Brandon, A. , & Meltzer‐Brody, S. (2016). Treatment of severe perinatal mood disorders on a specialized perinatal psychiatry inpatient unit. Archives of Women's Mental Health, 19, 645–653. [DOI] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. L. , & Williams, J. B. (2001). The PHQ‐9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, J. J. , Belelli, D. , Harney, S. C. , Peters, J. A. , & Frenguelli, B. G. (2001). Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Research. Brain Research Reviews, 37, 68–80. [DOI] [PubMed] [Google Scholar]
- Luisi, S. , Petraglia, F. , Benedetto, C. , Nappi, R. E. , Bernardi, F. , Fadalti, M. , … Genazzani, A. R. (2000). Serum allopregnanolone levels in pregnant women: Changes during pregnancy, at delivery, and in hypertensive patients. Journal of Clinical Endocrinology and Metabolism, 85, 2429–2433. [DOI] [PubMed] [Google Scholar]
- Maguire, J. , & Mody, I. (2008). GABAAR plasticity during pregnancy: Relevance to postpartum depression. Neuron, 59, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer‐Brody, S. , Brandon, A. R. , Pearson, B. , Burns, L. , Raines, C. , Bullard, E. , & Rubinow, D. (2014). Evaluating the clinical effectiveness of a specialized perinatal psychiatry inpatient unit. Archives of Women's Mental Health, 17, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misri, S. , Swift, E. , Abizadeh, J. , & Shankar, R. (2016). Overcoming functional impairment in postpartum depressed or anxious women: A pilot trial of desvenlafaxine with flexible dosing. Ther Adv Psychopharmacol, 6, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux, E. , Howard, L. M. , Mcgeown, H. R. , Karia, A. M. , & Trevillion, K. (2014). Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews, CD002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi, R. E. , Petraglia, F. , Luisi, S. , Polatti, F. , Farina, C. , & Genazzani, A. R. (2001). Serum allopregnanolone in women with postpartum “blues”. Obstetrics and Gynecology, 97, 77–80. [DOI] [PubMed] [Google Scholar]
- O'connor, T. G. , Monk, C. , & Burke, A. S. (2016). Maternal affective illness in the perinatal period and child development: Findings on developmental timing, mechanisms, and intervention. Current Psychiatry Reports, 18, 24. [DOI] [PubMed] [Google Scholar]
- Paoletti, A. M. , Romagnino, S. , Contu, R. , Orrù, M. M. , Marotto, M. F. , Zedda, P. , … Melis, G. B. (2006). Observational study on the stability of the psychological status during normal pregnancy and increased blood levels of neuroactive steroids with GABA‐A receptor agonist activity. Psychoneuroendocrinology, 31, 485–492. [DOI] [PubMed] [Google Scholar]
- Posner, K. , Brown, G. K. , Stanley, B. , Brent, D. A. , Yershova, K. V. , Oquendo, M. A. , … Mann, J. J. (2011). The Columbia‐Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. American Journal of Psychiatry, 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, C. E. , Meltzer‐Brody, S. , & Rubinow, D. R. (2014a). The role of reproductive hormones in postpartum depression ( pp. 1–12). September: CNS Spectr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, C. E. , Schmidt, P. J. , & Rubinow, D. R. (2014b). Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berlin), 5, 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, C. E. , Meltzer‐Brody, S. , & Rubinow, D. R. (2015). The role of reproductive hormones in postpartum depression. CNS Spectrums, 20, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schule, C. , Nothdurfter, C. , & Rupprecht, R. (2014). The role of allopregnanolone in depression and anxiety. Progress in Neurobiology, 113, 79–87. [DOI] [PubMed] [Google Scholar]
- Spitzer, R. L. , Kroenke, K. , Williams, J. B. W. , & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD‐7. Archives of Internal Medicine (Chicago, Ill.: 1908), 166, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Zimmerman, M. , Martinez, J. H. , Young, D. , Chelminski, I. , & Dalrymple, K. (2013). Severity classification on the Hamilton Depression Rating Scale. Journal of Affective Disorders, 150, 384–388. [DOI] [PubMed] [Google Scholar]


