Abstract
Introduction:
In 2017, neoadjuvant, cisplatin-based chemotherapy followed by radical cystectomy (RC) is considered the gold standard therapy for muscle-invasive bladder based on randomized controlled trials. Across all tumor stages, this approach has been associated with the highest rates of disease-specific survival. However, RC is one of the most challenging procedures performed by urologic surgeons and carries with it significant risks of complications, hospital readmission, and even a small risk of mortality, in addition to lifestyle changes that can have long-term effects on well-being. For these reasons, bladder-sparing approaches are utilized in some highly selected patients. We reviewed the most recent evidence for bladder-sparing modalities for muscle-invasive urothelial bladder cancer and summarize those findings in this review article.
Methods:
We performed a PubMed literature review utilizing the key words “bladder preservation,” “trimodal therapy,” “muscle-invasive bladder cancer,” and “partial cystectomy” written in English, dating back to 1990. We excluded case reports.
Results:
Our search yielded more than 2000 articles which we screened. Some articles were then rejected due to inappropriate topic. In addition, we reviewed the most recent American Urological Association, National Comprehensive Cancer Network (NCCN), and European guidelines on muscle-invasive bladder cancer. We identified fifty relevant articles which are summarized in this text. In some rare instances, recommendations are based on expert opinion.
Conclusions:
Bladder preservation is often considered for quality of life considerations or in the setting of multiple medical comorbidities, and this remains oncologically appropriate even in 2016 in highly selected patients with muscle-invasive urothelial carcinoma of the bladder.
INTRODUCTION
Neoadjuvant, cisplatin-based chemotherapy followed by radical cystectomy (RC) is considered the gold standard therapy for muscle-invasive bladder based on randomized controlled trials.[1,2] Across all tumor stages, this approach has been associated with the highest rates of disease-specific survival.[3,4] However, RC is one of the most challenging procedures performed by urologic surgeons and carries with it significant risks of complications, hospital readmission, and even a small risk of mortality, in addition to lifestyle changes that can have long-term effects on well-being.[3,5,6,7]
Patients with bladder cancer are older and are often frail, with comorbidities that accompany long-time tobacco use. As a greater percentage of the worldwide population ages, bladder cancer patients will continue to present at advanced age and may no longer be considered ideal candidates for RC. The literature supports that older patients are more likely to be undertreated: one study found that older patients with muscle-invasive bladder cancer (MIBC) were treated with observation alone in 25% of patients aged 70–79 and 40% of patients aged 80–89.[8] In addition, younger patients who otherwise may be good surgical candidates may also desire to avoid the complications and physical changes associated with RC, especially those for whom quality of life (QoL) supersedes oncologic outcome. Bladder preservation is an option for MIBC in patients unfit or unwilling to undergo cystectomy as it may lessen the effects on sexual and bowel function while avoiding the cosmetic changes of urinary diversion. Clearly, careful patient selection and extensive counseling are paramount to successful intervention.
“Bladder preservation” as a treatment paradigm includes a range of therapies: partial cystectomy, radical transurethral resection, chemotherapy, and radiation therapy. Emerging data suggest that an approach using a combination of these therapies results in the best oncologic outcomes. In highly selected patients, metastasis-free and cancer-specific survivals appear to be similar to RC.[9,10]
METHODS
We performed a PubMed literature review utilizing the key words “bladder preservation,” “trimodal therapy,” “muscle-invasive bladder cancer,” and “partial cystectomy” written in English, dating back to 1990. We excluded case reports. This yielded more than 2000 articles which we screened. Some articles were then rejected due to inappropriate topic. In addition, we reviewed the most recent American Urological Association, NCCN, and European guidelines on muscle-invasive bladder cancer. We identified fifty relevant articles which are summarized in this text. In some rare instances, recommendations are based on expert opinion.
PARTIAL CYSTECTOMY
The ideal candidates for partial cystectomy are those who present with a solitary lesion (<5 cm) in a region that can be excised with adequate, 2-cm margins (such as the dome of the bladder). Those patients with multiple lesions or concomitant carcinoma in situ (CIS) are typically excluded from the study.[4] In addition, patients with defunctionalized or contractile bladders may also not be appropriate candidates. The literature demonstrates that approximately 2.8%–5% of patients will have tumor characteristics that meet these criteria.[11,12] Historically, patients with tumors in bladder diverticula have been considered ideal candidates for partial cystectomy, but diverticular tumors in bladders with associated CIS or multifocal tumors remain at similarly high risk of recurrence.[13] As multifocal tumors and CIS preclude patients from partial cystectomy, adequate bladder and prostatic urethral mapping should be considered before partial cystectomy. In addition, all patients need to be counseled before surgery for the possibility of RC in certain scenarios (unable to achieve negative margins on frozen section and inadequate residual bladder volume).
Pelvic lymphadenectomy is an integral component of partial cystectomy to increase survival and to complete cancer staging. Historically, lymphadenectomies performed in partial cystectomies have been less extensive than those performed in RC and therefore inadequate.[10] Surgeons preparing for partial cystectomy must understand that the borders of lymphadenectomy should mirror that of RC. At a minimum, lymphadenectomy should include the external, internal, obturator, and common iliac nodes. Standard lymphadenectomy compared to minimal or no lymphadenectomy has been shown to dramatically increase survival in the RC population.[14]
For well-selected patients, outcomes appear to be reasonable for partial cystectomy. In an analysis of population data, Capitanio et al. found 5-year overall survival and cancer-specific survival for partial cystectomy matched those for RC (57.2% and 70.3% compared to 54.6% and 69.2%) in patients matched for age, race, TNM stage, grade, and number of removed lymph nodes.[10] As summarized in Table 1, overall 5-year survival for patients undergoing partial cystectomy for MIBC is between 50% and 70%, with up to 81% of patients retaining their native bladder at 10 years.[9,10,12,13,15,19,20,21]
Table 1.
Modern studies of partial cystectomy
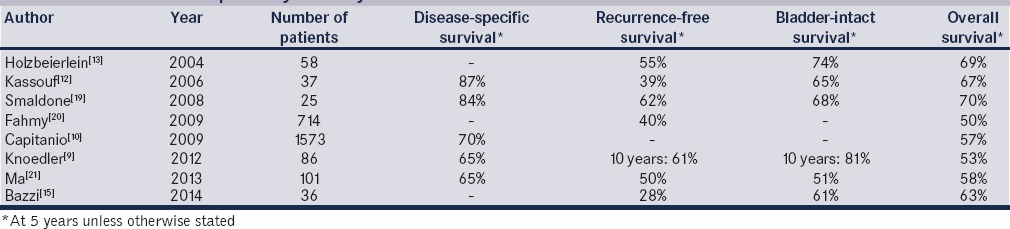
In addition, the question of whether or not to utilize neoadjuvant chemotherapy before partial cystectomy has also been addressed in a small, nonrandomized series. Bazzi et al. reported on their contemporary experience with the use of partial cystectomy postneoadjuvant chemotherapy as a bladder-sparing modality in 36 highly selected patients. They found that at last follow-up, 19 (53%) patients had recurrence, 15 (42%) had advanced recurrences (defined as recurrence that could not be addressed with intravesical therapy or RC), 10 (28%) died of disease, and 1 died of another cause.[15] Five-year recurrence-free survival and overall survival were 28 and 63%, respectively. Furthermore, 56% of patients had no evidence of disease after a median follow-up of 17 months, and 22 (61%) had an intact bladder.[15] The authors concluded that these results were consistent with similar previous small series of oncologic outcomes in patients receiving neoadjuvant chemotherapy followed by partial cystectomy and established this modality as a viable alternative to neoadjuvant chemotherapy and RC in carefully selected patients.[15,16]
Because the bladder remains as a source of recurrence, patients counseled for partial cystectomy must understand that postoperative surveillance is mandatory. In addition to imaging of the chest, upper tracts, abdomen, and pelvis, NCCN guidelines recommend cystoscopy and urine cytology, ± mapping biopsies, in addition to laboratory testing every 3–6 months for 2 years and then increasing intervals as appropriate.[4] Recurrence rates (noninvasive + invasive) have been as high as 38%–49% with 7%–30% of partial cystectomy patients ultimately undergoing RC; salvage RC is usually performed within 2 years after PC.[9,12,17,18] Survival after salvage cystectomy varies within published studies, often due to differences in the amount of available patients. Bruins et al. compared 72 patients with salvage RC to 2218 patients with up-front RC and found the 5-year recurrence-free survival to be similar at 56% and 66%, respectively.[18] Overall 5-year survival for salvage RC was 41% in that series. These findings underscore the great importance of appropriate patient selection and patient compliance with follow-up in implementing partial cystectomy for MIBC.
TRIMODAL THERAPY
Trimodal therapy – so named because it utilizes surgical, chemotherapeutic, and radiation interventions – is the bladder-sparing treatment motif with the widest application. Its use is currently supported by multiple major United States and international guidelines for the treatment of MIBC.[4,22] Although the target population for trimodal therapy is typically larger than for partial cystectomy, there are still critical patient selection factors that must be considered. Ideal selection criteria include traditional urothelial carcinoma pathology without variant pathology such as micropapillary, minimally invasive T2 disease, absence of tumor-associated hydronephrosis, and absence of concurrent CIS.[4,22,23,24] Nevertheless, bladder sparing has been utilized in cT3 and cT4 disease in patients with overall poor performance status or those who have refused RC for advanced disease.[4,23,24,25] Concurrent CIS has been found to be a positive predictor of recurrence.[26,27] Furthermore, trimodal therapy should be avoided in patients with a small bladder as scarring and contracture from radiation therapy may result in a debilitating low-volume bladder and severe urinary symptoms.[28] Nonetheless, trimodal bladder-sparing protocols offer a reasonable alternative to patients who are either surgically unfit or otherwise opposed to RC with or without neoadjuvant chemotherapy.
Transurethral resection of bladder tumor
Aggressive transurethral resection of bladder tumor (TURBT) should be performed in patients pursuing trimodal therapy. The clinician should strive for maximal visible resection of bladder tumor to include the muscularis propria layer. Indeed, the presence of residual tumor after maximal resection with TURBT is an independent predictor of disease recurrence and progression as well as progression to cystectomy.[4,22,27,29,30] In one recent large series of outcomes of trimodal therapy at a major US academic center, patients undergoing visibly complete TURBT eventually required cystectomy in only 22% of cases compared to 42% in patients undergoing incomplete TURBT, P < 0.001.[30] Patients should undergo repeat TURBT 4–6 weeks after initial resection to remove any residual disease.[4,22,23,27]
Likewise, tumor involving the ureter or causing hydronephrosis may imply extravesical involvement. Consideration for RC is prudent in patients with these features on initial or repeat bladder tumor resection. Similarly, the presence of CIS on repeat resection is another poor prognostic factor, and strong consideration should be given to RC with or without neoadjuvant chemotherapy.[4,22,23,24]
Chemotherapy
In the setting of bladder-sparing treatment modalities, the addition of chemotherapy to radiation has been strongly associated with improvement in cancer-related outcomes.[31] This is likely due to elimination of distant micrometastases that cannot be fully controlled with tumor resection and local radiation. One autopsy study reviewed the pathology of 367 patients deceased from MIBC; distant metastases were noted in 68% of these cases.[32] Furthermore, it is well established that the frequency of metastases increases with tumor stage (pT2, 36%; pT3a, 45%; pT3b, 69%; and pT4, 79%).[5] Thus, clinical investigators have hypothesized that concomitant chemotherapy reduces and/or eradicates micrometastatic disease and thus decreases the likelihood of progression and recurrence. To this end, several clinical trials have investigated the effect of various combinations of chemotherapy and radiation versus radiation or chemotherapy alone.
James et al. reporting on the results of a multicenter, phase III randomized controlled trial of patients with pathologically confirmed T2, T3, or T4a bladder without evidence of nodal involvement or metastasis, observed that addition of synchronous chemotherapy with fluorouracil and mitomycin-C to radiotherapy improved locoregional control of bladder cancer as compared to radiotherapy alone.[31] In this trial reported in the New England Journal of Medicine, 2-year rates of locoregional disease-free survival were 67% in the chemo + radiotherapy group but only 54% in the radiotherapy alone group. With an excellent median overall follow-up 69.9 months, the hazard ratio in the chemoradiotherapy group was 0.68 (P = 0.03), and 5-year overall survival was 48% in the chemoradiotherapy group compared to 35% in the radiotherapy group. Although grade 3 or 4 adverse events were slightly more common in the chemoradiotherapy group than in the radiotherapy alone group during treatment (36.0% vs. 27.5%, P = 0.07), there was little difference in the rate of adverse events in follow-up (8.3% vs. 15.7%, P = 0.07).[31] This trial further underscores the assertion that radiation therapy and chemotherapy are superior to chemotherapy or radiation alone in urothelial carcinoma.
Seeking to evaluate the safety, efficacy, and tolerability of chemotherapy regimens in patients undergoing trimodal therapy, RTOG 0233 enrolled patients with T2-4a transitional cell carcinoma and randomly allocated patients to receive paclitaxel plus cisplatin or fluorouracil plus cisplatin with twice-daily radiation on the basis of clinical T-stage (T2 vs. T3-4). This trial demonstrated a lower overall level of treatment-related toxicity in the fluorouracil group versus the paclitaxel group with 34 of 40 patients (85%) in the paclitaxel group developing grade 3 or 4 toxicity during adjuvant chemotherapy versus 31 of 41 patients (76%) in the fluorouracil group.[33] Thus, current protocols typically utilize cisplatin with fluorouracil as a first-line regimen. Alternatively, a combination of methotrexate, vincristine, adriamycin, and cisplatin may also be utilized.[27] In patients who cannot tolerate or are poor candidates for cisplatin-based chemotherapy, 5-fluorouracil plus mitomycin-C or low-dose gemcitabine may be suitable alternatives although these regimens are less well studied than cisplatin-based protocols.[4,22,23,27]
Radiation therapy
There are two typical templates for radiation with radiosensitizing chemotherapy in trimodal therapy. The first involves administration of 40–45 Gy of radiation with induction chemotherapy, followed by cystoscopy and rebiopsy of any residual tumor, and then administration of consolidation chemoradiation with an additional 25 Gy or radiation if the biopsy is negative. In contrast, the alternative protocol includes full-dose chemoradiation to a total of 55–65 Gy followed by cystoscopic rebiopsy and then surveillance if the rebiopsy is negative.[4,22,23,24,29,34,35] Both protocols utilize standard fractionation of 1.8–2 Gy/fraction with the total radiation dose to the bladder being approximately 55–70 Gy and 45–50 Gy to the pelvic lymph nodes. Four retrospective reviews have identified total radiation dose >55–60 Gy to be associated with superior local control of MIBC.[36,37,38,39] In addition, Pos et al. found a dose–response relationship in invasive bladder cancer with increasing local control with radiation dose escalation.[40] Either conventional or accelerated hyperfractionation schemes are acceptable treatment options.[4,22,29] One important consideration is that salvage cystectomy may be more technically difficult in a patient undergoing full-dose chemoradiation versus those undergoing a split treatment protocol with induction and then mid-treatment cystoscopic examination of clinical response although there are no specific study data to support this claim.
Assessing response to trimodal therapy
After completing staged TURBT and either split- or single-course chemoradiation, patients should undergo repeat chest imaging, cystoscopy with rebiopsy of the primary tumor site, and abdominal/pelvic (contrast-enhanced computed tomography or magnetic resonance imaging) imaging to assess for treatment response. In patients with evidence of nonmetastatic residual disease or recurrence, the treating physician should strongly suggest proceeding to salvage cystectomy with urinary diversion. Patients who initially underwent split-course radiation and have a negative rebiopsy they may proceed to consolidative chemoradiation. Patients who underwent single-course chemoradiation may proceed to surveillance if repeat biopsy is negative.[4,22,23,24,27]
Surveillance
Patients who choose to undergo trimodal therapy should be counseled that they are committing to meticulous and likely lifelong surveillance. This follow-up should consist of regular cystoscopy every 3 months and imaging of the chest, abdomen, and pelvis every 3–6 months for the first 2 years. Patients with evidence of disease recurrence on repeat cystoscopy should be offered prompt salvage cystectomy.[4,23,26,27]
Outcomes of trimodal therapy
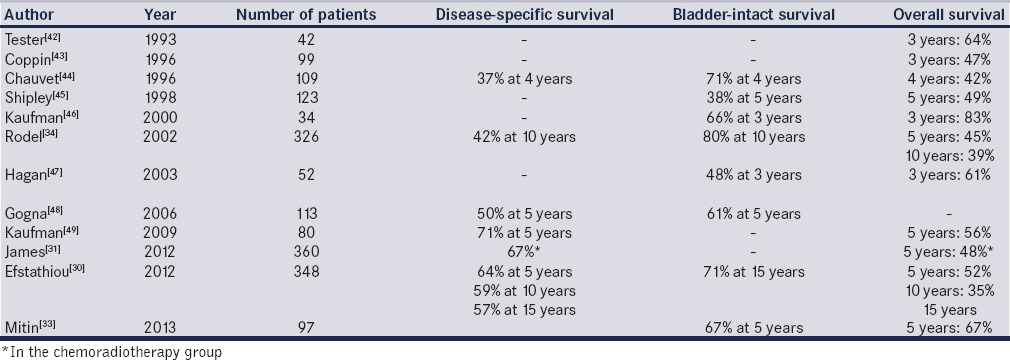
Long-term data from trials in the past 5 years suggest that approximately 70% (69%–78%) of patients treated with trimodal therapy will achieve a complete response with TURBT and chemoradiation, and we have summarized the outcomes of major modern trials in [Table 2].[30,31,33,35,41,42,43,44,45,46,47,48,49] Mak et al. report that in a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233, complete response to trimodal therapy was observed in 69% of patients, and the 5- and 10-year overall survival rates were 57% and 36%, respectively. Disease-specific survival rates at 5 and 10 years in this study were 71% and 65%, respectively, at a median follow-up of 4.3 years.[41] One large review found that 72% of patients had complete response to trimodal therapy, and 5-, 10-, and 15-year disease-specific survival rates were 64%, 59%, and 57%, respectively (T2 = 74%, 67%, and 63%; T3-4 = 53%, 49%, and 49%) This study also demonstrated 5-, 10-, and 15-year overall survival rates of 52%, 35%, and 22%, respectively (T2: 61%, 43%, and 28%; T3-4 = 41%, 27%, and 16%). Among those patients demonstrating complete response, 10-year rates of noninvasive, invasive, pelvic, and distant recurrences were 29%, 16%, 11%, and 32%, respectively.[30] These results are comparable with overall rates of disease-specific and overall survival in patients treated with early cystectomy in MIBC.[22,23,24,27,30,41]
Table 2.
Modern studies of bladder preservation with chemoradiotherapy (stage cT2-pT4a)
Another important consideration in patients undergoing trimodal therapy is long-term QoL. Although limited by small study populations, several reports to date have demonstrated good long-term QoL in patients who pursue trimodal for MIBC.[28,50] One contemporary study of patients who underwent trimodal therapy demonstrated statistically significant higher scores of QoL at a median follow-up of 6 years posttreatment on validated QoL surveys when compared to patients who underwent RC although this was not a randomized study.[7] This supports the need to at least discuss the option of trimodal therapy in well-selected patients.
CONCLUSIONS
In appropriately selected patients, bladder-sparing treatment modalities may play a useful role in the management of MIBC. Recent nonrandomized studies have demonstrated comparable overall survival in patients undergoing bladder preservation when compared to patients undergoing RC, even when adjusting for increasing clinical stage. Additional randomized controlled studies evaluating the long-term oncologic efficacy of bladder-sparing therapy for MIBC in subpopulations with more advanced comorbidities are needed to further define exactly whom to offer bladder-sparing modalities. Furthermore, re-evaluating QoL in each treatment pathway could yield additional useful information to help patients select the most appropriate clinical pathway to balance treatment-associated morbidity with cancer control. In highly selected patients, both partial cystectomy and trimodal therapy can serve as appropriate therapies, even in patients with invasive bladder cancer.
Acknowledgments
We would like to thank Dr. Ashish Kamat, MD, M.D. Anderson Cancer Center, Houston, Texas, USA.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 2.Yuh BE, Ruel N, Wilson TG, Vogelzang N, Pal SK. Pooled analysis of clinical outcomes with neoadjuvant cisplatin and gemcitabine chemotherapy for muscle invasive bladder cancer. J Urol. 2013;189:1682–6. doi: 10.1016/j.juro.2012.10.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–5. doi: 10.1016/j.eururo.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Clark PE, Spiess PE, Agarwal N, Bangs R, Boorjian S, Buyyounouski M, et al. Bladder cancer. NCCN Clinical Practice Guidelines in Oncology 2016. J Natl Compr Canc Netw. 2016;14:1213–1224. doi: 10.6004/jnccn.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 6.Stimson CJ, Chang SS, Barocas DA, Humphrey JE, Patel SG, Clark PE, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184:1296–300. doi: 10.1016/j.juro.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Caffo O, Fellin G, Graffer U, Luciani L. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma. A survey by a self-administered questionnaire. Cancer. 1996;78:1089–97. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1089::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 8.Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: Results from the National Cancer Data Base. Eur Urol. 2013;63:823–9. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Knoedler JJ, Boorjian SA, Kim SP, Weight CJ, Thapa P, Tarrell RF, et al. Does partial cystectomy compromise oncologic outcomes for patients with bladder cancer compared to radical cystectomy? A matched case-control analysis. J Urol. 2012;188:1115–9. doi: 10.1016/j.juro.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 10.Capitanio U, Isbarn H, Shariat SF, Jeldres C, Zini L, Saad F, et al. Partial cystectomy does not undermine cancer control in appropriately selected patients with urothelial carcinoma of the bladder: A population-based matched analysist. Urology. 2009;74:858–64. doi: 10.1016/j.urology.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney P, Kursh ED, Resnick MI. Partial cystectomy. Urol Clin North Am. 1992;19:701–11. [PubMed] [Google Scholar]
- 12.Kassouf W, Swanson D, Kamat AM, Leibovici D, Siefker-Radtke A, Munsell MF, et al. Partial cystectomy for muscle invasive urothelial carcinoma of the bladder: A contemporary review of the M. D. Anderson Cancer Center experience. J Urol. 2006;175:2058–62. doi: 10.1016/S0022-5347(06)00322-3. [DOI] [PubMed] [Google Scholar]
- 13.Holzbeierlein JM, Lopez-Corona E, Bochner BH, Herr HW, Donat SM, Russo P, et al. Partial cystectomy: A contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004;172:878–81. doi: 10.1097/01.ju.0000135530.59860.7d. [DOI] [PubMed] [Google Scholar]
- 14.Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: A cooperative group report. J Clin Oncol. 2004;22:2781–9. doi: 10.1200/JCO.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Bazzi W, Kopp R, Donahue T, Bernstein M, Russo P, Bochner B, et al. Partial cystectomy post neoadjuvant chemotherapy: Memorial Sloan-Kettering Cancer Center contemporary experience. J Urol. 2014;191:556. doi: 10.1155/2014/702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons MD, Smith AB. Surgical bladder-preserving techniques in the management of muscle-invasive bladder cancer. Urol Oncol. 2016;34:262–70. doi: 10.1016/j.urolonc.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Eswara JR, Efstathiou JA, Heney NM, Paly J, Kaufman DS, McDougal WS, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle invasive bladder cancer. J Urol. 2012;187:463–8. doi: 10.1016/j.juro.2011.09.159. [DOI] [PubMed] [Google Scholar]
- 18.Bruins HM, Wopat R, Mitra AP, Cai J, Miranda G, Skinner EC, et al. Long-term outcomes of salvage radical cystectomy for recurrent urothelial carcinoma of the bladder following partial cystectomy. BJU Int. 2013;111(3 Pt B):E37–42. doi: 10.1111/j.1464-410X.2012.11438.x. [DOI] [PubMed] [Google Scholar]
- 19.Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RL., Jr Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology. 2008;72:613–6. doi: 10.1016/j.urology.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 20.Fahmy N, Aprikian A, Tanguay S, Mahmud SM, Al-Otaibi M, Jeyaganth S, et al. Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol. 2010;28:419–23. doi: 10.1007/s00345-009-0478-x. [DOI] [PubMed] [Google Scholar]
- 21.Ma B, Li H, Zhang C, Yang K, Qiao B, Zhang Z, et al. Lymphovascular invasion, ureteral reimplantation and prior history of urothelial carcinoma are associated with poor prognosis after partial cystectomy for muscle-invasive bladder cancer with negative pelvic lymph nodes. Eur J Surg Oncol. 2013;39:1150–6. doi: 10.1016/j.ejso.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmström PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 24.Efstathiou JA, Saylor P, Wszolek M, Giacalone NJ. Bladder preservation treatement options for muscle-invasive urothelial bladder cancer. UpToDate. 2016. [Last accessed on 2015 Oct 27]. Available on: http://www.uptodate.com/contents/bladder-preservation-treatment-options-for-muscle-invasive-urothelial-bladder-cancer .
- 25.Chou R, Selph SS, Buckley DI, Gustafson KS, Griffin JC, Grusing SE, et al. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer. 2016;122:842–51. doi: 10.1002/cncr.29843. [DOI] [PubMed] [Google Scholar]
- 26.Choueiri TK, Raghavan D. Chemotherapy for muscle-invasive bladder cancer treated with definitive radiotherapy: Persisting uncertainties. Nat Clin Pract Oncol. 2008;5:444–54. doi: 10.1038/ncponc1159. [DOI] [PubMed] [Google Scholar]
- 27.Biagioli MC, Fernandez DC, Spiess PE, Wilder RB. Primary bladder preservation treatment for urothelial bladder cancer. Cancer Control. 2013;20:188–99. doi: 10.1177/107327481302000307. [DOI] [PubMed] [Google Scholar]
- 28.Zietman AL, Sacco D, Skowronski U, Gomery P, Kaufman DS, Clark JA, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: Results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772–6. doi: 10.1097/01.ju.0000093721.23249.c3. [DOI] [PubMed] [Google Scholar]
- 29.Ott OJ, Rödel C, Weiss C, Wittlinger M, St. Krause F, Dunst J, et al. Radiochemotherapy for bladder cancer. Clin Oncol (R Coll Radiol) 2009;21:557–65. doi: 10.1016/j.clon.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Efstathiou JA, Spiegel DY, Shipley WU, Heney NM, Kaufman DS, Niemierko A, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol. 2012;61:705–11. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 31.James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 32.Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urol Int. 1999;62:69–75. doi: 10.1159/000030361. [DOI] [PubMed] [Google Scholar]
- 33.Mitin T, Hunt D, Shipley WU, Kaufman DS, Uzzo R, Wu CL, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): A randomised multicentre phase 2 trial. Lancet Oncol. 2013;14:863–72. doi: 10.1016/S1470-2045(13)70255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rödel C, Grabenbauer GG, Kühn R, Papadopoulos T, Dunst J, Meyer M, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol. 2002;20:3061–71. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 35.Mitin T, George A, Zietman AL, Heney NM, Kaufman DS, Uzzo RG, et al. Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: A Pooled Analysis of NRG oncology/RTOG 9906 and 0233. Int J Radiat Oncol Biol Phys. 2016;94:67–74. doi: 10.1016/j.ijrobp.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greven KM, Solin LJ, Hanks GE. Prognostic factors in patients with bladder carcinoma treated with definitive irradiation. Cancer. 1990;65:908–12. doi: 10.1002/1097-0142(19900215)65:4<908::aid-cncr2820650415>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Moonen L, vd Voet H, de Nijs R, Horenblas S, Hart AA, Bartelink H. Muscle-invasive bladder cancer treated with external beam radiation: Influence of total dose, overall treatment time, and treatment interruption on local control. Int J Radiat Oncol Biol Phys. 1998;42:525–30. doi: 10.1016/s0360-3016(98)00263-6. [DOI] [PubMed] [Google Scholar]
- 38.Quilty PM, Kerr GR, Duncan W. Prognostic indices for bladder cancer: An analysis of patients with transitional cell carcinoma of the bladder primarily treated by radical megavoltage X-ray therapy. Radiother Oncol. 1986;7:311–21. doi: 10.1016/s0167-8140(86)80060-3. [DOI] [PubMed] [Google Scholar]
- 39.Smaaland R, Akslen LA, Tønder B, Mehus A, Lote K, Albrektsen G. Radical radiation treatment of invasive and locally advanced bladder carcinoma in elderly patients. Br J Urol. 1991;67:61–9. doi: 10.1111/j.1464-410x.1991.tb15071.x. [DOI] [PubMed] [Google Scholar]
- 40.Pos FJ, Hart G, Schneider C, Sminia P. Radical radiotherapy for invasive bladder cancer: What dose and fractionation schedule to choose? Int J Radiat Oncol Biol Phys. 2006;64:1168–73. doi: 10.1016/j.ijrobp.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32:3801–9. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tester W, Porter A, Asbell S, Coughlin C, Heaney J, Krall J, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma: Results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25:783–90. doi: 10.1016/0360-3016(93)90306-g. [DOI] [PubMed] [Google Scholar]
- 43.Coppin CM, Gospodarowicz MK, James K, Tannock IF, Zee B, Carson J, et al. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14:2901–7. doi: 10.1200/JCO.1996.14.11.2901. [DOI] [PubMed] [Google Scholar]
- 44.Chauvet B, Brewer Y, Félix-Faure C, Davin JL, Choquenet C, Reboul F. Concurrent cisplatin and radiotherapy for patients with muscle invasive bladder cancer who are not candidates for radical cystectomy. J Urol. 1996;156:1258–62. [PubMed] [Google Scholar]
- 45.Shipley WU, Winter KA, Kaufman DS, Lee WR, Heney NM, Tester WR, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576–83. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman DS, Winter KA, Shipley WU, Heney NM, Chetner MP, Souhami L, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: Phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–6. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 47.Hagan MP, Winter KA, Kaufman DS, Wajsman Z, Zietman AL, Heney NM, et al. RTOG 97-06: Initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665–72. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 48.Gogna NK, Matthews JH, Turner SL, Mameghan H, Duchesne GM, Spry N, et al. Efficacy and tolerability of concurrent weekly low dose cisplatin during radiation treatment of localised muscle invasive bladder transitional cell carcinoma: A report of two sequential Phase II studies from the Trans Tasman Radiation Oncology Group. Radiother Oncol. 2006;81:9–17. doi: 10.1016/j.radonc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman DS, Winter KA, Shipley WU, Heney NM, Wallace HJ, 3rd, Toonkel LM, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–7. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 50.Mak KS, Smith AB, Eidelman A, Clayman RH, Cheng JS, Matthews J, et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. J Clin Oncol. 2015;33 doi: 10.1016/j.ijrobp.2016.08.023. Supplemental 7: Abstract 319. [DOI] [PubMed] [Google Scholar]


