Abstract
Objectives
To describe our experience with non‐invasive prenatal testing (NIPT) in twin pregnancy.
Methods
Two sets of maternal blood samples from twin pregnancies were analyzed at our laboratory using NIPT: 115 stored samples from pregnancies with known outcome (Clinical Study A) and 487 prospectively collected samples for which outcomes were requested from providers (Clinical Study B). NIPT was used to screen for the presence of fetal aneuploidy on chromosomes 13, 18, 21, X and Y in all cases, and results were compared with outcomes when known.
Results
In Clinical Study A, all 115 samples were classified correctly by NIPT: three cases of trisomy 21 (one fetus affected), one of monochorionic trisomy 18 (both fetuses affected) and 111 euploid. In Clinical Study B, a NIPT result was reported for 479 (98.4%) of the 487 samples. Aneuploidy was detected or suspected in nine (1.9%) cases: seven cases of trisomy 21 detected, one case of trisomy 21 suspected and one case with trisomy 21 detected and trisomy 18 suspected. Information on aneuploidy outcome was available for 171 (35.7%) cases in Clinical Study B. Of the nine cases with aneuploidy detected or suspected, six were confirmed to be a true positive in at least one twin based on karyotype or birth outcome and two were suspected to be concordant based on ultrasound findings; the one known discordant result was for the aneuploidy suspected case. No false negatives were reported.
Conclusion
NIPT performed well in the detection of trisomy 21 in twin pregnancy, with a combined false‐positive frequency for trisomies 13, 18 and 21 of 0% for Clinical Study A and 0.2% for Clinical Study B. © 2016 Illumina. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: chromosomal aneuploidy, false‐positive rate, non‐invasive prenatal testing, trisomy 21, twin pregnancy
INTRODUCTION
Massively parallel next‐generation whole‐genome sequencing (WGS) of cell‐free DNA (cfDNA) from maternal plasma has been shown to have high sensitivity and specificity for the detection of trisomies 21, 18 and 13, and for sex chromosome analysis in singleton pregnancies1, 2, 3, 4. However, twins account for approximately 1 in 30 live births in the USA, and the rate of twin births is increasing5. With a high proportion of twin births thought to originate in women undergoing assisted reproductive technology (ART), the use of non‐invasive prenatal testing (NIPT) to screen for fetal aneuploidy is especially desirable. Traditionally, prenatal aneuploidy screening options have been less robust for twin pregnancies than for singletons6, whereas the miscarriage risk associated with invasive diagnostic procedures is higher in twins7. Preliminary data have suggested that NIPT is a feasible test option for twin gestations8, 9, 10. Currently, due to the paucity of reported studies in twins, professional societies and others have called for more studies on NIPT performance in twin gestations11, 12, 13, 14.
One of the factors governing NIPT performance is the fetal contribution to the cfDNA present in maternal plasma, known as the fetal fraction, with NIPT being offered from around 10 weeks of gestation because of the lower fetal contribution at earlier gestational ages. Although the total fetal fraction has been shown to be as much as 35% higher in twin pregnancies when compared with singletons10, the fetal fraction per twin is lower8, 9. Furthermore, it has been shown that individual cfDNA contribution from each twin could differ by as much as two‐fold15, 16. The complexity of the fetal fraction in twin gestations has raised concerns about a potentially increased false‐negative rate of NIPT in twin gestations. Furthermore, single‐nucleotide polymorphism‐based technologies or targeted sequencing technologies for NIPT are not currently offered clinically for twin gestations.
The primary objective of this study was to describe the clinical laboratory experience of a WGS‐based NIPT in twin gestations. The secondary objective was to estimate the fetal fraction in clinical samples from twin pregnancies.
METHODS
Patients and sample collection
Clinical Study A included frozen plasma samples from twin pregnancies with known outcomes that were collected as part of two independent clinical studies, MELISSA (MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy) and CARE (Comparison of Aneuploidy Risk Evaluations), in high‐risk and all‐risk pregnant populations, as described previously1, 17. Briefly, a minimum of 7 mL of whole blood was collected in acid citrate dextrose1 or cfDNA blood‐collection (Streck) tubes17 and shipped either overnight in temperature‐controlled (cooled) conditions (acid citrate dextrose tubes) or in ambient shippers within 5 days of blood draw (Streck tubes) to the Illumina R&D Laboratory (Redwood City, CA, USA), where samples were inspected and plasma was prepared and stored at –80°C until sequencing. cfDNA was isolated from the plasma by centrifugation at 1600 g for 10 min. A second 10‐min centrifugation step was performed on the supernatant after transfer to a fresh tube or plate1, 17, 18, 19. Institutional review boards at each collection site approved the studies and written informed consent was obtained from each patient. In both studies, women ≥ 18 years of age with a twin pregnancy ≥ 8 weeks' gestation were eligible for inclusion; 1.7% (n = 2) of samples were obtained at < 10 weeks' gestation. For the MELISSA study, patients with a gestational age > 22 weeks were excluded and eligibility criteria did not require an invasive procedure (amniocentesis or chorionic villus sampling) prior to enrollment1. For the CARE study, eligibility criteria required that a minimum of 2 weeks had elapsed between an invasive procedure, if performed, and the blood draw for cfDNA testing17; none of the samples in this study was drawn after an invasive procedure. Patients in the CARE study were also excluded if prenatal screening for aneuploidy was carried out by measurement of nuchal translucency only. Data on clinical outcome detailing fetal karyotype from invasive prenatal procedures and/or newborn physical examination were entered into an electronic database by research personnel. Research laboratory personnel who carried out the sequencing were blinded to the clinical outcome data. Classification by sequencing was compared with clinical outcome for all subjects; cfDNA‐based NIPT results were not reported to the patients.
Clinical Study B included fresh maternal blood samples indicated as twin gestation on the test requisition forms (TRFs) that were received during the study period at the College of American Pathologists‐accredited and Clinical Laboratory Improvement Act‐certified Illumina Laboratory from providers in the USA requesting the commercially available verifi® Prenatal Test (Illumina, Inc., San Diego, CA, USA). Samples received from distributor laboratories and/or health systems located in the USA were excluded due to the inability to obtain clinical follow‐up. Although unlikely, it is possible that some of the samples in Clinical Study B were obtained after an invasive procedure (for which the timing is unknown). Demographic information, such as maternal age, gestational age and clinical indication for testing, was obtained from the TRF. Reasons for testing indicated on the TRF were the following: advanced maternal age (AMA), abnormal ultrasound finding, previous affected pregnancy or positive serum screen (determined by local community standards); clinicians could select multiple options. NIPT yielded a report that was sent to physicians with results of aneuploidy status for chromosomes 21, 18 and 13 (‘aneuploidy detected’, ‘no aneuploidy detected’ or ‘aneuploidy suspected’, as described previously1) and presence of Y (‘detected’ or ‘not detected’), if requested. Testing of clinical samples could be canceled due to either administrative (insufficient sample quantity, gestational age at sampling < 10 weeks or patient or physician request) or technical (failure to meet quality‐control metrics, laboratory processing issue or insufficient or high cfDNA concentration) reasons18, 20. Providers were notified if the test was canceled and offered the option to submit a second sample.
Sample preparation and analysis
cfDNA was extracted from frozen (Clinical Study A) and fresh (Clinical Study B) plasma samples; 1 mL of plasma was required for analysis. Sequencing libraries were prepared using the Illumina TruSeq DNA Sample Prep Kit and sequencing was performed on Illumina HiSeq 2000 sequencers (Illumina, Inc.), as described previously for singleton pregnancies1, 17, 18. Sequence alignment and tag counting methods have been described previously1, 19. Analysis was performed using SAFeR (Selective Algorithm for Fetal Results), which incorporated several important updates to the analytic platform, including improved genomic filtering, removal of systematic biases and improved normalization and classification approaches. These updates were designed to improve the assay limitation of detection, theoretically enabling sufficient sensitivity for aneuploidy detection in twin gestations.
Fetal fraction estimates were made using tags on the X chromosome and/or chromosome 21, as described previously21. Fetal fraction estimates based on X chromosome tags were made for samples in which aneuploidy was not detected and the presence of Y was detected, and for samples reported as aneuploidy detected for trisomy 21 with presence of Y for which fetal karyotype was known. Fetal fraction estimates using tags on chromosome 21 were performed for samples reported as trisomy 21 detected for which fetal karyotype or birth outcome was known. Fetal fraction estimates were not determined for samples that did not have either presence of Y or trisomy 21 detected. These estimates were made for the purpose of this study analysis only; no fetal fraction cut‐off limit for reporting was applied to samples. At the time of the study, fetal fraction estimates were not reported to patients by the Illumina laboratory.
Clinical outcomes
An active follow‐up process was utilized to obtain information on fetal karyotype and sex (confirmed by invasive diagnostic procedure, newborn testing/physical examination or ultrasound evaluation) for all clinical cases according to standard laboratory practice and quality procedures, as described previously18, 22. The estimated delivery date had passed for all samples at the time of outcome collection. Fetal sex of each twin was requested for all cases in which sex chromosome status by NIPT was ordered on the TRF.
When aneuploidy was detected or suspected for chromosomes 13, 18 and/or 21, cases were categorized as (1) ‘concordant’ if NIPT results matched the karyotype or birth outcome for one or both twins (true positive); (2) ‘discordant’ if NIPT results did not match the karyotype or birth outcome of either twin (false positive); (3) ‘suspected to be concordant’ if karyotype information was unavailable but other indicators suggestive of aneuploidy, such as abnormal ultrasound findings, were present; or (4) ‘no information’ if outcome information was insufficient to determine or suspect concordance, or was not available to the laboratory because a practice failed to respond to our request for outcomes. When aneuploidy was not detected, cases were categorized as (1) ‘concordant’ if both twins were determined to be unaffected by karyotyping or birth outcome (true negative); (2) ‘discordant’ if one or both twins were determined to be affected by karyotyping or birth outcome (false negative); or (3) ‘no information’, as above.
For fetal sex, reported as the presence or absence of Y, cases were categorized as (1) ‘concordant’ if presence of Y was reported and one or both twins were determined from clinical outcomes or ultrasound evaluation to have a Y chromosome (XY, XXY or XYY), or absence of Y was reported and both twins lacked the Y chromosome (monosomy X, XX or XXX); (2) ‘discordant’ if presence of Y was reported and both twins lacked a Y chromosome (monosomy X, XX or XXX), or absence of Y was reported and one or both twins was determined to have a Y chromosome (XY, XXY or XYY); or (3) ‘no information’, as above. Sex chromosome abnormality testing was not available to patients with twin gestations; these were reported as presence or absence of Y only.
Statistical analysis
Statistical significance was determined by an unpaired Student's t‐test for continuous variables, a chi‐square test for categorical variables and the Wilcoxon test for non‐parametric values. A P‐value < 0.05 was considered statistically significant. Analyses were performed using the R statistical package (version 2.12.0; R Foundation for Statistical Computing, Vienna, Austria) and Microsoft Excel statistical tool.
Results
Demographic characteristics
Demographic characteristics of the two study cohorts of Clinical Studies A and B are shown in Table 1. Mean maternal age was similar between the two cohorts. Mean gestational age at sampling was significantly lower in Clinical Study B (P < 0.0001).
Table 1.
Demographic characteristics of twin pregnancies with non‐invasive prenatal testing included in Clinical Studies A and B
| Characteristic | Clinical Study A (n = 115) | Clinical Study B (n = 487) | P |
|---|---|---|---|
| Maternal age (years) | 34.4 ± 6.1 (18.9–48.9) | 35.5 ± 4.9 (18.3–53.5) | 0.1016 |
| Gestational age (weeks) | 16.6 ± 6.5 (8–35) | 13.7 ± 3.9 (9–32)* | < 0.0001 |
| Trimester | < 0.0001 | ||
| First (≤ 13 weeks) | 55 (47.8) | 333 (68.4) | |
| Second (14–27 weeks) | 49 (42.6) | 149 (30.6) | |
| Third (28–40 weeks) | 11 (9.6) | 5 (1.0) |
Data are given as mean ± SD (range) or n (%).
Testing of samples obtained at < 10 weeks' gestation was canceled.
Clinical Study A
Clinical Study A included frozen plasma samples from 115 twin gestations: three from trisomy 21‐affected pregnancies, one from a trisomy 18‐affected pregnancy and 111 from unaffected twin gestations (Table 2). In this cohort, 53.0% (61/115) of twin pregnancies were patients undergoing ART, including six cases of ovum donor pregnancies. Conventional prenatal aneuploidy screening results (serum biomarkers ± ultrasound measure of nuchal translucency thickness), carried out using various screening types including sequential and first‐trimester combined testing, were available for 82 patients. Of these results, 20 (24.4%) were false positives and two (2.4%) were false negatives; of the false‐positive results, 90% (18/20) were for trisomy 21 and 10% (2/20) were for trisomy 18. Trisomy 18 results for twin gestations could not be provided by conventional screening approaches in 32.9% (27/82) of patients, depending on the laboratory and type of screening used. In comparison, all 115 samples in Clinical Study A were sequenced and analyzed, and generated an NIPT result (Table 2). All four samples from pregnancies with at least one aneuploid fetus were identified correctly by NIPT for the appropriate aneuploid chromosome (sensitivity, 100%); one of these cases was from a patient undergoing ART. These four affected samples were obtained at an average gestational age of 17.3 (range, 11.6–32.9) weeks. None of the clinically defined unaffected samples was classified as aneuploidy detected or suspected (specificity, 100%). Additionally, the presence or absence of the Y chromosome was identified correctly in all samples (Table 2).
Table 2.
Results of non‐invasive prenatal testing (NIPT) in 115 twin pregnancies with known clinical outcome included in Clinical Study A
| Sample size (n) | Clinical outcome | NIPT result | ||
|---|---|---|---|---|
| Twin A | Twin B | Aneuploidy | Chromosome Y | |
| 24 | 46,XX | 46,XX | Not detected | Absent |
| 45 | 46,XX | 46,XY | Not detected | Present |
| 42 | 46,XY | 46,XY | Not detected | Present |
| 2 | 47,XY + 21 | 46,XY | T21 detected | Present |
| 1 | Mosaic 47,XY + 21 [7]/46,XY [11] | 46,XX | T21 detected | Present |
| 1 | 47,XY + 18 | 47,XY + 18 | T18 detected | Present |
| DR: 91/91 (100%); Spec: 24/24 (100%) | ||||
DR, detection rate; Spec, specificity; T18, trisomy 18; T21, trisomy 21.
Clinical Study B
A total of 487 fresh maternal blood samples from twin gestations met the inclusion criteria for the clinical outcome study. Zygosity, chorionicity and method of conception were not reported to the laboratory for most samples. NIPT results were reported in 98.4% (479/487) of cases with an average turnaround time of 3.2 business days. Eight (1.6%) tests were canceled, none due to technical reasons. Of the 479 reported NIPT results, seven (1.5%) were reported as aneuploidy detected for trisomy 21, one (0.2%) as aneuploidy suspected for trisomy 21, one (0.2%) as aneuploidy detected for trisomy 21 and aneuploidy suspected for trisomy 18 and 470 (98.1%) as no aneuploidy detected. Fetal sex determination was requested in 87.5% (419/479) of cases. Of these, presence of Y was reported in 70.9% (297/419) and absence of Y reported in 29.1% (122/419).
Information on aneuploidy outcome was available for 171 (35.7%) cases; no false negatives were reported. Of the nine cases reported as aneuploidy detected or suspected, six were confirmed as true positives, one was a false positive and two were unconfirmed but were suspected to be concordant based on ultrasound findings (Table 3). All aneuploidy detected cases were either confirmed or had ultrasound findings suggestive of aneuploidy; the one discordant result was an aneuploidy suspected case. In this single discordant case, a fetal microduplication of 1.27 megabases on chromosome 20q11 was reported to the laboratory; the significance of this finding with respect to the discordant NIPT result is unclear. No information on maternal genotype was available for this case so we were unable to determine whether the microduplication was maternally inherited. Both unconfirmed aneuploidy detected/suspected cases, including the double aneuploidy case, had ultrasound findings that could be consistent with the NIPT result; in one case there was demise of the twin post blood draw. Based on the one reported false‐positive case, the observed false‐positive frequency in the clinical study population was 0.2% (1/479); if the two unconfirmed cases were also false positives, the false‐positive frequency would have been 0.6% (3/479). However, for aneuploidy detected cases only, the observed false‐positive frequency was 0.0% (0/479).
Table 3.
Clinical outcome in nine twin pregnancies with non‐invasive prenatal testing (NIPT) result of aneuploidy detected or suspected included in Clinical Study B
| Sample | NIPT result | Clinical outcome | ||||
|---|---|---|---|---|---|---|
| Aneuploidy | Chromosome Y | Twin A | Twin B | Source | Details | |
| 1 | T21 detected | Present | XY + 21 | XY | CVS | |
| 2 | T21 detected | Present | XY + 21 | XY | CVS | |
| 3 | T21 detected | Present | XY + 21 | XX | Amniocentesis | Triplet to dizygotic twins |
| 4 | T21 detected | Present | XY + 21 | XX | Amniocentesis | |
| 5 | T21 detected | Present | XX + 21 | XY | Visual exam at birth | |
| 6 | T21 detected | NA* | XY + 21 | XX | Cord blood analysis | |
| 7 | T21 suspected | Present | XY | XX | Amniocentesis | 1.27 Mb microduplication of 20q11 in one fetus |
| 8 | T21 detected | Absent | Abnormal findings† | Normal findings | Ultrasound exam | |
| 9 |
T21 detected, T18 suspected |
Present | Demise | NA | ||
Of the six clinical cases with confirmed fetal trisomy, three underwent selective reduction of affected twin, two delivered both twins and the other case began as a triplet pregnancy with demise of one fetus at 6 weeks; amniocentesis of the remaining two viable fetuses confirmed trisomy 21 in one and normal karyotype in the other.
Fetal sex information not requested.
Included increased nuchal translucency, absent stomach, pyelectasis and polyhydramnios.
CVS, chorionic villus sampling; Mb, megabases; NA, not available; T, trisomy.
Of the 419 cases for which fetal sex information was requested, 156 (37.2%) had this information reported to the laboratory (Table 4); fetal sex was identified by ultrasound in 16 of these 156 cases. For three cases with a NIPT report indicating an absence of Y chromosome, one twin was confirmed to be female but the sex of the other twin was not reported because of cotwin demise. Of the 153 cases with sufficient clinical information for comparison with NIPT results, concordance for the presence of Y (one or both twins were male) was confirmed in 99.1% (115/116) of cases and concordance for absence of Y (both twins were female) was confirmed in 97.3% (36/37); there were two cases of fetal gender discordance reported (Table 4). Therefore, in the majority of cases, presence or absence of Y was indicative of the true status of the fetuses.
Table 4.
Results of non‐invasive prenatal testing (NIPT) for presence or absence of Y chromosome in 479 twin pregnancies included in Clinical Study B
| Clinical sex | NIPT result | Total | ||
|---|---|---|---|---|
| Presence of Y | Absence of Y | Sex not requested | ||
| XX | 0 | 3 | 0 | 3 |
| XX/XX | 1 | 36 | 2 | 39 |
| XY | 4 | 0 | 0 | 4 |
| XX/XY | 58 | 1 | 2 | 61 |
| XX/XXY | 1 | 0 | 0 | 1 |
| XY/XY | 52 | 0 | 1 | 53 |
| No information | 181 | 82 | 55 | 318 |
| Total | 297 | 122 | 60 | 479 |
| DR: 115/116 (99.1%)* | Spec: 36/37 (97.3%)* | |||
Excludes cases with insufficient outcome data to determine detection rate (DR) and specificity (Spec).
Fetal fraction
Fetal fraction estimates were calculated for clinical samples for which the presence of Y was reported as detected and fetal gender outcome was known for both twins (Figure 1). For twin gestations with one male and one female, the fetal fraction being measured was that of the male fetus. For twin gestations with two male fetuses, the fetal fraction being measured was the combined fetal fraction from the two male fetuses. The average fetal fraction in samples from pregnancies with one female (XX) and one male (XY) twin was 7.8 ± 4.0% (range, 0.8–17.4%; n = 58). The average fetal fraction in samples from pregnancies with two confirmed male (XY) twins was 16.1 ± 6.7% (range, 2.8–31.9%; n = 51).
Figure 1.

Fetal fraction
of cell‐free DNA in maternal plasma according to gestational age at non‐invasive prenatal testing in 58 twin pregnancies with one male and one female fetus (XX/XY,  ) and 51 with two male fetuses (XY/XY,
) and 51 with two male fetuses (XY/XY,  ), confirmed by clinical outcome. The XX/XY fetal fraction estimate is derived solely from the male twin, while the XY/XY fetal fraction is the combined value for both twins.
), confirmed by clinical outcome. The XX/XY fetal fraction estimate is derived solely from the male twin, while the XY/XY fetal fraction is the combined value for both twins.
Fetal fraction estimation using chromosome 21 and the X chromosome was performed in six samples with confirmed trisomy 21 karyotype and at least one male fetus (Table 5). Fetal fraction measurement using chromosome 21 provides an estimate of the fetal fraction derived from the affected twin only. Similarly, fetal fraction estimation using the X chromosome provides an estimate of the fetal fraction derived from fetuses carrying one X chromosome (in this case, male fetuses).
Table 5.
Fetal fraction (FF) estimates in six twin pregnancies with confirmed trisomy 21 and at least one male fetus, included in Clinical Study B
| Fetal karyotype (Twin A/B) | FF by X (%) | FF by Chr 21 (%) | Source of FF | Correlation with clinical findings |
|---|---|---|---|---|
| XY/XY + 21 | 31.9 | 12.0 |
FF by X is from Twins A and B FF by Chr 21 is from Twin B |
Expectation: higher FF by X*
Finding: higher FF by X |
| XY/XY + 21 | 13.5 | 6.4 |
FF by X is from Twins A and B FF by Chr 21 is from Twin B |
Expectation: higher FF by X*
Finding: higher FF by X |
| XX/XY + 21 | 11.3 | 4.8 | Both FF measurements are from Twin B |
Expectation: similar FF†
Finding: higher FF by X‡ |
| XX/XY + 21 | 6.7 | 7.2 | Both FF measurements are from Twin B |
Expectation: similar FF†
Finding: similar FF |
| XY/XX + 21 | 6.6 | 9.6 |
FF by X is from Twin A FF by Chr 21 is from Twin B |
Expectation: FF within 1.5 of each other§
Finding: FF within 1.5 of each other |
| XX/XY + 21 | 15.1 | 14.8 | Both FF measurements are from Twin B |
Expectation: similar FF†
Finding: similar FF |
FF by X is combined contribution from both twins and FF by chromosome (Chr) 21 is only from affected twin.
Because both FF measurements are from same twin, we would expect similar values for each method.
Originally triplet pregnancy with one demise at around 6 weeks; if the demised twin was male, without trisomy 21, this could account for higher FF by X.
As shown in Table 5, the pattern of fetal fraction estimates seen in the analysis was consistent with what would be expected and demonstrates that the fetal fraction contribution per fetus is not necessarily equal. Interestingly, in one outlier case of a male fetus with trisomy 21 and a female euploid fetus, the fetal fractions by X and chromosome 21 were not similar, at 11.3% and 4.8%, respectively. This pregnancy started as a triplet gestation with demise of one fetus, and therefore it can be speculated that the demised triplet was male, leading to the increased X chromosome‐based fetal fraction estimate.
Clinical Study B test indications
Indications for NIPT were reviewed for the 479 reported cases in Clinical Study B, with 443 (92.5%) cases listing one or more indications on the TRF. Amongst a variety of indications (not all listed here), the most common was AMA in 63.3% (303/479), followed by abnormal ultrasound findings in 16.9% (81/479), previous affected pregnancy in 5.4% (26/479) and positive serum screening result in 2.7% (13/479) of cases. Indications were not mutually exclusive and 7.5% (36/479) of cases had more than one indication; all had AMA in addition to one or more of the other indications. Of the six confirmed affected clinical cases, all had AMA as an indication for NIPT, one case also had an ultrasound abnormality and one case also had abnormal ultrasound findings, a positive serum screening result and a previous affected pregnancy. For the two aneuploidy detected/suspected cases with unconfirmed clinical outcomes, clinical indications for testing were AMA in one case and abnormal ultrasound findings in the other.
Evaluation of the gestational age at which patients requested NIPT revealed that women with AMA or a previous affected pregnancy as the test indication predominantly underwent NIPT in the first trimester (Figure 2). In contrast, women with a positive serum screening result or an abnormal ultrasound finding as the test indication most frequently underwent NIPT in the second trimester.
Figure 2.
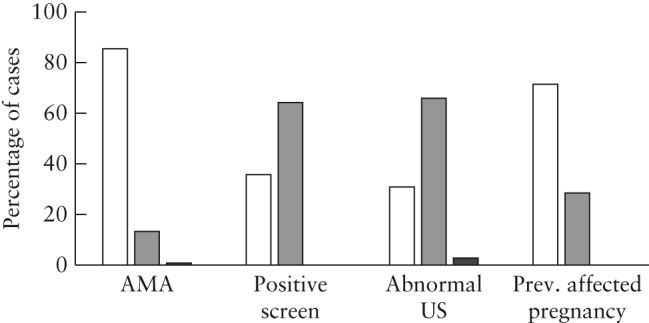
Proportion of cases with twin pregnancy undergoing non‐invasive prenatal testing indicated by advanced maternal age (AMA), positive serum screening result, abnormal ultrasound findings (US) or a previous (Prev.) affected pregnancy, presenting in the first (10–13 weeks;  ), second (14–27 weeks;
), second (14–27 weeks;  ) or third (28–40 weeks;
) or third (28–40 weeks;  ) trimester.
) trimester.
DISCUSSION
Although there is considerable evidence for robust NIPT performance in singleton pregnancies1, 17, 22, 23, 24, 25, there is still relatively little published about its performance in twins8, 9, 10, 26. Here, we demonstrate the feasibility and clinical application of a cfDNA WGS‐based NIPT for fetal aneuploidy screening in twin pregnancies. Standard serum screening approaches have lower detection rates in twin pregnancies when compared with singletons, high false‐positive rates27 and often cannot provide a result for trisomies 18 or 13. Therefore, there is a need for an accurate non‐invasive method for fetal aneuploidy detection of trisomies 21, 18 and 13 in twin pregnancies. This is a particularly desirable option for patients who are risk averse, such as ART patients, for whom fear of procedure‐related loss is heightened due to difficulties achieving pregnancy. Over half of the cases in Clinical Study A were ART pregnancies; information on the proportion of ART pregnancies in Clinical Study B was unavailable. In Clinical Study A, the method identified correctly all four trisomic twin pregnancies with no false positive or negative. In Clinical Study B, evaluation of the 479 reported clinical twin samples revealed nine with aneuploidy detected or suspected, no reported false positive in the aneuploidy detected samples, one reported false positive in aneuploidy suspected samples and no reported false negative.
For Clinical Study B, evaluation of population demographics and indications for testing provided insights into the patient population choosing NIPT. In this population, 68.4% of samples originated from women in their first trimester of pregnancy and the average maternal age was 35.5 years. This is consistent with singleton samples received by the laboratory during the same study period (data not shown). The finding that 92.5% of clinical patients had at least one high‐risk indication on the TRF supports the view that, at present at this laboratory, NIPT in twin pregnancies is being utilized primarily by patients at high risk for fetal aneuploidy.
This is the first known study detailing NIPT reporting of fetal sex information for twins. In this clinical population, the majority (87.5%) of patients requested fetal sex information in addition to aneuploidy screening results. Combining results from Clinical Studies A (Table 2) and B (Table 4) revealed a detection rate for chromosome Y of 99.5% (206/207; 91/91 + 115/116) and specificity of 98.4% (60/61; 24/24 + 36/37). Thus, although NIPT has a high degree of accuracy, discordant results, including inaccurate fetal sex prediction, can occur. For cases with discordance in fetal sex results between NIPT and ultrasound, there are several steps that clinicians can consider before invasive diagnostic testing is carried out22. These include assessment of maternal history, checking for a possible demised twin and performance of a detailed ultrasound exam. The benefits and limitations of fetal sex prediction by NIPT should be explained clearly to all patients before consent for testing22.
One of the principal areas of discussion surrounding the application of NIPT in twin pregnancies is that of fetal fraction. Although the total fetal fraction of twin pregnancies might be higher than that of singletons10, the individual contribution from each twin is generally lower than that of a singleton8, 9; however, there may be exceptions. In our study, the average combined fetal fraction of both twins (16.1%) was higher than determined previously for singletons over similar gestational‐age ranges (12.6%; P = 0.001)21, and higher still than the average fetal fraction for a single twin (7.8%; P < 0.0001), consistent with the previously mentioned studies. Rava et al. described fetal‐fraction thresholds for this WGS‐based NIPT approach, demonstrating the capacity for this approach to detect aneuploidy at low fetal fractions in singleton pregnancies21. Fetal fraction estimates using chromosomes X and 21 can provide insight into the fetal fraction contribution per fetus and also allow independent checks of the value for samples confirmed to be trisomy 21 with at least one male fetus, as outlined in Table 5. Here, the technical cancellation rate for twin samples was 0%. In contrast, studies using different NIPT approaches, which apply a fetal‐fraction cut‐off because of reduced sensitivity at low fetal fractions, reported first‐draw technical failure rates of 5.6% and 7.3%8, 9. Importantly, no trisomic pregnancies were missed in Clinical Study A, no false negatives were reported for Clinical Study B and no false positives were reported for aneuploidy detected findings in either study. In contrast, three other studies in twin pregnancies reported at least one false negative8, 9, 26, despite two of the studies applying a 4% fetal‐fraction threshold (applied to the lower of the two fetal fraction values) for reporting8, 9. Here, fetal fraction estimates using chromosomes X and 21 were used for study analysis purposes only, and these estimates were not reported to the patient. However, we are developing better methods to assess reliably fetal fraction in response to physician interest.
The work reported here has some limitations. Like other published twin studies8, 9, 10, 26, the number of affected pregnancies was small and the majority were trisomy 21. This precluded determination of detection rates for trisomies 13 and 18. Although there were no positive calls for trisomy 13, this study does allow an evaluation of the specificity and false‐positive frequency for trisomy 13. It is also important to note that we had no reports of false‐negative calls for trisomy 13. These studies also allow determination of the overall observed trisomy false‐positive frequency: 0% in Clinical Study A and 0.2% in Clinical Study B. These values are in line with the 0–0.3% combined false‐positive rate described for singletons1, 17. Another limitation was incomplete clinical outcomes, with aneuploidy outcome information available for only 35.7% (171/479) of cases in Clinical Study B; obtaining outcomes remains a challenge for clinical laboratories. Also, neither zygosity nor chorionicity information was available for the majority of patients in Clinical Study B.
In this study, we successfully demonstrated detection of fetal trisomies and the Y chromosome by NIPT in twin pregnancies. The detection rate for trisomy 21 in twins appears to be in line with that in singletons. The limited number of affected cases for other trisomies precluded conclusive determination of those detection rates. In summary, the findings reported here support the view that cfDNA WGS‐based NIPT performs well in twin pregnancies, with overall very low false‐positive frequencies.
DISCLOSURES
L. Fosler, P. Winters, K. W. Jones, K. J. Curnow, A. J. Sehnert and S. Bhatt are, or were, employees of, and hold equity in, Illumina. L. D. Platt is a paid consultant for Illumina. This study was funded by Illumina.
REFERENCES
- 1. Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome‐wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol 2012; 119: 890–901. [DOI] [PubMed] [Google Scholar]
- 2. Mazloom AR, Dzakula Z, Oeth P, Wang H, Jensen T, Tynan J, McCullough R, Saldivar JS, Ehrich M, van den Boom D, Bombard AT, Maeder M, McLennan G, Meschino W, Palomaki GE, Canick JA, Deciu C. Noninvasive prenatal detection of sex chromosomal aneuploidies by sequencing circulating cell‐free DNA from maternal plasma. Prenat Diagn 2013; 33: 591–597. [DOI] [PubMed] [Google Scholar]
- 3. Palomaki GE, Deciu C, Kloza EM, Lambert‐Messerlian GM, Haddow JE, Neveux LM, Ehrich M, van den Boom D, Bombard AT, Grody WW, Nelson SF, Canick JA. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med 2012; 14: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palomaki GE, Kloza EM, Lambert‐Messerlian GM, Haddow JE, Neveux LM, Ehrich M, van den Boom D, Bombard AT, Deciu C, Grody WW, Nelson SF, Canick JA. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med 2011; 13: 913–920. [DOI] [PubMed] [Google Scholar]
- 5. Martin JA, Hamilton BE, Osterman MLK. Three Decades of Twin Births in the United States, 1980–2009. US Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, 2012. [Google Scholar]
- 6. Spencer K, Nicolaides KH. Screening for trisomy 21 in twins using first trimester ultrasound and maternal serum biochemistry in a one‐stop clinic: a review of three years experience. BJOG 2003; 110: 276–280. [PubMed] [Google Scholar]
- 7. Yukobowich E, Anteby EY, Cohen SM, Lavy Y, Granat M, Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis. Obstet Gynecol 2001; 98: 231–234. [DOI] [PubMed] [Google Scholar]
- 8. del Mar Gil M, Quezada MS, Bregant B, Syngelaki A, Nicolaides KH. Cell‐free DNA analysis for trisomy risk assessment in first‐trimester twin pregnancies. Fetal Diagn Ther 2014; 35: 204–211. [DOI] [PubMed] [Google Scholar]
- 9. Bevilacqua E, Gil MM, Nicolaides KH, Ordonez E, Cirigliano V, Dierickx H, Willems PJ, Jani JC. Performance of screening for aneuploidies by cell‐free DNA analysis of maternal blood in twin pregnancies. Ultrasound Obstet Gynecol 2015; 45: 61–66. [DOI] [PubMed] [Google Scholar]
- 10. Canick JA, Kloza EM, Lambert‐Messerlian GM, Haddow JE, Ehrich M, van den Boom D, Bombard AT, Deciu C, Palomaki GE. DNA sequencing of maternal plasma to identify Down syndrome and other trisomies in multiple gestations. Prenat Diagn 2012; 32: 730–734. [DOI] [PubMed] [Google Scholar]
- 11. American College of Obstetricians and Gynecologists . Committee Opinion No. 640: Cell‐free DNA screening for fetal aneuploidy. Obstet Gynecol 2015; 126: e31–e37. [DOI] [PubMed] [Google Scholar]
- 12. Salomon LJ, Alfirevic Z, Audibert F, Kagan KO, Paladini D, Yeo G, Raine‐Fenning N. ISUOG consensus statement on the impact of non‐invasive prenatal testing (NIPT) on prenatal ultrasound practice. Ultrasound Obstet Gynecol 2014; 44: 122–123. [DOI] [PubMed] [Google Scholar]
- 13. Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for fetal aneuploidies: updated meta‐analysis. Ultrasound Obstet Gynecol 2015; 45: 249–266. [DOI] [PubMed] [Google Scholar]
- 14. American College of Obstetricians and Gynecologists . Practice Bulletin No. 163: Screening for Fetal Aneuploidy. Obstet Gynecol 2016; 127: 979–981. [DOI] [PubMed] [Google Scholar]
- 15. Qu JZ, Leung TY, Jiang P, Liao GJ, Cheng YK, Sun H, Chiu RW, Chan KC, Lo YM. Noninvasive prenatal determination of twin zygosity by maternal plasma DNA analysis. Clin Chem 2013; 59: 427–435. [DOI] [PubMed] [Google Scholar]
- 16. Leung TY, Qu JZ, Liao GJ, Jiang P, Cheng YK, Chan KC, Chiu RW, Lo YM. Noninvasive twin zygosity assessment and aneuploidy detection by maternal plasma DNA sequencing. Prenat Diagn 2013; 33: 675–681. [DOI] [PubMed] [Google Scholar]
- 17. Bianchi DW, Parker RL, Wentworth J, Madankumar R, Saffer C, Das AF, Craig JA, Chudova DI, Devers PL, Jones KW, Oliver K, Rava RP, Sehnert AJ. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med 2014; 370: 799–808. [DOI] [PubMed] [Google Scholar]
- 18. Futch T, Spinosa J, Bhatt S, de Feo E, Rava RP, Sehnert AJ. Initial clinical laboratory experience in noninvasive prenatal testing for fetal aneuploidy from maternal plasma DNA samples. Prenat Diagn 2013; 33: 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sehnert AJ, Rhees B, Comstock D, de Feo E, Heilek G, Burke J, Rava RP. Optimal detection of fetal chromosomal abnormalities by massively parallel DNA sequencing of cell‐free fetal DNA from maternal blood. Clin Chem 2011; 57: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 20. Taneja PA, Snyder HL, de Feo E, Kruglyak KM, Halks‐Miller M, Curnow KJ, Bhatt S. Noninvasive prenatal testing in the general obstetric population: clinical performance and counseling considerations in over 85 000 cases. Prenat Diagn 2015; 36: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rava RP, Srinivasan A, Sehnert AJ, Bianchi DW. Circulating fetal cell‐free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem 2013; 60: 243–250. [DOI] [PubMed] [Google Scholar]
- 22. Bianchi DW, Parsa S, Bhatt S, Halks‐Miller M, Kurtzman K, Sehnert AJ, Swanson A. Fetal sex chromosome testing by maternal plasma DNA sequencing: Clinical laboratory experience and biology. Obstet Gynecol 2015; 125: 375–382. [DOI] [PubMed] [Google Scholar]
- 23. Porreco RP, Garite TJ, Maurel K, Marusiak B, Ehrich M, van den Boom D, Deciu C, Bombard A. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol 2014; 211: 365.e361–365.e312. [DOI] [PubMed] [Google Scholar]
- 24. Lau TK, Cheung SW, Lo PS, Pursley AN, Chan MK, Jiang F, Zhang H, Wang W, Jong LF, Yuen OK, Chan HY, Chan WS, Choy KW. Non‐invasive prenatal testing for fetal chromosomal abnormalities by low‐coverage whole‐genome sequencing of maternal plasma DNA: review of 1982 consecutive cases in a single center. Ultrasound Obstet Gynecol 2014; 43: 254–264. [DOI] [PubMed] [Google Scholar]
- 25. Zhang H, Gao Y, Jiang F, Fu M, Yuan Y, Guo Y, Zhu Z, Lin M, Liu Q, Tian Z, Zhang H, Chen F, Lau TK, Zhao L, Yi X, Yin Y, Wang W. Non‐invasive prenatal testing for trisomies 21, 18 and 13: clinical experience from 146 958 pregnancies. Ultrasound Obstet Gynecol 2015; 45: 530–538. [DOI] [PubMed] [Google Scholar]
- 26. Huang X, Zheng J, Chen M, Zhao Y, Zhang C, Liu L, Xie W, Shi S, Wei Y, Lei D, Xu C, Wu Q, Guo X, Shi X, Zhou Y, Liu Q, Gao Y, Jiang F, Zhang H, Su F, Ge H, Li X, Pan X, Chen S, Chen F, Fang Q, Jiang H, Lau TK, Wang W. Noninvasive prenatal testing of trisomies 21 and 18 by massively parallel sequencing of maternal plasma DNA in twin pregnancies. Prenat Diagn 2014; 34: 335–340. [DOI] [PubMed] [Google Scholar]
- 27. Audibert F, Gagnon A; Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada; Prenatal Diagnosis Committee of the Canadian College of Medical Geneticists . Prenatal screening for and diagnosis of aneuploidy in twin pregnancies. J Obstet Gynaecol Can 2011; 33: 754–767. [PubMed] [Google Scholar]


