Abstract
Introduction
Links between preclinical Alzheimer's disease (AD) and driving difficulty onset would support the use of driving performance as an outcome in primary and secondary prevention trials among older adults (OAs). We examined whether AD biomarkers predicted the onset of driving difficulties among OAs.
Methods
One hundred four OAs (65+ years) with normal cognition took part in biomarker measurements, a road test, clinical and psychometric batteries, and self-reported their driving habits.
Results
Higher values of cerebrospinal fluid (CSF) tau/Aβ42 and phosphorylated tau (ptau181)/Aβ42 ratios, but not uptake on Pittsburgh compound B amyloid imaging (P = .12), predicted time to a rating of marginal or fail on the driving test using Cox proportional hazards models. Hazards ratios (95% confidence interval) were 5.75 (1.70–19.53), P = .005 for CSF tau/Aβ42; 6.19 (1.75–21.88), and P = .005 for CSF ptau181/Aβ42.
Discussion
Preclinical AD predicted time to receiving a marginal or fail rating on an on-road driving test. Driving performance shows promise as a functional outcome in AD prevention trials.
Keywords: Alzheimer's disease, Biomarker, Driving, Older adults, Aged, Preclinical, Cerebrospinal fluid, Amyloid imaging, Tau, Ptau, Road test, Functional outcome
1. Introduction
Driving among older adults (OAs) is a critical and timely public health issue. Thirty-eight million US drivers are now aged 65 years or older [1], and their number will double within the next 40 years [2], when they will make up 25% of all drivers. Longitudinal studies show that OAs have deterioration in driving performance over time [3] and that motor vehicle crashes are a leading cause of injury and death [4]. Driving fatalities increase with OA age, such that risk of death for drivers 85 years or older is nine-fold greater in a crash than it is for drivers 69 years or younger [5]. The annual lifetime costs associated with these deaths and injuries are estimated at $80 billion [6].
Spurred by postmortem studies indicating that the brains of many OA drivers killed in car accidents had the neuropathological changes of Alzheimer's disease (AD) [7], we recently identified preclinical AD as cross-sectionally linked with driving difficulty among OAs [8]. “Preclinical AD,” the condition in which abnormal levels of AD biomarkers are present without concurrent detectable dementia symptoms, is present in about 30% [9] of adults over age 65 years. Molecular biomarkers reflect the presence of brain plaques and tangles, which are the signature lesions of AD. In vivo imaging of fibrillar amyloid plaques can be obtained using radiotracers, including Pittsburgh compound B (PIB) [10], together with positron emission tomography (PET). Soluble, rather than fibrillar, levels of brain amyloid (Aβ42) are obtained from cerebrospinal fluid (CSF). CSF biomarkers are based on assays of proteins that are pathologically misfolded, and in addition to Aβ42, include tau and phosphorylated tau (ptau181), the principal components of neurofibrillary tangles [11]. Our prior study results have indicated that road test performance [8] and self-reports of previous involvement in crashes [12] are cross-sectionally associated with abnormality of these biomarker levels among persons who are cognitively normal.
A critical next step is to determine whether these biomarkers can predict the future onset of driving difficulties among cognitively normal OAs. If so, this finding could have important long-term consequences. First, the ability to identify who will be at most risk of decline in driving performance and to forecast when decline will occur would allow intervention before or at the time of early decline, which could extend driving life expectancy and prevent motor vehicle crashes, injuries, and deaths. The preservation of safe driving ability would also prolong the independence and enhance the well-being of elders.
Second, due to the failure of all previous clinical trials of AD-modifying therapies [13], trials designed to prevent or slow the AD pathologic process are now being conducted among symptom-free persons at high risk for AD development [14], before substantial neurodegeneration has occurred [15]. Measures of very early functional change in preclinical AD are needed for these trials [15], and these measures must be relevant to the disease process as well as clinically meaningful [16]. Driving is a common functional activity that depends on a combination of cognitive, perceptual, and motor skills working in concert. These skills are essential to driving and many decline with the onset and progression of AD [17].
If longitudinally associated with AD biomarkers, assessment of driving skills may have important implications for safety and quality of life of OAs and would have demonstrated potential as a functional outcome in AD primary or secondary prevention trials. We therefore tested whether AD biomarkers could predict the onset of future problems with driving among cognitively normal OAs.
2. Methods
2.1. Design
Participants with normal cognition (clinical dementia rating [CDR] = 0) [18], aged 65 years and older, with a valid driver's license, and who were driving an automobile at least once per week, were recruited from the pool of individuals already participating in longitudinal studies at the Knight Alzheimer's Disease Research Center. Study protocols were approved by the Washington University Human Research Protection Office, and written informed consent was obtained. Participants are offered a stipend for their participation.
At baseline and every subsequent annual visit, participants took part in clinical assessments, on-road driving tests [19], and their current driving practices were assessed using the Driving Habits Questionnaire (DHQ) [20]. Existing biomarker data were used for participants who had received amyloid imaging and CSF collection via their enrollment in other studies within the two years preceding their baseline driving assessment session. If existing data were not available, participants were asked to complete amyloid imaging and/or CSF collection for inclusion in the current study. Data from participants with processed amyloid imaging and/or CSF biomarker results, and at least one follow-up assessment after baseline, are reported here.
2.2. Clinical assessment
A CDR was derived by experienced clinicians who synthesize information obtained from interviews with the participant and separately with a collateral source who knows the participant well. The clinician's judgment about the presence of dementia is based on the principle of intraindividual change where the individual is used as his or her own control. The CDR reflects whether the participant has dementia, and if so, the severity of that dementia. CDRs are derived in accordance with a standard scoring algorithm; CDR 0 = no dementia, CDR 0.5 = very mild, CDR 1 = mild, CDR 2 = moderate, and CDR 3 = severe dementia. The Sum of Boxes (CDR-SB) is a global measure of cognition obtained by summing the scores on the six CDR domains, resulting in a score ranging from 0 to 18 [18]. The CDR-SB is sensitive to the earliest changes in symptomatic AD and is used in clinical trials to assess longitudinal change in cognition over time [21]. The clinical assessment battery also includes the Mini–Mental State Examination [22] and psychometric tests from the uniform data set [23].
2.3. Measurement of AD biomarkers
2.3.1. Imaging
PET-PIB imaging was used to determine brain amyloid burden [24]. Detailed information on our PET-PIB methodology is available [25]. Briefly, alignment of PET-MRI within a participant is used to create three-dimensional regions-of-interest (ROIs), which are applied to images of the PET dynamic data, yielding regional time-activity curves [25]. Using the cerebellum ROI data as the reference tissue input function, a time-activity curve for each ROI is analyzed for specific PIB binding. The slope of each curve reflects the tracer distribution volume (DV) in the tissue of interest relative to the input function [25]. A binding potential (BP) value reflecting the ROI binding value proportional to the number of binding sites for each ROI is calculated using the equation BP = DV − 1. The mean cortical binding potential (MCBP) is obtained by taking the mean of the BPs from brain regions known to have high uptake among participants with AD: the prefrontal cortex, gyrus rectus, lateral temporal cortex, and precuneus [25]. The FreeSurfer image analysis suite (version 5.3) is used to obtain estimates of hippocampal volume, total brain volume, and intracranial volume [26].
2.3.2. CSF biomarkers
CSF analytes [27] (Aβ42, tau and ptau181; Innotest, Fujirebio [formerly Innogenetics], Ghent, Belgium) were measured using sensitive and quantitative enzyme-linked immunosorbent assays. CSF was obtained by trained neurologists via standard lumbar puncture using a 22-gauge Sprotte spinal needle to draw 20–30 mL of CSF at 8:00 AM following an overnight fast. CSF samples were gently inverted and centrifuged at low speed to avoid possible gradient effects and frozen at −84°C after aliquoting into polypropylene tubes. Biomarker assays included a common reference standard, within-plate sample randomization, and standardized protocol adherence. Samples were reanalyzed if coefficients of variability exceed 25% (per Alzheimer's Disease Neuroimaging Initiative criteria), if there were “edge artifacts” or if the pooled common CSF sample yielded widely discrepant values.
2.3.3. APOE genotyping
Briefly, all DNA samples underwent stringent quality control, before genotyping with the Illumina 610 or the OmniExpress chip [28]. More detailed information regarding apolipoprotein E (APOE) genotyping has been published [28].
2.4. Driving test
The 12-mile, modified Washington University Road Test (mWURT) takes about an hour to complete [19]. The course begins in a closed parking lot so that the participant becomes familiar with the study car, a 4-door sedan, then proceeds to a public in-traffic route [19]. The participant drives through the mWURT route as directed by an examiner sitting in the front seat. The examiner can take control of the wheel if needed, and the research vehicle is outfitted with a second, passenger-side brake so that the examiner can apply the brake if necessary. The examiner is blinded to the participant's biomarker, clinical, and psychometric test results.
The driving examiner assigns a pass, marginal, or fail rating after each driving test. A pass rating is defined as successfully demonstrating competency in all aspects of the road test and across all levels of traffic density with minimal to no errors, and no safety concerns. A marginal rating occurs when errors occur indicating low to moderate risk for safety concerns (e.g., rolling stop, speed variability, inconsistent scanning). A fail rating is given when the driver demonstrates errors indicating moderate to maximum risk for safety concerns (e.g., goes out of the lane, runs a stop sign/traffic light).
2.5. Statistical analyses
Portions of the data were collected and managed using REDCap electronic data capture tools [29]. For all analyses, SAS statistical software version 9.4 (SAS Institute Inc.) was used, α = 0.05 was taken to indicate statistical significance, and all tests were two tailed.
Based on significant cross-sectional associations found in our previous research [8], we tested whether three biomarkers MCBP for PET using PIB [24], CSF tau/Aβ42, and CSF ptau181/Aβ42 predicted longitudinal change in driving-related behaviors. Higher values of these three biomarkers are commonly used to define pathology due to preclinical AD [30]. Similar to our previous work [8], [31], we compared the highest one-third of the biomarker values with the lower two-thirds in our statistical analyses.
Two primary driving decline outcomes were examined: time from baseline to receiving a rating of marginal or fail [19] on the road test, and self-reported decline over time on everyday driving practices (driving space, miles driven, number of places visited, and number of trips over the previous year) [20] assessed using the DHQ.
Kaplan-Meier curves [32] illustrating the time from baseline to first rating of marginal or fail on the yearly driving test as a function of higher and lower biomarker values were calculated, and the difference between the curves was analyzed using the log-rank test. Cox proportional hazards models [33] tested whether each biomarker variable was associated with time from baseline to receiving a rating of marginal or fail on the driving test while adjusting for, and simultaneously testing the effects of age, education, gender, race, and APOE ε4 genotype. In the Cox models, data from participants who did not return for follow-up, or who did not receive a rating of marginal or fail on the driving test by the end of the follow-up period, were censored at the date of the most recent driving assessment session.
Linear mixed models [34], likewise adjusted for age, education, gender, race, and APOE ε4, tested whether higher baseline biomarker values predicted a more rapid rate of future decline in the continuous composite scores of interest from the DHQ. These models included the participant, and intercept and slope terms, as random effects.
2.5.1. Secondary analyses
Secondary analyses tested the extent of biomarker-associated declines in global cognitive measures in the same individuals over the same follow-up period. In these analyses, a psychometric composite score was calculated for each assessment for each participant using methods similar to those previously published [35]. The composite reflects tests measuring the domains of attention, processing speed, executive function, episodic memory, and language [23]. Adjusted linear mixed models tested whether the slope of change across time on the psychometric composite, CDR-SB, and MMSE differed for the higher and lower biomarker groups. Because CDR-SB and MMSE are sometimes subject to ceiling effects among participants who are cognitively normal, we also used Kaplan-Meier and adjusted Cox proportional hazards models to test whether the biomarker values were associated with time to a one-or-more unit increase in the CDR-SB, and a two-or-more unit decrease in the MMSE.
3. Results
Data were collected from August 28, 2012 through April 6, 2016. One hundred four individuals aged 65.8–88.2 years met inclusion criteria (Table 1). Follow-up time ranged from 0.96 to 3.45 years, with a mean of 1.86 years. Cutoff values for the higher and lower biomarker groups were 0.436 pg/mL, 0.089 pg/mL, and 0.140 units for CSF tau/Aβ42, CSF ptau181/Aβ42, and MCBP for PIB, respectively.
Table 1.
Demographics (N = 104)
| Age at driving assessment, mean (SD), years | 72.5 (4.6) |
| Women, no. (%) | 52 (50.0) |
| African American,∗ no. (%) | 10 (9.6) |
| Education, mean (SD), years | 16.1 (2.5) |
| APOE ε4+, no. (%) | 31 (29.8) |
| MMSE, mean (SD)† | 29.3 (1.0) |
| CDR Sum of Boxes, mean (SD)‡ | 0.01 (0.05) |
| Follow-up time, mean (SD), years | 1.9 (0.6) |
Abbreviations: SD, standard deviation; APOE ε4, apolipoprotein E ε4; MMSE, Mini–Mental State Examination; CDR, clinical dementia rating.
All remaining participants reported their race as Caucasian.
MMSE scores range from 0 (worst performance) to 30 (best performance).
CDR Sum of Boxes scores range from 0 (best performance) to 18 (worst performance).
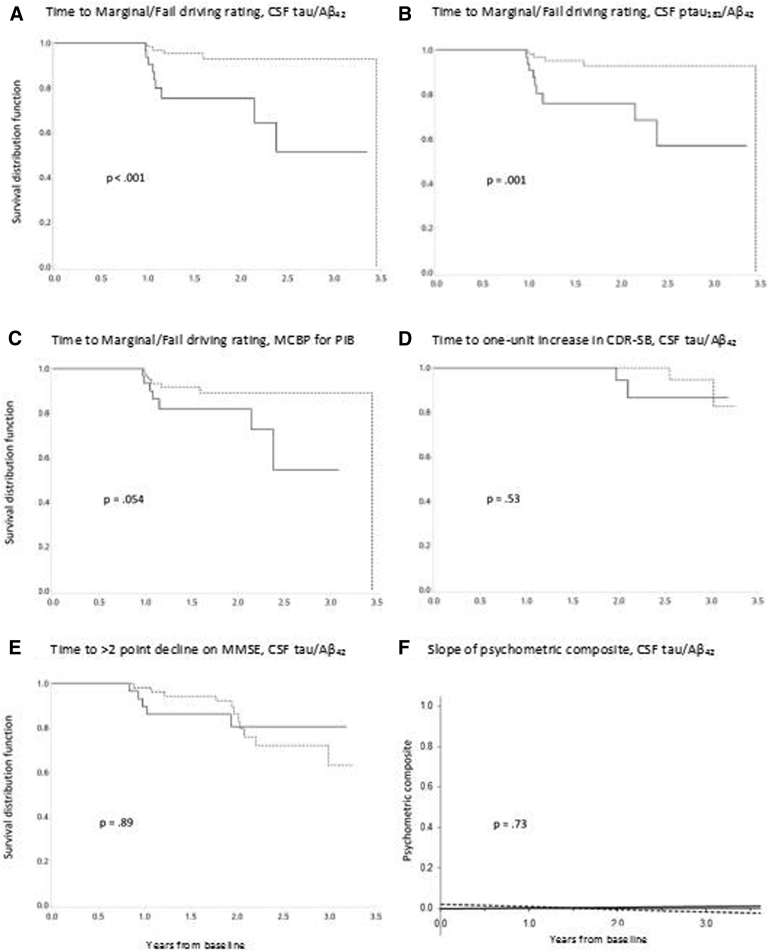
Fig. 1 shows Kaplan-Meier survival curves for the unadjusted association of the biomarker groups with the driving test results. Of the 104 participants with two or more driving tests, 88 (84.6%) passed all driving tests administered during the follow-up period, 12 (11.5%) received a marginal rating, and 4 (3.9%) received a fail rating.
Fig. 1.
Kaplan-Meier curves. Kaplan-Meier curves showing relationships between biomarkers and time to a rating of marginal or fail on the road test (A–C) and change in some of the cognitive outcomes as a function of baseline CSF tau/Aβ42 values (D–F). Dotted lines = lower biomarker values, solid lines = higher biomarker values. Abbreviations: CDR, clinical dementia rating; CSF, cerebrospinal fluid; MCBP, mean cortical binding potential; PIB, Pittsburgh compound B.
The Cox proportional hazards models indicated that higher values of the ratios of CSF tau/Aβ42 and ptau181/Aβ42, but not MCBP for PIB, predicted time to a rating of marginal or fail on the driving test. Hazards ratios (95% confidence interval) were 5.75 (1.70–19.53), P = .005 for CSF tau/Aβ42; 6.19 (1.75–21.88), P = .005 for CSF ptau181/Aβ42; and 2.65 (0.73–9.00), P = .12 for MCBP for PIB (Fig. 1, panels A, B, and C). No significant effects of age, education, gender, race, or APOE ε4 were found (P > .08). Mixed linear model analyses indicated no difference between the biomarker groups in self-reported change across time in driving space, miles driven, number of places visited, and number of trips (P > .15) as measured by the DHQ. There was no significant decline in self-reported driving across the follow-up period (P > .25).
Secondary analyses showed no difference between the high and low groups for any of the biomarkers on the slopes of change across time for the psychometric composite score (P > .57), the CDR-SB (P > .56), and the MMSE (P = .66). Time to a one-or-more point increase in the CDR-SB (P > .20) and to a two-or-more point increase on the MMSE (P > .18) were not associated with the biomarker groups in the Cox proportional hazards models.
The associations of CSF tau/Aβ42 and CSF ptau181/Aβ42 with time to receiving a marginal or fail rating on the driving test were confirmed using the Benjamini-Hochberg [36] method to correct for multiple testing.
Given these results, several post hoc tests were conducted. First, to examine whether specific types of cognitive processing predicted time to a marginal or fail driving test rating, we repeated the Cox models including scores on an episodic memory test and on an attention and processing speed subscale and its component tests (described here [35]) in turn. There was no association between the episodic memory score (P > .241), the attention and processing subscale (P > .191), scores on the individual tests comprising that subscale (P > .090), and time to receiving a marginal or fail rating. Second, we examined whether the AD biomarkers were associated with changes on tests assessing specific cognitive domains by repeating the mixed models using the episodic memory measure, the attention and processing subscale, and its component tests, as the cognitive outcomes. No significant relationships were found (P > .064).
There have been three driving examiners involved over the course of the study. Each used the same trichotomous scale, but because there was no overlap between examiners, we were unable to calculate a kappa statistic. However, a separate study found strong kappa (κ = 0.84) on the same pass/marginal/fail ratings when comparing an evaluator in the front and one in the back seat [19].
4. Discussion
Among cognitively normal OAs, we found that higher values of CSF tau/Aβ42 and ptau181/Aβ42 predicted time to a marginal or fail rating on an on-the-road driving test, indicating that driving performance declines more rapidly among participants with preclinical AD. Thus, as with cognitive outcomes [9], the risk of developing driving problems appears to be greatest when the preclinical AD biomarker profile is characterized by both cerebral beta-amyloidosis and markers of neural injury, such as elevated levels of CSF tau and ptau181, rather than cerebral beta-amyloidosis levels alone.
Although driving problems occurred faster for those with preclinical AD, these participants did not correspondingly change their everyday driving behavior over the same time period. As self-reported on the DHQ, changes in miles driven, number of trips, number of places visited, and driving space were similar for the normal and abnormal biomarker groups. There are several possible reasons for this result. First, individuals with preclinical AD may not have noticed any change in their driving skills over time. Or, participants could have noticed changes in their driving but did not believe that those changes warranted any modification of their driving behavior. Another possibility is that the effects of preclinical AD on driving may not occur in everyday driving but only manifest when an individual is “pushed” or “stressed,” such as driving an unfamiliar vehicle, on unfamiliar roads, while being evaluated under controlled conditions (as in a road test) [37]. Future research should test this hypothesis via driving simulators, which can be used to safely vary the level of demandingness of the traffic situation and crash risk [38].
Importantly, although study participants showed biomarker-linked decline in driving performance across the study period, there was no association between biomarkers and global cognitive test scores (CDR-SB, MMSE, a psychometric composite) for the same participants over the same period. These results suggest that the effects of preclinical AD may be detected earlier when driving, rather than global cognition, is tested. Larger samples examined over a longer period are needed to thoroughly examine this question.
There has been some skepticism regarding whether suitable functional outcomes for preclinical AD trials can be found [39]. However, driving test performance meets two essential criteria for functional outcomes [16], [40] in primary and secondary prevention trials for AD: driving is a clinically meaningful outcome and is related to the underlying biological disease process. Driving tests may allow earlier and more rapid testing of potential treatments in primary and secondary AD prevention trials.
In addition to their potential usefulness in clinical trials research, our results help inform the appropriate timing of driving interventions targeted at individuals with preclinical AD. If interventions occur too long before the onset of driving problems, the skills learned during the intervention may not be maintained. If occurring too late, once frank dementia symptoms are present, it may be difficult for an individual to learn and remember to successfully use the intervention components. Given that the association between preclinical AD and driving performance is newly demonstrated, there are no existing interventions designed to maintain driving skills among individuals with preclinical AD. However, promising interventions for individuals who are cognitively normal as well as those with very mild or mild dementia may be appropriate for those with preclinical AD [41]. By being able to predict when driving decline is likely to occur, these interventions can be applied when people are cognitively normal, or only very mildly demented. Although CSF biomarkers are now primarily used in clinical trials, standardization work is underway to develop automated clinical-grade assays that will hopefully become as clinically useful as chemistry tests for other common human diseases [30].
Our study has limitations. Participants were willing to undergo amyloid imaging and/or lumbar puncture tests, many had a friend or relative with AD, and therefore may not be representative of the larger US population of OAs. We used on-road driving tests as our measure of driving performance. Therefore, our results are subject to the same limitations as other research using that methodology [37], including the extent to which associations between preclinical AD and results obtained using driving tests will generalize to everyday driving. Driving research has therefore increasingly shifted focus to naturalistic outcomes using global positioning systems methodologies [42], [43], [44]. It is likely that driving measures such as these will improve objectivity, replication, and generalizability to the real world.
The effect of amyloid imaging, but not CSF Aβ42, on time to driving problems approached significance in the unadjusted (P = .054) analysis, with the significance of the effect lessened (P = .12) when adjusted for demographic variables in the Cox model. Therefore, although the effect of amyloid imaging on time to driving problems may be weaker than that found for the CSF biomarkers reflecting both amyloid and tau/ptau181, a significant relationship may be found in studies using a larger sample size, or longer follow-up.
Unfortunately, clinical use of driving tests (together with other evaluation procedures) for fitness-to-drive purposes is currently paid out-of-pocket in most cases and can be prohibitively expensive for persons on a fixed income. However, the cost of driving tests conducted in a research setting can be affordable. In our research program, the cost of an on-road driving assessment by a professional driving instructor is less than the costs of imaging, CSF studies, and of administering the clinical and psychometric assessment batteries.
Finally, driving tests would not be suitable outcomes for persons who have never driven or have ceased driving. Thus, inclusion of driving tests as the primary outcome in clinical trials would not be generalizable to the larger OA population. The value of using road tests as clinical trial outcomes will need to be weighed against this restriction in generalizability in future research.
In summary, our findings indicated that preclinical AD is related to time to receiving a marginal or fail rating on an on-road driving test. Participants with preclinical AD did not report changing their daily driving habits, and there was no association of preclinical AD with change in cognition, over the same follow-up period. Driving performance shows promise as a functional outcome in primary and secondary AD prevention trials.
Research in Context.
-
1.
Systematic review: After reviewing the literature, most publications on driving performance center on older adults (OAs) with symptomatic Alzheimer's disease (AD), mild cognitive impairment, or are cognitively normal without biomarkers. No study has examined whether decline in driving performance is associated with AD biomarkers among cognitively normal OAs.
-
2.
Interpretation: AD biomarkers predict time to a marginal/fail rating on a road test indicating that driving performance declines more rapidly among OAs with preclinical AD. OAs with preclinical AD did not report changing their daily driving habits, and there was no association of preclinical AD with change in cognition, over the same follow-up period.
-
3.
Future directions: Driving performance has promise as a functional outcome in primary and secondary AD prevention trials. Examination of driving behavior can inform the appropriate timing of driving interventions targeted at individuals with preclinical AD.
Acknowledgments
Funding for this study was provided by the National Institute on Aging (R01AG043434, R01AG43434-03S1, P50AG005681, P01AG003991, and P01AG026276); Fred Simmons and Olga Mohan, the Farrell Family Research Fund, and the Charles and Joanne Knight Alzheimer's Research Initiative of the Washington University Knight Alzheimer's Disease Research Center (ADRC). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article. The authors thank the participants, investigators, and staff of the Knight ADRC Clinical Core for participant assessments, Genetics Core for APOE genotyping, Biomarker Core for cerebrospinal fluid analysis, and the Imaging Core for amyloid imaging. Imaging facilities were supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). Imaging analyses used the services of the Neuroimaging Informatics and Analysis Center, supported by NIH grant 5P30NS048056. Catherine M. Roe had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Ghoshal has participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfullness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease) trial. She receives funding from NICHD K12 HD001459. Dr. Barco receives funding from the Missouri Department of Transportation; NIH (RO1ETO26199); NIMH/OBSSR (R01MH099011); board membership: American Occupational Therapy Board and Specialty Certification (BASC); consultant: Traffic Injury Research Foundation (TIRF). Dr. Holtzman receives funding from C2N Diagnostics SAB, Genentech SAB, and Neurophage SAB. He is a consultant for AbbVie. His laboratory receives grants from the NIH, the JPB Foundation, Cure Alzheimer's Fund, the Tau Consortium, Eli Lilly, and C2N Diagnostics. Dr. Benzinger is funded by NIH grants #P50AG005681; UF1AG032438; U01AG042791; 2P01AG003991; P01AG026276; R01AG043434; U54 MH091657; and the Barnes-Jewish Hospital Foundation. Dr. Fagan is on the scientific advisory boards of IBL International and Roche and is a consultant for AbbVie, Novartis, and DiamiR. Dr. Ott receives research funding from the NIH/NIA, Alzheimer's Association, Long Term Care Group. He has participated or is participating in clinical trials sponsored by Merck, Avid, Eli Lilly, and TauRx. He is a consultant for Amgen (Steering Committee), Accera (DSMB). He is a speaker for, and/or provides education for, DHHS/NHTSA, Medscape, American Academy of Neurology, and the Alzheimer's Association. Dr. Carr receives support from NIA (R01 AG043434; Roe-PI and K23; Betz-PI), NEI (R01 EY026199-01; Bhorade-PI), Missouri Department of Transportation (16-M2PE-05-002; Carr-PI, 16-DL-02-002; Carr-PI and 16-DL-02-003; Barco-PI), State Farm Insurance (Carr-PI), and HealthSouth (Carr-PI) and has past consulting relationships in the last 2 years with The Traffic Injury Research Foundation, Medscape, the AAA Foundation for Traffic Safety, and the American Geriatric Society. Dr. Morris and his family do not own stock or have equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris has participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Janssen Immunotherapy, Pfizer, Eli Lilly/Avid Radiopharmaceuticals, SNIFF (The Study of Nasal Insulin to Fight Forgetfullness) study, and A4 (The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease) trial. Dr. Morris has served as a consultant for Lilly USA and Charles Dana Foundation. He receives research support from Eli Lilly/Avid Radiopharmaceuticals and is funded by NIH grants #P50AG005681; P01AG003991; P01AG026276; and UF1AG032438. All other authors report no conflicts of interest.
Authors' contributions: Dr. Roe contributed to conception and design of the study; collection, management, analysis, and interpretation of the data; preparation of the final article, supervision, and obtaining funding. Dr. Babulal contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Dr. Head contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Ms. Stout contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Ms. Vernon contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Dr. Ghoshal contributed to interpretation of the data and critical revision of the article for important intellectual content. Mr. Garland contributed to collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Dr. Barco contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content. Dr. Williams contributed to interpretation of the data and critical revision of the article for important intellectual content. Ms. Johnson contributed to interpretation of the data and critical revision of the article for important intellectual content. Ms. Fierberg contributed to interpretation of the data and critical revision of the article for important intellectual content. Mr. Fague contributed to collection, management, and interpretation of the data and critical revision of the article for important intellectual content. Dr. Xiong contributed to conception and design of the study, analysis, and interpretation of the data and preparation of the final article. Dr. Mormino contributed to interpretation of the data and critical revision of the article for important intellectual content. Dr. Grant contributed to analysis and interpretation of the data and critical revision of the article for important intellectual content. Dr. Holtzman contributed to collection and interpretation of the data and critical revision of the article for important intellectual content. Dr. Benzinger contributed to conception and design of the study; collection, management, and interpretation of the data and critical revision of the article for important intellectual content. Dr. Fagan contributed to conception and design of the study; collection, management, and interpretation of the data and critical revision of the article for important intellectual content. Dr. Ott contributed to interpretation of the data and critical revision of the article for important intellectual content. Dr. Carr contributed to conception and design of the study; interpretation of the data; and critical revision of the article for important intellectual content. Dr. Morris contributed to conception and design of the study; collection, management, and interpretation of the data; and critical revision of the article for important intellectual content.
References
- 1.National Center for Statistics and Analysis . National Highway Traffic Safety Administration; Washington, DC: 2016. Older population: 2014 data. Report No.: DOT HS 812 273. [Google Scholar]
- 2.U.S. Census Bureau. Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050 [online]. Available at: http://www.census.gov/population/www/projections/summarytables.html. Accessed August 8, 2001.
- 3.Duchek J.M., Carr D.B., Hunt L., Roe C.M., Xiong C., Shah K. Longitudinal driving performance in early stage dementia of the Alzheimer type. J Am Geriatr Soc. 2003;51:1342–1347. doi: 10.1046/j.1532-5415.2003.51481.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayhew D.R., Simpson H.M., Ferguson S.A. Collisions involving senior drivers: high-risk conditions and locations. Traffic Inj Prev. 2006;7:117–124. doi: 10.1080/15389580600636724. [DOI] [PubMed] [Google Scholar]
- 5.Insurance Institute for Highway Safety. Fatality facts 2013, Older people [online]. Available at: http://www.iihs.org/iihs/topics/t/older-drivers/fatalityfacts/older-people/2013. Accessed December 23, 2016.
- 6.Naumann R.B., Dellinger A.M., Zaloshnja E., Lawrence B.A., Miller T.R. Incidence and total lifetime costs of motor vehicle–related fatal and nonfatal injury by road user type, United States, 2005. Traffic Inj Prev. 2010;11:353–360. doi: 10.1080/15389588.2010.486429. [DOI] [PubMed] [Google Scholar]
- 7.Gorrie C.A., Rodriguez M., Sachdev P., Duflou J., Waite P.M. Mild neuritic changes are increased in the brains of fatally injured older motor vehicle drivers. Accid Anal Prev. 2007;39:1114–1120. doi: 10.1016/j.aap.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Roe C.M., Barco P.P., Head D.M. Amyloid Imaging, cerebrospinal fluid biomarkers predict driving performance among cognitively normal individuals. Alzheimer Dis Assoc Disord. 2016 doi: 10.1097/WAD.0000000000000154. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 11.Carrillo M.C., Blackwell A., Hampel H., Lindborg J., Sperling R., Schenk D. Early risk assessment for Alzheimer's disease. Alzheimers Dement. 2009;5:182–196. doi: 10.1016/j.jalz.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Ott B.R., Jones R.N., Noto R.B. Amyloid deposition in preclinical Alzheimer's disease is associated with increased driving risk. Alzheimers Dement (Amst) 2016 doi: 10.1016/j.dadm.2016.10.008. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich M.J. Researchers test strategies to prevent Alzheimer disease. JAMA. 2014;311:1596–1598. doi: 10.1001/jama.2014.3891. [DOI] [PubMed] [Google Scholar]
- 14.Bateman R.J., Xiong C., Benzinger T.L.S., Fagan A.M., Goate A., Fox N.C. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder P.J., Kahle-Wrobleski K., Brannan S., Miller D.S., Schindler R.J., DeSanti S. Assessing cognition and function in Alzheimer's disease clinical trials: do we have the right tools? Alzheimers Dement. 2014;10:853–860. doi: 10.1016/j.jalz.2014.07.158. [DOI] [PubMed] [Google Scholar]
- 17.Ott B.R., Daiello L.A. How does dementia affect driving in older patients? Aging Health. 2010;6:77–85. doi: 10.2217/ahe.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris J.C. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Carr D.B., Barco P.P., Wallendorf M.J., Snellgrove C.A., Ott B.R. Predicting road test performance in drivers with dementia. J Am Geriatr Soc. 2011;59:2112–2117. doi: 10.1111/j.1532-5415.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owsley C., Stalvey B., Wells J., Sloane M.E. Older drivers and cataract: driving habits and crash risk. J Gerontol A Biol Sci Med Sci. 1999;54:M203–M211. doi: 10.1093/gerona/54.4.m203. [DOI] [PubMed] [Google Scholar]
- 21.Williams M.M., Roe C.M., Morris J.C. Stability of the Clinical Dementia Rating: 1979-2007. Arch Neurol. 2009;66:773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental State: a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub S., Salmon D., Mercaldo N., Ferris S., Graff-Radford N.R., Chui H. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychological test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klunk W.E. Amyloid imaging as a biomarker for cerebral β-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol Aging. 2011;32:S20–S36. doi: 10.1016/j.neurobiolaging.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mintun M.A., LaRossa G.N., Sheline Y.I., Dence C.S., Lee S.Y., Mach R.H. [11 C] PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B., Salat D.H., Busa E., Dieterich M., Haselgrove C., van der Kouwe A. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 27.Fagan A.M., Mintun M.A., Mach R.H., Lee S.Y., Dence C.S., Shah A.R. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aá42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 28.Cruchaga C., Kauwe J.S., Nowotny P., Bales K., Pickering E.H., Mayo K. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer's disease. Hum Mol Genet. 2012;21:4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blennow K., Dubois B., Fagan A.M., Lewczuk P., De Leon M.J., Hampel H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer's disease. Alzheimers Dement. 2015;11:58–69. doi: 10.1016/j.jalz.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snider B.J., Fagan A.M., Roe C.M., Shah A.R., Grant E.A., Xiong C. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–645. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 33.Cox D.R. Breakthroughs in statistics. Springer; New York: 1992. Regression models and life-tables; pp. 527–541. [Google Scholar]
- 34.Verbeke G., Molenberghs G. Springer Science & Business Media; New York: 2009. Linear mixed models for longitudinal data. [Google Scholar]
- 35.Obermann K.R., Morris J.C., Roe C.M. Exploration of 100 commonly used drugs and supplements on cognition in older adults. Alzheimers Dement. 2013;9:724–732. doi: 10.1016/j.jalz.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y., Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple. J R Stat Soc Ser B Method. 1995;57:289–300. [Google Scholar]
- 37.Hird M.A., Egeto P., Fischer C.E., Naglie G., Schweizer T.A., Pachana N. A systematic review and meta-analysis of on-road simulator and cognitive driving assessment in Alzheimer's disease and mild cognitive impairment. J Alzheimers Dis. 2016;53:713–729. doi: 10.3233/JAD-160276. [DOI] [PubMed] [Google Scholar]
- 38.Casutt G., Martin M., Keller M., Jäncke L. The relation between performance in on-road driving, cognitive screening and driving simulator in older healthy drivers. Transportation Res F Traffic Psychol Behav. 2014;22:232–244. [Google Scholar]
- 39.Kozauer N., Katz R. Regulatory innovation and drug development for early-stage Alzheimer's disease. N Engl J Med. 2013;368:1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 40.Silverberg N.B., Ryan L.M., Carrillo M.C., Sperling R., Petersen R.C., Posner H.B. Assessment of cognition in early dementia. Alzheimers Dement. 2011;7:e60–e76. doi: 10.1016/j.jalz.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott B.R., Davis J.D., Bixby K. Video feedback intervention to enhance the safety of cognitively impaired older adults. Am J Occup Ther. 2016 doi: 10.5014/ajot.2017.020404. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Babulal G., Addison A., Ghoshal N., Stout S.H., Vernon E.K., Sellan M. Development and interval testing of a naturalistic driving methodology to evaluate driving behavior in clinical research. F1000Res. 2016;5:1716. doi: 10.12688/f1000research.9150.1. [version 2; referees: 2 approved] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aksan N., Dawson J.D., Emerson J.L., Yu L., Uc E.Y., Anderson S.W. Naturalistic distraction and driving safety in older drivers. Hum Factors. 2013;55:841–853. doi: 10.1177/0018720812465769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babulal G., Traub C., Webb M., Stout S.H., Addison A., Carr D.B. Creating a driving profile for older adults using GPS devices and naturalistic driving methodology. F1000Res. 2016;5:2376. doi: 10.12688/f1000research.9608.1. [version 1; referees: 1 approved, 1 approved with reservations] [DOI] [PMC free article] [PubMed] [Google Scholar]