Abstract
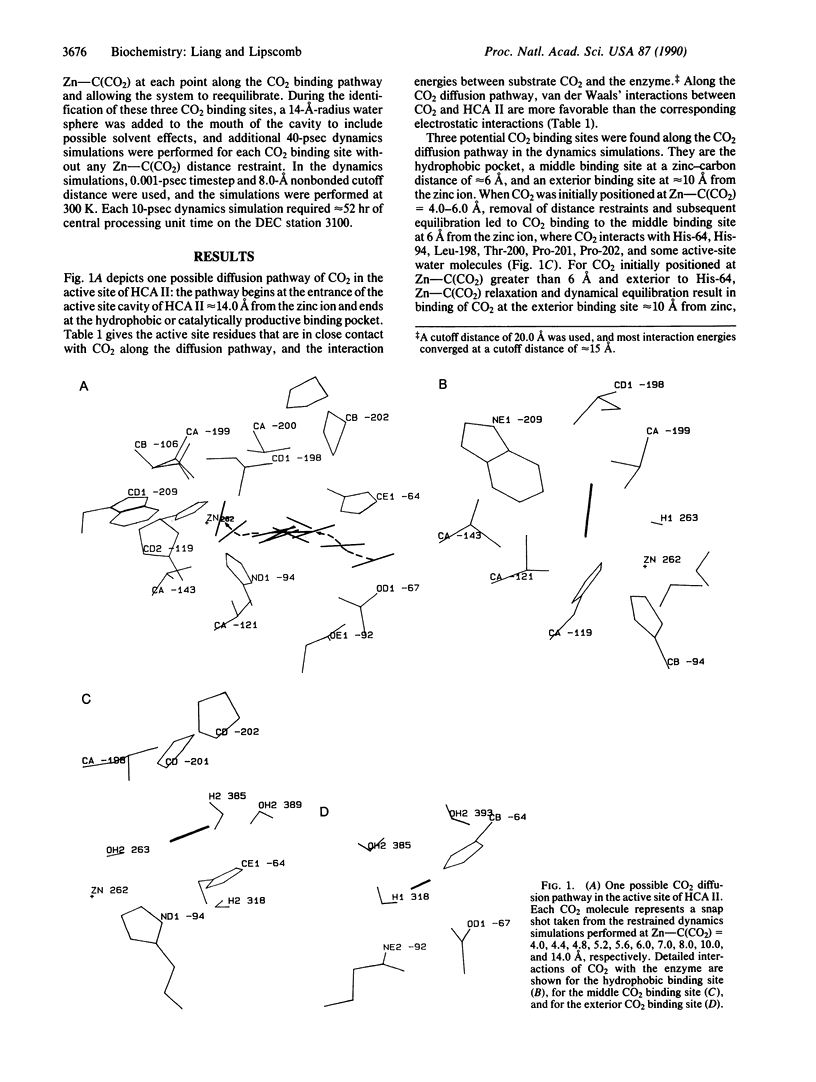
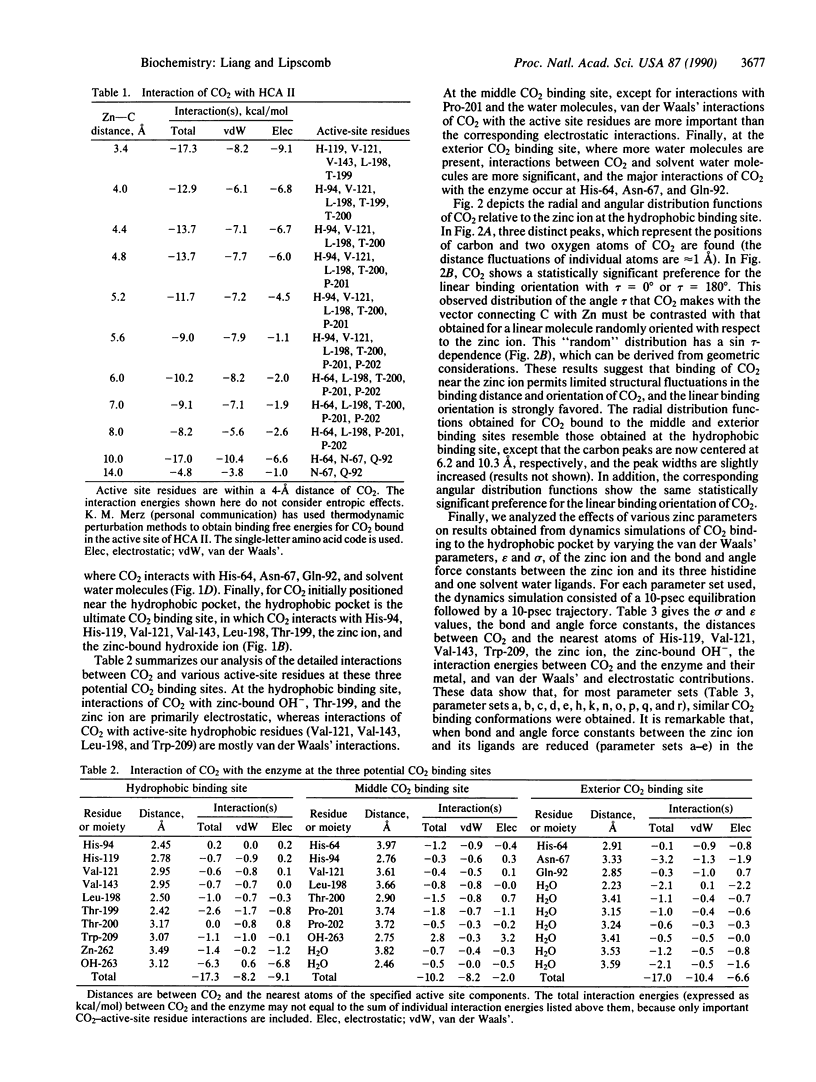
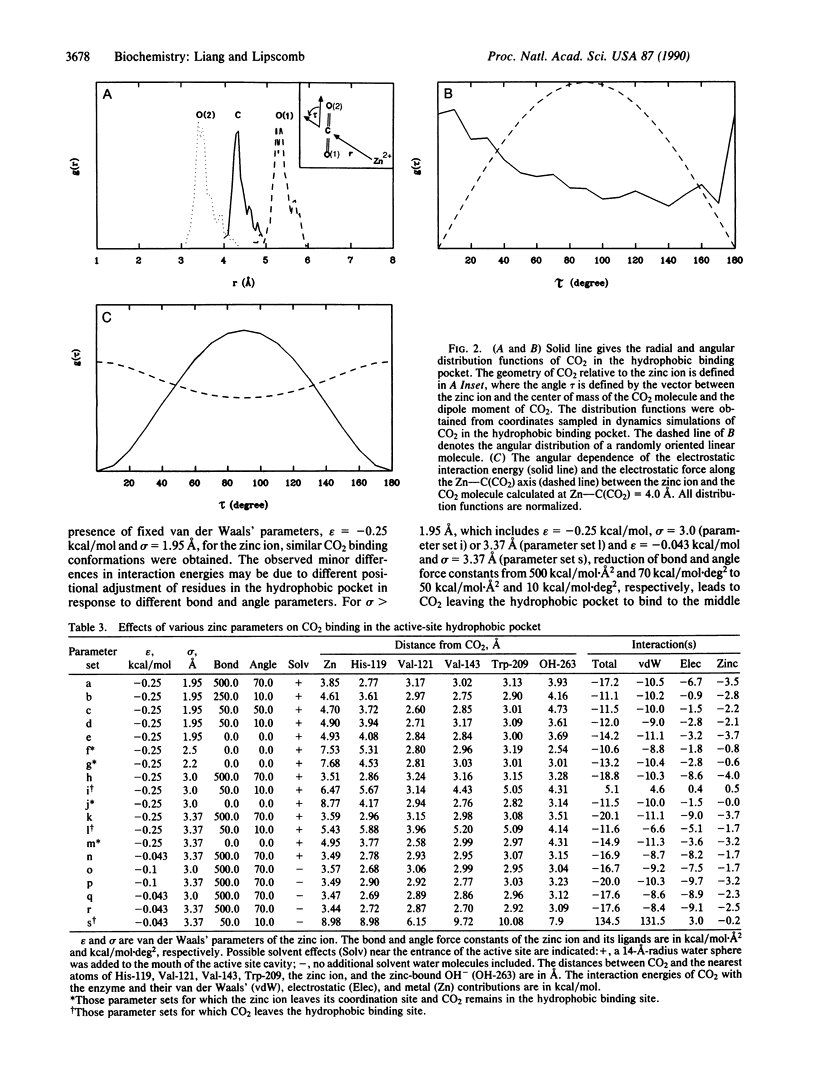
Molecular dynamics has been used to study binding of substrate CO2 to the active site of human carbonic anhydrase II. Three potential CO2 binding sites have been located. The first is at the active-site hydrophobic pocket (the catalytically productive site), where CO2 is approximately 3.5 A from the zinc ion and interacts with His-94, His-119, Val-121, Val-143, Leu-198, Thr-199, the zinc ion, and the zinc-bound hydroxide ion. The second CO2 binding site is approximately 6 A from the zinc ion, where CO2 interacts with His-64, His-94, Leu-198, Thr-200, Pro-201, Pro-202, and some active-site water molecules. The third CO2 binding site is approximately 10 A from the zinc ion, is largely solvated by water molecules, and interacts with His-64, Asn-67, and Gln-92. At these three CO2 binding sites, the CO2 molecule is highly localized (the average Zn-CO2 distance fluctuation is approximately 1 A) and favors the linear binding orientation toward the zinc ion. This linear binding orientation of CO2 and its electrostatic interaction with the zinc ion direct diffusion of CO2 toward the zinc ion and facilitate the nucleophilic attack from O of the zinc-bound OH- to C of CO2 in the productive hydrophobic binding site. Finally, the two CO2 binding sites outside the hydrophobic binding pocket, which may represent two intermediate states along the CO2 binding pathway, could play important roles as a CO2 relay.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Kylsten P. M., Jones T. A., Liljas A. Crystallographic studies of inhibitor binding sites in human carbonic anhydrase II: a pentacoordinated binding of the SCN- ion to the zinc at high pH. Proteins. 1988;4(4):283–293. doi: 10.1002/prot.340040407. [DOI] [PubMed] [Google Scholar]
- Kannan K. K., Petef M., Fridborg K., Cid-Dresdner H., Lövgren S. Structure and function of carbonic anhydrases. Imidazole binding to human carbonic anhydrase B and the mechanism of action of carbonic anhydrases. FEBS Lett. 1977 Jan 15;73(1):115–119. doi: 10.1016/0014-5793(77)80027-6. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. Structure and catalysis of enzymes. Annu Rev Biochem. 1983;52:17–34. doi: 10.1146/annurev.bi.52.070183.000313. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Adv Enzymol Relat Areas Mol Biol. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- Stein P. L., Merrill S. P., Henkens R. W. Carbon-13 nuclear magnetic relaxation study on cobalt carbonic anhydrase: Evidence on the location of enzyme bound CO2 and HCO3. J Am Chem Soc. 1977 Apr 27;99(9):3194–3196. doi: 10.1021/ja00451a070. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Henkens R. W. Dynamic 13C NMR investigations of substrate interaction and catalysis by cobalt(II) human carbonic anhydrase I. Biochemistry. 1985 May 7;24(10):2459–2462. doi: 10.1021/bi00331a010. [DOI] [PubMed] [Google Scholar]



