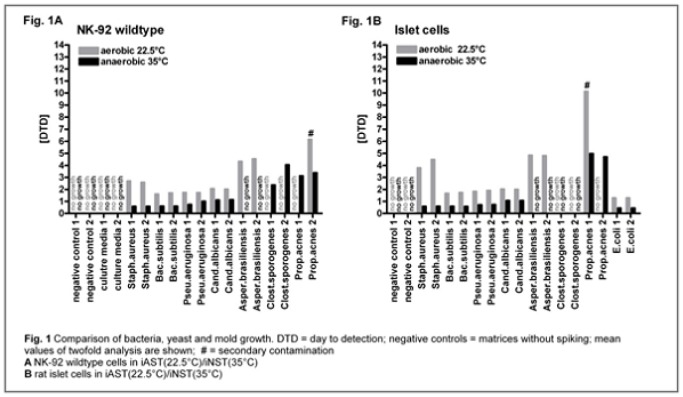
Transfus Med Hemother 2016;43(suppl 1):1–84
A
Abel F. R. JS-03-4
Abels W. C. SI-09-7
Achenbach S. P06-5, SI-14-4
Adair J. E. JS-09-2
Ahrens N. P01-8, P02-20, P03-10, P03-13, P09-6
Aigner M. SI-14-4
Albert M. H. SI-14-6
Albrecht J. P10-14
Alex J. SI-04-3
Aljabri A. SI-09-1
Altermann W. JS-10-5, P08-4
Althaus K. P09-9, SI-04-3, SI-04-5
Alt-Mayer T. SI-09-5
Amann E. M. JS-03-3
Ampofo E. P06-2
Amrein K. P07-2
Andres O. SI-04-4
Andresen S. P03-3, SI-12-5
Anliker M. SI-06-4, SI-13-5
Arendt A. P10-20
Arenskrieger K. P07-5
Arlt N. P09-12, P10-21, P11-5
Arnold M.-L. P08-6, JS-10-3, JS-10-6
Arnold R. SI-09-9
Auberger J. P09-10
Aurich K. P09-9
Avendańo C. JS-01-2
Axt-Fliedner R. SI-13-3
B
Baade M. JS-11-3
Baal N. P09-13, P10-10, P10-22, JS-02-4
Babikeir Eln Mustafa K. JS-01-2
Bach C. P08-6, JS-08-8, JS-10-3, JS-10-6
Bade-Döding C. SI-09-6
Bade-Doeding C. SI-09-7
Bader P. P11-15
Bakchoul T. P02-25, SI-04-3, SI-04-4, SI-04-5,
SI-13-4, SI-14-5
Balola A. P02-21, P02-30
Bartolmäs T. P02-18, P02-21, P02-30
Bauhaus M. SI-12-5
Baumann-Baretti B. P03-3, P03-12
Bazhin A. P08-3
Becker J. U. SI-09-1
Becker P. P11-15
Beckmann M. W. P06-5
Beer A. P09-14
Bein G. P01-9, P05-2, P09-13, P10-10, P10-22,
JS-02-4, SI-11-3, SI-13-3, JS-11-5, SI-16-5
Beinhauer A.-K. P10-20
Berens C. P09-17
Berger K. SI-07-4
Besler K. P09-6
Beth L. SI-09-6
Beyer S. JS-08-5
Bieback K. JS-01-1, P10-11, P10-18
Bienemann K. P02-2
Binder L. P11-3
Binder T. M. C. SI-09-3, P08-2, P11-7
Biswas A. P06-1
Bittner A.-K. P11-6
Bittner R. P02-7
Blasczyk R. P10-6, P10-26, SI-09-1, SI-09-6,
SI-09-7
Blessing F. P03-6
Blumentritt C. SI-04-5
Böck M. P01-3, P01-5
Boenig H. JS-05-1, P09-2, P09-11, P10-14,
JS-08-5, JS-09-5
Bolbasov E. P10-5
Bonifacio E. P07-1
Boenig H. JS-09-5
Börger A.-K. P10-6
Börger V. P11-17, P11-18
Bornhäuser M. P09-5
Bosser M. P01-6
Brachtl G. P10-11
Bramhoff A. P03-6
Braumüller S. JS-03-3
Braune J. P01-1
Brauninger S. P09-11
Bräuninger A. SI-13-3
Brendel C. P09-13, JS-02-3, JS-02-4
Bremer M. P11-17, P11-18
Brennan M. JS-01-2
Brenner R. JS-03-3
Breyer M. P11-14
Brixner V. P04-3, SI-07-5
Brosig A. M. P03-10
Brys B. P03-3, P03-12
Bucher J. P08-3
Budde H. P10-1, P11-3, JS-08-4
Budde K. SI-09-4
Bugert P. SI-13-2, P02-4, P02-5, P02-8, P02-27, P06-7, P10-17, SI-04-4, JS-11-3
Buhmann R. SI-14-6
Bujnoch D. P05-5
Buldakov M. P02-6
Bunjes D. P09-14, SI-09-9
Bunos M. JS-08-5
Bunse C. P10-26
Burger M. P10-19
Burkhardt T. P11-2, P11-8
Burkhart J. P09-10
Busch D. P10-14
Buwitt-Beckmann U. P09-16
Bux J. P02-2
C
Caesar A. P02-12
Castro S.V.C. P11-16
Celik A. A. SI-09-6
Cherdyntseva N. P02-6
Chevalier E. P09-11
Cichutek K. JS-07-3
Cohen S. P10-7
Colic L. P02-27
Conradi R. P02-13
Cooper N. SI-11-3
D
D'Amato Tóthovį J. P04-6
Dankerl H. P11-9
De Miroschedji K. P11-18
Decristoforo P. P07-4
Degenhardt J. SI-13-3
Dehl J. SI-13-3
Deisting C. SI-13-3
Deitenbeck R. SI-16-4
Deitmer A. SI-16-5
Delcea M. P02-17
Delle-Chiaie L. P02-8
Dembowsky K. P09-11
Dengler T. P10-17
Denker K. SI-11-5
Depre F. P06-4
Depré F. P06-6
Detsch R. P10-4
Dhople V. P01-1
Dick A. JS-10-3
Diekmann J. JS-11-4
Diel A. P02-4
Dieplinger B. P02-11
Dietrich D. P10-20
Dirkmann D. P14-11
Doescher A. P02-1, P02-3, SI-07-3
Dombos S. P04-3, SI-07-5
Dormann F. P01-8, P03-10
Döscher A. P02-13
Dössinger G. P10-14
Douglas G. P09-11
Drawz A. P06-2
Dreier J. JS-11-4
Drexler C. P07-2
Dunai Z. A. P10-11
Duong Y. C. JS-11-5
Dührsen U. P11-14
Dürig J. JS-09-1
Dürrenfeld A. P03-12
Dütsch M. JS-03-4
Dziodzio T. P08-1
E
Ebell W. SI-09-8
Eckstein R. SI-07-1, P01-6, P03-7, P03-8,
P05-4, P06-3, P06-5, P10-4, P10-23,
P10-25, P11-9, SI-06-5, SI-14-4
Egger-Salmhofer M. P02-11
Egle A. P05-1
Ehrhardt H. SI-11-3
Ehrnthaller C. JS-01-2
Eichler H. P06-2, P06-7, P06-8, SI-06-3
Eichner R. P05-3
Eicke D. P10-6
Eiermann T. H. P11-7
Einsele H. P10-14, SI-09-9
Eisenberger U. JS-08-3
Eiz-Vesper B. JS-05-2, P09-7, P10-6, P10-26
Elvers-Hornung S. P10-18
Emmanuel K. P02-11
Engström C. P02-12
Enkel S. P02-25
Erickson A. P04-3
Escot C. P09-11
Eugster A. P07-1
F
Fabricius D. P10-9
Falk J. SI-12-4
Faltus T. JS-09-3
Fecher T. P10-9
Fechner J. SI-02-1
Feichtner M. P10-11, P10-16
Fenchel K. P01-10
Fiedler J. JS-03-3
Fiedler M. P08-5
Figueiredo C. P10-6, P10-26, SI-09-1, JS-09-6,
SI-10-2
Filimonov V. P10-5
Fischer E. G. P11-8
Fischer J. SI-10-3, P01-6, P03-6
Fischer M. P09-4
Flesch B. K. P02-9, P02-10, P02-19, P02-29,
SI-09-5
Fleury-Cappellesso S. JS-01-2
Flommersfeld S. P05-2, P06-1
Foley R. P10-7
Frank K. P04-5, SI-12-3
Frers I. C. P06-1
Frey B. M. SI-13-1, P02-12, P04-4, P09-1
Fritz L. JS-02-3
Fröhner V. SI-13-3
Fuchs B. P09-10
Fuchs N. P11-14
Fuchs T. SI-12-5
Funk M. SI-12-1
Funk W. P10-17
Fürst D. P01-4, P09-14, JS-03-3, SI-09-9
G
Ganslandt T. SI-05-3, P05-4
Gassner C. SI-13-1, P02-12, P09-1
Gathof B. P03-1, P03-11, P04-2, P07-5
Gattenlöhner S. SI-13-3
Gatto C. P04-6
Gebhard F. JS-01-2
Geithner M. P08-6
Gerez L. P10-7
Germeroth L. P10-14
Giebel B. JS-05-3, JS-09-1, P11-6, P11-16,
P11-17, P11-18
Gielen J. P04-2
Giers G. P03-6
Gimona M. P10-11
Giordano R. JS-01-2
Giptner A. JS-11-5
Giurgola L. P04-6
Gjerde C. G. JS-01-2
Gloger D. P07-3
Göbel S. SI-09-5
Gökbuget N. P10-19
Gomez Barrena E. JS-01-2
Gonzalo Daganzo R. M. JS-01-2
Gorelashvili M. SI-09-8
Görgens A. JS-09-1, P11-16, P11-17,
P11-18, P11-19
Goslings D. P04-4
Gourri E. P09-1
Gowland P. P02-16, P04-1
Graber J. P02-16
Grabmer C. P05-1
Grabowski C. P01-10
Graf D. P06-1
Graff J. P09-11
Gramatzki M. JS-07-2, P09-16, SI-09-9
Graminske S. P04-3
Gravemann U. SI-07-5
Grebhardt C. P01-7
Greger N. P02-23
Greil R. P05-1
Greinacher A. SI-16-2, P01-1, P02-17, P07-3,
P09-9, SI-04-3, SI-04-5, SI-11-5, SI-14-5
Greinix H. JS-06-3
Grigoleit G. U. P10-14
Großgebauer T. P04-7
Guba M. P08-3
Gubbe K. P04-5, SI-12-3
Guberina H. JS-08-3
Gudima A. P10-5, P10-13
Gül S. P08-1
Günther A. P09-16
Gupta S. P06-1
Guzman C. A. P10-6
H
Hackstein H. JS-06-1, P09-13, P10-10, P10-22,
JS-02-3, JS-02-4, SI-11-3, SI-13-3, JS-11-5,
SI-16-5
Hähnel V. P01-8, P03-10
Halimeh S. P06-1
Halle K. P02-22
Haltmayer M. P02-11
Hammer E. P01-1
Hammes H.-P. P10-18
Han W. JS-03-4
Handke W. SI-14-4
Hang R. JS-02-5
Hansen A.-L. P11-7
Harder M. SI-06-4
Harmsen M. P02-14
Hartl A. P10-11
Hasenkamp J. JS-08-4
Hatz R. SI-09-2
Hauber D. P09-14
Hauck-Dlimi B. P03-8
Hauser-Kronberger C. P10-11
Heiden M. SI-12-1, SI-16-4
Heidenreich F. P07-1
Heil U. P03-4
Heinrichs S. P11-14
Heim A. P09-7
Heinemann F. M. P08-5, JS-08-3, JS-10-3
Heinold A. P08-5, JS-08-3
Heinrich C. P09-6
Helbig C. P04-7
Heldke S. P04-3
Hellem S. JS-01-2
Hellstern P. P06-7
Hellweg R. JS-03-4
Helmberg W. SI-12-5
Hemmer S. P09-13
Henkenius K. JS-02-3
Hennig H. SI-14-1
Henny C. P02-16
Henschler R. P10-12
Herber M. P08-6
Herbert K. SI-13-4
Herbst L. SI-06-5
Herkt S. P10-24
Hermann S. P01-5
Hermann W. P01-7
Hersmann G. P04-7
Heuft H.-G. P11-8
Hiller O. SI-09-1
Hitzler W. P02-13
Höchsmann B. SI-06-4
Hoffmann T. SI-02-2
Hoffmann W. P07-3
Hölig K. P05-3, P07-1, P09-5
Holland H. JS-03-5
Holloschi A. JS-03-4
Hooftman L. P09-11
Höppel U. P10-4
Horn P. A. JS-09-1, P11-14, P08-5, P09-15,
P11-6, P11-16, P11-17, P11-18,
P11-19, JS-08-3
Houareau C. SI-14-2, SI-16-4
Hourfar K. P04-5, SI-12-3
Hoyer P. P01-10
Huber-Lang M. JS-03-3
Humbel T. P03-5
Humpe A. JS-07-2, P09-16
Huppert V. P10-20
Hussain S. P11-13
Hutschenreuter G. P02-3
Hütter G. JS-03-5
Huyton T. SI-09-6
I
Ignatius A. JS-01-2
Ihrig K. P10-19
Immenschuh S. SI-09-1
Ivaskevicius V. SI-06-2, P06-1
J
Jahrsdörfer B. P10-8, P10-9, JS-02-5
Jänigen B. JS-08-2
Janzen L. SI-04-3, SI-14-5
Jorks S. P01-10
Jouni R. SI-04-3, SI-13-4
Juhl D. SI-14-1
Jungk H. P03-4
Juretzek T. P09-12, P10-21, P11-5
Jurisic M. SI-13-4
Just B. P02-9
K
Kabisch H. P11-7
Kagan K. O. P02-25, SI-13-4
Kahaly G. J. SI-09-5
Kahlenberg F. P03-3, P03-12
Kaltenmeier C. T. P10-8
Kappert G. P06-1
Karagianni M. SI-14-6
Karl A. P04-5, P11-2, SI-12-3
Karnot A. P02-10, P02-19
Karpova D. P09-2, P09-11
Kasimir-Bauer S. P11-6
Kauke T. P08-3, SI-09-2
Kauling B. P10-20
Kelle N. P03-12
Keller K. P10-17
Kellner C. JS-07-2
Kelsch R. SI-09-3, P11-7
Ketter R. P06-8
Kibler E. P10-5
Kiefel V. P02-23, P07-3
Kiem H.-P. JS-09-2
Kiessig S. T. P11-1
Kießig S. SI-16-3
Killer M. JS-02-3
Kimmig R. P11-6
Klesse C. P07-1
Klöcker S. P08-6
Klopocki E. SI-04-4
Klump H. JS-02-1, P09-15
Klümpers V. P02-27, P06-7
Klüter H. P02-5, P02-6, P02-14, P10-5,
P10-13, P10-18, JS-11-3
Knabbe C. JS-11-4
Kneidinger N. SI-09-2
Knöfler R. SI-04-4
Knowles L. M. P06-2, P06-8, SI-06-3
Kobsar A. P01-3, P01-5
Koburger M. P04-7
Kollar K. P10-12
König E.-M. SI-04-4
König I. R. JS-11-5
König L. P11-6
König S. P02-15
Konrad H. JS-10-5, P08-4
Kontermann R. P10-2
Kordes U. P11-7
Körmöczi G. P02-28
Körper S. P09-14
Kößler A. P01-3, P01-5
Kößler J. JS-06-2, P01-3, P01-5
Kraemer A. P09-11
Kramer M. P05-3
Kramer-Zucker A. JS-08-2
Krautwurst A. SI-13-3
Kreibich U. P06-1
Kremer H. P10-18
Krenn P. P11-15
Kribben A. JS-08-3
Kripp H. P08-3
Kroll H. P01-10, P04-7
Kronstein N. P09-3, SI-11-4
Kronstein-Wiedemann R. P09-3, SI-11-4
Krüger W. P09-9
Küchler S. JS-03-5
Kudryavtseva V. P10-5
Kugel T. JS-03-5
Kühl J.-S. SI-09-8
Kuhn M. P07-1
Kühnl P. P11-7
Kunze-Schumacher H. SI-09-6, SI-09-7
Kurnik K. P06-1
Kuvardina O. N. P10-24
Kwoczek J. C. P10-26
Kzhyshkowska J. P02-6, P02-14, P10-5, P10-13
L
Lachance S. P10-7
Lachmann N. P08-1, SI-09-4, JS-08-3
Lahrberg J. P10-26
Lakshmipathi S. P10-14
Lambrecht B. SI-14-4
Laner-Plamberger S. P10-11, P10-16
Lang K. P07-1
Larmann J. SI-09-1
Lauer B. P08-6
Lausen J. P10-24
Laws H.-J. P02-2
Layrolle P. JS-01-2
Legler T. J. SI-11-2, P10-1, P11-3, JS-08-4
Leibacher J. P04-3, SI-07-5
Leisch M. P05-1
Leithäuser M. P02-23
Leitner G. P11-8
Lenz V. JS-08-3, P11-14
Lessig D. P06-8, SI-06-3
Lewalle P. P10-7
Li F. P02-14
Lindemann M. P08-5, JS-08-3
Lindlbauer N. P05-1
Link C. S. P07-1
Linke K. P04-7
Lippitsch A. P10-22
Litviakov N. P02-6
Liu T. P02-6
Lohmann A. SI-12-1
Lotfi R. JS-01-2, P10-17, JS-02-5, SI-12-4
Lotsch M. SI-13-5
Ludwig B. P10-21
Luz B. P02-8
M
Macher S. P07-2
Madla W. P03-4
Maecker-Kolhoff B. P09-7
Maertens J. P10-7
Malakhova D. P09-4
Manadhar T. SI-09-7
Mannhalter C. P06-7
Mansouri Taleghani B. P11-8
Marais I. P02-29
Marandiuc D. P02-13
Marini I. P10-2, SI-04-3
Marschall R. P05-2
Martin A. P11-9
Matheis N. SI-09-5
Matko S. P10-3, P10-15
Mayer B. P02-18, P02-21, P02-22, P02-24, P02-26, P02-30, P06-4, P06-6
Mayer G. P05-1
Mayr-Wohlfart U. P04-5, SI-12-3
Medina Escobar P. P01-7
Medvedev N. P02-17
Meyer S. SI-13-1, P02-12, Meyer zu Bexten E. SI-16-5
Michel G. P10-10
Mielke S. P10-7
Miettinen J. P09-2
Mildenberg I. P10-19
Miller N. P10-3
Mitrofanova I. P02-6
Mittelbronn M. P10-19
Moerman N. SI-12-5
Moganti K. P02-14
Mohrez M. P03-13
Moldenhauer A. JS-03-4
Moldenhauer S. JS-03-4
Möllmann M. JS-09-1
Montemurro T. JS-01-2
Moog R. P01-2, P01-4, P09-12, P10-21, P11-2, P11-5
Moritz M. P07-2
Mufti N. P04-3
Mughal N. P11-12, P11-13
Müller B. SI-14-5
Müller I. P06-2, P06-7, P06-8
Müller J. P09-17
Müller K. JS-11-5
Müller S. SI-12-1
Müller T. P02-1, SI-07-3
Müller V. P09-6
Murke F. P11,19, JS-09-1
Mynarek M. P09-7
Mytilineos J. P01-4, P09-14, SI-09-9
N
Nazar R. P11-13
Nepal B. P11-10, P11-11, P11-12, P11-13
Netz U. P03-9
Neubauer A. JS-02-3
Neuberger P. P02-8
Neuchel C. P01-4, SI-09-9
Neuenhahn M. P10-14
Neurohr C. SI-09-2
Nguyen T.-H. P02-17
Niederacher D. SI-10-3
Niederhauser C. P02-16, P04-1
Niederwieser D. SI-09-9
Niemann M. SI-09-4
Nold P. P09-13, JS-02-3, JS-02-4
North A. P04-3
Northoff H. SI-16-4
Nowak-Harnau S. P02-25
Nyadu B. JS-08-3
Nydegger U. P01-7
O
Odendahl M. P10-3, P10-14, P10-15, P10-21
Oelke M. P10-26
Oergel T. P09-9
Offergeld R. SI-14-2, SI-14-3, SI-16-4
Offner R. P03-10, P03-13
Olavarria E. P10-7
Oldenburg J. P06-1, P09-17
Olivieri M. P06-1
Öller M. P10-11, P10-16
Öllinger R. P08-1
Opitz A. P03-4
Ostermann H. SI-07-4
Oustianskaia L. P03-1, P07-5
P
Palmer A. JS-03-3
Pamler I. P03-13, P09-6
Papenkort E.-M. JS-11-5
Papert S. P10-1, JS-08-4
Paranikulangara P. P04-3, P11-15
Pasini E. P09-3
Paul A. JS-08-3
Paul-Konietzko K. SI-14-5
Payrat J. M. P03-3
Peipp M. JS-07-2
Peisl J. P06-5
Peltroche H. P09-12, P10-21, P11-5
Peter W. P11-7
Peters J. P11-14
Petershofen E. K. SI-07-3
Petrescu-Jipa V.-M. P03-1
Petters O. JS-03-5
Pfeiffer A. P10-4
Pfeiffer H. P03-7, SI-06-5, SI-14-4
Pfizenmaier K. P10-2
Pichl L. SI-14-5
Picksak G. P09-7
Pilch J. P06-2, P06-8, SI-06-3
Pisarski P. JS-08-2
Platz A. P03-2
Pleyer L. P05-1
Pohler P. SI-07-5
Pollet D. P10-12
Poppe C. JS-08-5
Portegys J. P02-27
Portmann C. P02-12
Posset M. P02-20
Pötzsch B. P09-17
Pratschke J. P08-1
Preußel K. SI-14-2, SI-14-3
Pruß A. P02-21, P04-6
Q
Qiu D. SI-11-3
R
Raab S. P03-2
Rabe A. SI-16-3
Radojska S. P03-1, P03-11, P07-5
Radtke S. JS-09-1
Raninger A. P10-11
Rauh C. P06-5
Rauh M. P10-25
Ravanat C. P04-3
Rebmann V. P11-6
Reichenberg S. SI-14-4
Reichhardt H. P10-1
Reil A. P01-2, P02-2
Reinert D. P04-4
Reinhardt P. P09-14
Reinke P. SI-09-4
Reitsma K. P10-7
Riabov V. P10-5, P10-13
Richter E. P02-4, P02-8, P02-27
Rieger C. SI-07-4
Riewaldt J. P09-18
Riggert J. P10-1, P11-3, JS-08-4
Ringel F. P06-4
Ringwald J. P06-3
Rink G. P02-4, P02-5, P02-8, P02-27, JS-11-3
Risch L. P01-7
Risch M. P01-7
Rodi S. JS-03-3
Rohde E. P05-1, P10-11, P10-16
Rojewski M. JS-01-2, P10-17, JS-02-5, JS-03-3
Romagnoli B. P09-11
Roppelt D. P08-6
Rösler W. P09-8
Rosner A. P05-3, P09-5
Rosskopf K. SI-12-5
Röth A P11-14
Rotem A. P07-5
Rothe R. P09-12, P10-21, P11-2, P11-5
Rothenberger S. P02-4, P02-8, P02-27
Rott H. P06-1
Rouard H. JS-01-2
Rovers J. P10-7
Roy D. C. P10-7
Roy J. P10-7
Rücker-Braun E. P07-1
Ruediger M. P10-7
Rühl H. JS-09-4
Russe E. P10-11
Rüster B. SI-12-3
S
Sachs U. J. JS-10-2, P01-9, P05-2
Sachs U. SI-11-3, SI-13-3, JS-11-5
Salama A. P02-18, P02-21, P02-22, P02-24, P02-26, P02-30, P06-4, P06-6
Sallaj B. SI-06-5
Santos Manvailer L. F. P11-6
Santoso S. SI-13-3, JS-11-5
Sanzenbacher R. JS-07-3
Saragih H. SI-09-1
Sauer M. P09-7
Sayed S. P09-18
Schäfer M. P11-7
Schallmoser K. P10-11, P10-16
Schanz U. P09-1
Scharberg E. A. P02-4, P02-8, P02-27
Scharf C. P01-1
Scharler C. P10-11
Schauwecker P. P09-14
Scheffel T. JS-03-4
Scheffler A. SI-14-6
Schellerer V. P01-6
Schennach H. P07-4
Schetelig J. P07-1, P09-5
Schiemann M. P10-14
Schiffer K. P03-8
Schlaf G. JS-10-5
Schlenke P. P07-2, SI-12-5
Schlott F. P10-14
Schmid-Horch B. SI-13-4
Schmidt A. P03-2
Schmidt C. JS-03-5
Schmidt C. Q. SI-06-4
Schmidt M. P04-5, SI-07-5, SI-12-3
Schmiedgen M. P07-1
Schmieger T. P01-6
Schmitzer M. SI-09-2
Schönbacher M. P02-28
Schönborn L. P07-3
Schönefeld S. SI-12-1
Schönemann C.P08-1, SI-09-4, SI-09-8, JS-08-3
Schopohl D. SI-07-4
Schottstedt V. P02-9
Schramm R. SI-09-2
Schramm S. P11-6
Schramm W. SI-07-4
Schranz D. SI-11-3
Schrezenmeier H. JS-01-2, P01-4, P04-5, P09-6, P09-14, P10-8, P10-9, P10-17, JS-02-5, JS-03-3, SI-06-4, SI-09-9, SI-12-3, SI-12-4, SI-13-5
Schröck M. P07-2
Schroeter J. P04-6
Schultze-Florey R. P09-7
Schulz A. P09-14
Schulz R. P11-4, JS-03-5
Schulz U. P01-2, P01-4
Schulze H. SI-04-4, SI-09-8
Schulze K. P10-6
Schulze T. J. P02-5, JS-11-3
Schütte-Nütgen K. SI-09-3, P08-2
Schwarz K. P09-14
Schwarze B. SI-06-5
Schwertz H. SI-04-2
Schwind P. P02-12
Seidl C. P11-15
Seifried E. P04-3, P04-5, P10-24, P11-15, SI-12-3, JS-08-5
Selleng K. SI-11-5
Selleslag D. P10-7
Seltsam A. SI-11-1, SI-07-5, SI-14-4
Senft C. P10-19
Sensebé L. JS-01-2
Seyboth S. P02-8
Siegemund M. P10-2
Sielaff S. P09-12
Sinzger C. SI-12-4
Six M. P10-23
Skenderi Z. P04-6
Smida D. SI-16-5
Solleder M. P02-20
Sommer M. SI-12-5
Song B. P02-6
Song W. P02-5, JS-11-3
Spiegel-Cürten I. P10-20
Spierings E. SI-09-4
Spohn G. P09-2
Spranger R. SI-12-1
Spriewald B. M. P09-8, JS-10-3, JS-10-6
Staeck O. SI-09-4
Stangenberg W. P07-3
Stankevich K. P10-5
Steffen M. P08-6, P09-8
Steil L. P01-1
Steinbach J. P10-19
Steinhauser J. P10-10
Steininger P. A. JS-09-7
Steppe L. JS-02-5
Stock B. P09-11
Stoecklein N. H. SI-10-3
Stöhr D. SI-12-4
Stolz M. P04-1
Störmer M. P03-1, P03-11, P04-2, P07-5
Stötzer F. SI-16-4
Straka C. P09-10
Strasser E. F. P03-7, P10-25, SI-14-4, P05-5, P06-3, P10-4, P10-23, SI-15-3
Strathmann K. P02-3
Strauß G. SI-09-8
Streif W. SI-04-4
Streit F. P11-3
Strobel J. SI-05-3, P03-7, P03-8, P05-4, P10-23, P11-9
Strobel U. SI-04-5
Strunk D. P10-11, P10-16
Stürtzel A. P02-8
Stützer T. P01-6
Sumian C. SI-14-4
Sümnig A. SI-16-4
Suwelack B. SI-09-3, P08-2
Sykora K.-W. P09-7
T
Tast B. P09-2
Teichweyde N. P09-15
Theilmeier G. SI-09-1
Thiele T. P01-1, SI-14-5
Thierbach J. P03-3
Thomas H. P04-7
Thomas K. P10-15
Thomas S. P10-26
Thornton N. M. P02-29
Tischer S. P09-7, JS-09-6
Todorova K. SI-09-8
Tolios A. P02-28
Tolksdorf F. SI-07-5
Tonn T. P01-4, P04-5, P09-3, P09-12, P09-18, P10-3, P10-14, P10-15, P10-19, P10-21, P11-2, P11-5, SI-07-5, SI-10-1, SI-11-4, SI-12-3
Trobisch H. P06-1
Tröger S. P10-3
Trost B. P09-16
Trost N. SI-13-1, P02-12
Tryankowski R. P03-4
Trzaska T. P10-9
Tsamadou C. P01-4, SI-09-9
Tschapikow I. JS-02-4
Türkmen T. SI-11-3
Tverdokhlebov S. P10-5
U
Ul Ebad Khan P11-12, P11-13
Ulrich E. P03-12, SI-16-3
Ünlü S. P08-1
Urban E. P01-4
Urbschat S. P06-8
Url C. SI-12-5
V
Valek A. P04-4
Van Sandt V. P02-15
Vasic B. SI-12-5
Velthuis J. P10-7
Verdonck K. P07-5
Vijayan V. SI-09-1
Vitoriano S. JS-09-1
Völker U. P01-1
Vollmer T. JS-11-4
Von Dossow V. SI-09-2
Von Zabern I. SI-06-4
Vrana N. E. P10-13
W
Wagner A. P09-16
Wagner B. P11-6, SI-14-6
Wagner E. SI-09-9
Wagner F. F. P02-1
Wagner F. P02-7
Wagner M. P10-19
Wahle A. JS-10-5, P08-4
Wahler A. P03-3
Walker I. P10-7
Waterstradt M. P09-9
Weber I. P04-3, P10-12, SI-07-5
Weber K. P01-3, P01-5
Wehrmann K. SI-14-5
Weinauer F. P09-10
Weingand T. P03-5, P11-8
Weinstock C. SI-06-4, SI-13-5
Weisbach V. SI-06-5
Weiss D. SI-06-1, P06-3, P11-9
Weiss L. P05-1
Weiss S. P08-1
Weitmann K. P07-3
Weitzendorfer M. P02-11
Wels W. S. P10-3
Wels W. P10-19
Wenzel F. P03-6
Werner J. P08-3
Wesche J. SI-14-5
Wessiepe M. P02-3
Weyand M. P05-5
Wichmann C. SI-14-6
Widmer N. P04-1
Wieckhusen C. P02-4, P02-8
Wiedemann H. P10-16
Wiederschein H. P08-6
Wienzek-Lischka S. SI-13-3, JS-11-5
Wiercinska E. P09-2, P09-11
Wiesinger K. P02-11
Wiesneth M. P09-14
Wilde B. P08-5
Wingenfeld E. JS-08-5
Winkelmann M. SI-12-4
Winkler J. P09-8
Winter H. SI-09-2
Winterstein K. P01-10
Wittmann G. SI-07-4, SI-14-6
Witzenhausen C. SI-12-1
Witzke O. P08-5, JS-08-3
Woestmann S. J. P02-19, P02-29
Wolf D. P09-17
Wolf S. P08-3
Wolff J.-C. P01-9
Wolter C. P06-8
Worel N. JS-06-3
Wößmann W. SI-11-3
Wu M. JS-03-4
Wulf G. JS-08-4
Y
Yard B. P02-14
Yürek S. P02-18
Z
Zainab Mukhtar Z. P11-12
Zaslavskaya M. P08-5
Zavjalova M. P02-6
Zehnder T. P10-4
Zeiler T. SI-14-5
Zelenski T. JS-02-5
Zeplin P. P10-17
Zhang C. P10-19
Zhuravlev M. P10-5
Ziehe B. P10-25
Ziemann M. SI-12-2
Ziemssen T. P10-15
Zilian E. SI-09-1
Zimmer K.-P. SI-11-3
Zimmermann B. P02-25
Zimmermann R. SI-07-1, SI-05-3, SI-15-4, P01-6, P03-7, P03-8, P05-4, P06-5, P10-23, P11-9, SI-14-4
Zingsem J. SI-07-1, JS-10-1, P05-4
Zschiedrich S. JS-08-2