Abstract
Cognitive dysfunction is a major comorbidity of the epilepsies; however, treatments targeting seizure-associated cognitive dysfunction, particularly deficits in learning and memory are not available. Isoketals and Neuroketals, collectively known as gamma-ketoaldehydes are formed via the non-enzymatic, free radical catalyzed oxidation of arachidonic acid and docosahexaenoic acid, respectively. They are attractive candidates for oxidative protein damage and resultant cognitive dysfunction due to their formation within the plasma membrane and their high proclivity to form cytotoxic adducts on protein lysine residues. We tested the hypothesis that gamma-ketoaldehydes mechanistically contribute to seizure-associated memory impairment using a specific gamma-ketoaldehyde scavenger, salicylamine in the kainic acid and pilocarpine rat models of temporal lobe epilepsy. We show that gamma-ketoaldehydes are increased following epileptogenic injury in hippocampus and perirhinal cortex, two brain regions imperative for learning and memory. Treatment with an orally bioavailable, brain permeable scavenger, salicylamine attenuated 1) spatial memory deficits 2) reference memory deficits and 3) neuronal loss and astrogliosis in two mechanistically distinct models of epilepsy without affecting the epileptogenic injury or the development of chronic epilepsy. We have previously demonstrated that reactive oxygen species and the lipid peroxidation biomarkers, F2-isoprostanes are produced following status epilepticus. However, which reactive species specifically mediate oxidative damage to cellular macromolecules remains at large. We provide novel data suggesting that memory impairment occurs via gamma-ketoaldehyde production in two models of epilepsy and that treatment with a gamma-ketoaldehyde scavenger can protect vulnerable neurons. This work suggests a novel target and therapy to treat seizure-induced memory deficits in epilepsy.
Introduction
Up to half of people with temporal lobe epilepsy (TLE) report experiencing one or more cognitive deficits (Gilliam et al., 2003). Quality of life correlates with the comorbid cognitive conditions rather than the seizures themselves, indicating that memory impairment represents a larger burden to patients than experiencing unpredictable seizures (Gilliam et al., 2003). Cognitive comorbidities of epilepsy, particularly memory impairment, are widely recognized for their frequent and debilitating nature, however there are currently no therapies to treat them and the mechanism(s) by which cognitive dysfunction arises during epilepsy are unknown.
A growing body of research has implicated oxidative stress as a contributing factor to cognitive impairment in disorders including Alzheimer’s disease (AD) and recently TLE (Berr et al., 2000; Pearson et al., 2015; Praticò et al., 2002). However, the precise mechanism(s) and species mediating oxidative damage remains unknown. Identification of specific species mediating damage is imperative as some reactive oxygen species (ROS) have recently been recognized for their signaling roles (SG, 2006; Thiels et al., 2000). The brain is uniquely susceptible to oxidative damage, specifically lipid peroxidation, due to its high oxygen demand and polyunsaturated fatty acid (PUFA) composition. Arachidonic acid is one such PUFA that is highly enriched in the brain and together with docosahexaenoic acid accounts for a large portion of the brain’s fatty acid content. Under conditions of oxidative stress, tissue esterified arachidonic acid and docosahexaenoic acid are susceptible to free radical-induced peroxidation forming F2-Isoprostanes (F2-IsoPs) and F4-Neuroprostanes, respectively (Milne et al., 2011). F2-IsoPs serve as reliable biomarkers of lipid peroxidation in vivo and have been detected in a variety of neurodegenerative conditions (Milne et al., 2005; Reich et al., 2001). Data from our laboratory demonstrate that levels of F2-IsoPs increase in the hippocampus as an acute consequence of status epilepticus (SE) (Patel et al., 2001). Thus, there is evidence for increased lipid peroxidation in the hippocampus following SE. While F2-IsoPs are well recognized biomarkers of oxidative stress, they are not known mediators of oxidative damage.
The pathway of free-radical induced peroxidation of arachidonic acid and docosahexaenoic acid also results in the formation of extremely reactive isoketals (IsoKs) and neuroketals (NeuroKs) respectively. IsoKs and NeuroKs, collectively known as γ-ketoaldehydes (γ-Ks), are attractive candidates as mediators of oxidative damage. They are among the most reactive products of lipid peroxidation identified, adduct almost instantaneously to protein lysine residues and readily induce protein-protein cross-links (Brame et al., 1999; Davies et al., 2004a). γ-K protein adducts are known to alter mitochondrial respiration, calcium homeostasis, ion channel function, inhibit proteasome function and induce cell death (Boyden PA, 2007; Brame et al., 2004; Davies et al., 2002; Stavrovskaya et al., 2010; Sullivan et al., 2010). In vitro, oxidation of arachidonic acid produces IsoKs and F2-IsoPs in similar abundance, suggesting that they are simultaneously produced (Brame et al., 1999). Based on this information, we hypothesize that γKs increase as a consequence of seizure activity and contribute to cognitive dysfunction associated with TLE.
Salicylamine (SA), is a lipophilic scavenger of γ-Ks that reacts specifically with γ-Ks and does not interfere with prostaglandin synthesis or other oxidative processes (Zagol-Ikapitte et al, 2010). In vitro, esterified γ-Ks have been shown to be about 1000 times more reactive to SA than lysine residues (Davies et al, 2006). Importantly, at concentrations ranging from 10–100μM, SA protected cells against oxidant-induced sodium channel dysfunction and at 500μM, SA protected HepG2 cells from oxidant-induced cell death (Davies et al, 2006). SA is orally bioavailable, crosses the blood brain barrier and when supplemented in drinking water (1–10g/L) results in tissue concentrations that span the range of concentrations shown to inhibit oxidant-induced damage (10–500 μM) (Zagol-Ikapitte et al, 2010). That SA is a specific scavenger of γKs and can be orally delivered in concentrations known to be effective, make it an excellent tool for dissecting out the role of γ-Ks in the pathology of seizure-induced cognitive dysfunction and epileptogenesis itself.
Materials & Methods
Reagents
SA was synthesized as previously described (Davies et al., 2006). The D11 ScFv antibody was a generous gift from Dr. Raymond Mernaugh. Kainic acid (KA) was purchased from AG Scientific. All other reagents were purchased from Sigma unless otherwise noted. Animals: All rats were handled in accordance with the Guide for the Care and Use of Laboratory Animals and all protocols were approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. Power analyses were performed to determine the minimal number of animals required in each group to reach the desired statistical power. Pilot experiments revealed large deficits in KA-treated animals on all behavioral tests. Based on these studies, a power analysis was performed (r= 0.8; α= 0.05; Power= 0.8) for determination of sample sizes. Upon arrival, adult, male Sprague-Dawley rats (250–300g; Harlan Sprague-Dawley, Indianapolis, Indiana) were singly housed on a 14/10 light/dark schedule and allowed ad libitum access to standard rat chow and filtered water. After one week, rats were randomly (using a random number generator, random.org) assigned to groups treated with either saline for control animals or KA (11mg/kg s.c.) to induce SE. To determine if effects were model specific, a separate cohort of rats was treated with another chemoconvulsant which acts through the cholinergic system, pilocarpine. Specifically, rats were treated with scopolamine methyl bromide (1mg/kg) followed 30 minutes later by pilocarpine hydrochloride (340mg/kg s.c.) before receiving diazepam (10mg/kg) 90 minutes after pilocarpine to stop the seizures, whereas controls rats received scopolamine and saline rather than pilocarpine. Progression into SE, for both groups, was visually monitored and behavioral seizures were scored according to a modified Racine Scale as previously described (Pearson et al., 2015; Pearson et al., 2014). Only rats experiencing SE defined as: at least five P3 or higher seizures followed by a period of continuous seizure activity for at least two hours, were included in further analyses as SE is the best predictor for the development of chronic epilepsy. Experimenters were blinded to all animal and sample treatment groups during testing by assigning a unique animal number to each animal/sample.
Salicylamine Treatment
Control and KA-treated or pilocarpine-treated rats were randomly assigned to groups treated with vehicle (PBS) or SA (200mg/kg i.p.). Treatment with SA was initiated 30 minutes after KA or pilocarpine injection followed by SA (1g/L) in the sole source of drinking water for the duration of the experiment. For the delayed treatment time point, rats were treated with SA in drinking water starting 3 weeks after SE. Rats were allowed free access to SA starting one week prior to cognitive testing and continuing throughout the duration of testing. Control rats treated with SA were included in select behavioral tasks and discernable differences were not detected, therefore they were excluded from further analyses.
HPLC determination of SA concentration
Dosing of SA was determined based on the known plasma half-life and bioavailability of the compound (Zagol-Ikapitte et al., 2010). HPLC was used to assess whether this dosing paradigm resulted in therapeutic concentrations in plasma and brain in control and KA-treated rats allowed ad libitum access to water supplemented with SA (1g/L). Samples were analyzed with an ESA (Chelmsford, MA.) 5600 CoulArray HPLC equipped with eight electrochemical cells following the company instruction (ESA Application Note 70-3993). The potentials of the electrochemical cells were set at 50/150/250/350/450/550/650/750 mV vs. Pd. Analyte separation was conducted on an YMC ODS-A (Montgomeryville, PA) reverse-phase C-18 analytical column (4.6 mmx150 mm; 5 μm particle size). The mobile phase was composed of 50 mM NaH2PO4 pH 2.7 and 1% methanol.
Assessment of γ-K production by mass spectrometry
Quantification of IsoK and NeuroK adducts was performed in whole hippocampus, hippocampal sub-regions and perirhinal cortex at 24 hours and 6 weeks after SE. These time points correspond to the acute and chronic phase of epilepsy, respectively. To attain sufficient tissue for liquid chromatography tandem mass spectrometry (LC/MS/MS) analysis in hippocampal sub-regions, samples from each region were pooled from 4 rats of the same treatment group. Purification and analysis of γ-K adducts was performed as previously described (Davies et al., 2007). Briefly, after enzymatic proteolysis of tissue samples and purification via HPLC, IsoK-lysyl-lactam adducts are quantified by LC/MS/MS against an internal standard.
Video-EEG Implantation and Monitoring
EEG implantation and monitoring of SE and spontaneous seizures was performed as previously described (Pearson et al., 2015). Rats were anesthetized and implanted with bilateral, dural electrodes (PlasticsOne; coordinates- 4.0 mm posterior, 2.5 mm lateral relative to the bregma). Animals were allowed to recover for one week before video (Virtual Dub) and EEG (Pinnacle Technology Inc.) were obtained. To assess the progression of SE, rats were monitored for a one hour baseline recording, followed by injection with KA. Thirty minutes after the KA injection, half of the animals received an acute injection of SA followed by SA in drinking water as detailed above. All EEG data were analyzed for average maximum electrographic power associated with SE (maximum amplitude-baseline) and power within various frequency bands using a Fast Fourier Transformation algorithm and custom-written software (White et al., 2006). Rats were continually monitored via 24/7 video-EEG (V-EEG) for the following month to assess the development of spontaneous seizures.
Behavioral Studies
Novel object recognition (NOR) testing was performed blinded and as previously described to assess non-hippocampal dependent memory (Pearson et al., 2015; Pearson et al., 2014). Rats were acclimated to conditions approximating those of the NOR task over a series of days starting 4 days after SE. These acclimations included exposure to the dedicated testing room, behavioral arenas (open field task) and stimulus objects. NOR testing occurred one week after SE and consisted of two phases. During the first phase, rats were allowed to investigate two identical objects for 5 minutes, before being placed back into their home cage for a one hour delay period. After the delay, rats were returned to the same behavioral arena however one object was replaced with an entirely novel object. Rats with intact recognition memory will notice this change and spend a greater proportion of time investigating the novel object (Berlyne, 1950; Mumby et al., 2002). All tests were video recorded and behaviors of interest were quantified using specialized behavior recognition software (Topscan by Cleversys Inc., Reston, VA.). To determine the percent of total investigation time spent with the novel object the following equation was used: (duration sniffing novel object/[duration sniffing novel object + duration sniffing familiar object]).
To assess hippocampal-dependent spatial memory, a separate cohort of KA-treated rats was tested in a Morris Water Maze (MWM) as described (Morris, 1984; Pearson et al., 2014). Testing in the MWM was initiated 14 days following SE to assess cognition once spontaneous seizures had begun. To test spatial memory performance in the delayed treatment cohort (i.e. SA treatment starting 3 weeks after SE) and in pilocarpine-treated rats, the standard Y-Maze (matte black, expanded PVC, 120° arm angles) was used. The Y-maze task was chosen in the delayed treatment cohort as pilot testing revealed frequent stress-induced seizures in the MWM starting 3 weeks after SE. Y-maze was also chosen for the pilocarpine-treated rats as previous data suggests these animals are unable to learn the MWM (Pearson et al., 2014). Rats were placed into one arm of the Y-shaped maze with distal cues in sight and allowed to explore for eight minutes as described (Pearson et al., 2015). Rats that have intact hippocampal-dependent spatial memory will remember the arm that was previously visited and will prefer to enter a new, unexplored arm. Total arm entries and sequence of arm entries was recorded and percent alternation was calculated as follows: (the number of actual alternations ÷ the maximum possible alternations) × 100.
Immunohistochemistry
Upon completion of the NOR task, approximately 10 days after SE, whole brains were rapidly dissected and formalin fixed before being Paraffin-embedded for all IHC studies. Every 6th coronal section (15 μm) through hippocampus and perirhinal cortex was collected and stained with Fluoro-Jade B (FJB; Histo-Chem) as previously described (Pearson et al., 2015; Schmued and Hopkins, 2000). Images were captured using a Nikon Eclipse TE2000-U microscope at 20× magnification. Quantification of FJB positive neurons in a given area was measured using Image J software. Objects with neuronal morphology and FJB signal twice the background were counted in CA1, CA3, hilus and perirhinal cortex. Every 7th section was collected for GFAP staining of reactive astrocytes. Sections were deparaffinized and rehydrated in sequential steps. Sections were blocked using 10% goat serum in PBST before incubation with the primary antibody diluted 1:100 in PBST. After an overnight incubation with the primary antibody, the sections were rinsed and incubated with the secondary antibody, rhodamine red for one hour. Sections were washed, cleared in xylene and coverslipped using DPX mounting medium. A Nikon Eclipse TE2000-U microscope was used to capture images at 10× magnification. Quantification of the average florescence intensity within a given area was performed using Image-J software. Data were compared using a one-way ANOVA and significant effects were probed using Newman-Keuls multiple comparison. To visualize the ability of SA to attenuate γ-K adducts, immunohistochemistry using the single-chain D11 ScFv anti-γ-K antibody was performed as previously described (Davies et al., 2011; Davies et al., 2004b). Briefly, 5 μm sections were deparaffinized, rehydrated and incubated in a 2% hydrogen peroxide solution in sequential steps. Sections were blocked in PBS containing 5% BSA and 5% normal mouse serum before being incubated with the primary antibody diluted 1:100 in PBS. Peroxidase activity of the secondary (Anti-FLAG/HRP 1:100) was detected by 3,3-diaminobenzidine tetrahydrochloride. Sections were imaged by a Nikon Eclipse 80i at 10× magnification. Cells were counted when staining at least twice the background was observed in a ring like structure indicative of membrane staining. Quantification was performed by an investigator blinded to experimental conditions.
Statistical analyses
Effects of group treatment and time on electrographical power during SE were compared using two-way ANOVA. Effects of group treatment (KA+PBS and KA+SA) on levels of IsoK/NeuroK protein adducts and neuronal loss were compared using an unpaired t-test. The effects of seizures (control and KA or pilocarpine) and treatment group (vehicle and SA) on learning and memory were compared using two-way ANOVA. Significant main effects and interactions (p < 0.05) were probed using Bonferroni post-tests. All data are expressed as mean ± SEM unless otherwise noted. All analyses were performed using GraphPad Prism 5 software.
Results
Gamma-ketoaldehydes are elevated in the kainic acid model of temporal lobe epilepsy
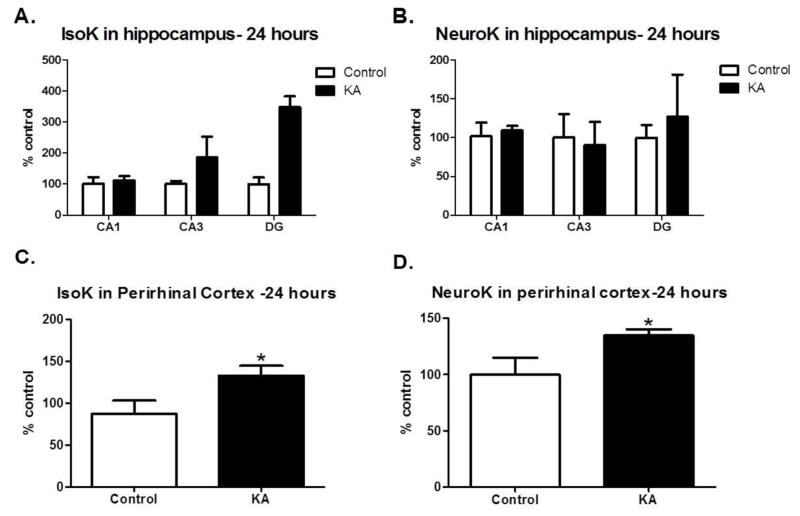
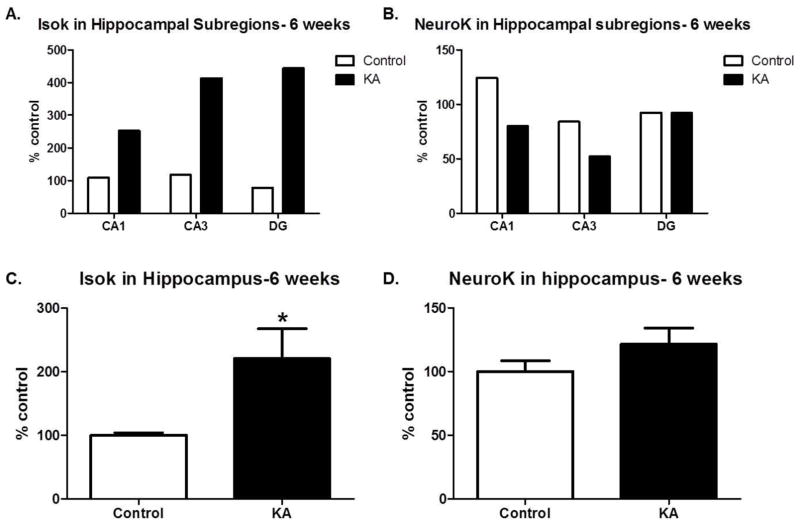
To determine whether IsoK and/or NeuroK protein adducts were elevated in the KA model of TLE, we analyzed levels of adducts in hippocampal sub-regions, perirhinal cortex and whole hippocampus. An initial study was conducted to determine if mass spectrometry could detect levels of IsoK protein adducts in brain areas (i.e. hippocampal sub-regions) where their precursors, IsoPs, are known to form. IsoKs and NeuroKs were detected in the CA1, CA3 and DG regions of the hippocampus (Figure 1A and 1B). Although the absolute magnitude of IsoK, but not NeuroK levels were higher in DG>CA3>CA1 in the KA vs control group, pooling of samples did not permit statistical analyses. Therefore, we assessed levels of protein adducts in the perirhinal cortex, 24 hours after SE. In this brain region, both IsoK and NeuroK protein adducts were significantly increased relative to control (p=0.03, Figure 1C and p=0.04, Figure 1D, respectively). To determine if protein adducts were altered in brain regions governing learning and memory during epilepsy, brains were taken 6 weeks after SE, once chronic spontaneous seizures were confirmed. An initial study confirmed our ability to detect the levels of IsoK and NeuroK protein adducts in all sub-regions of the hippocampus (Figures 2A and 2B). To ascertain whether γ-K adducts were significantly increased in KA-treated animals 6 weeks after SE, whole hippocampus was collected. Compared to control rats, KA-treated rats showed significantly elevated levels of IsoK protein adducts (p=0.04, Figure 2C) while no changes were detected in levels of NeuroK protein adducts (p=0.22, Figure 2D). Taken together, the data suggest that γ-K protein adducts are increased in experimental TLE in brain regions important for learning and memory.
Figure 1. Gamma-ketoaldehydes are increased acutely after SE.
Pooled measurement of IsoK adducts (A) and NeuroK adducts (B) in sub-regions of the hippocampus, collected 24 hours after SE n=2 samples/group of 4 rats/sample. IsoK adducts (C) and NeuroK adducts (D) measured in perirhinal cortex 24 hours after SE. n= 6–8/group. *p<0.05 compared to control by t-test.
Figure 2. Gamma-ketoaldehydes are increased chronically after SE.
Pooled measurement of IsoK adducts (A) and NeuroK adducts (B) in sub-regions of the hippocampus, collected 6 weeks after SE n=1 sample/group of 4 rats/sample. IsoK adducts (C) and NeuroK adducts (D) measured in whole hippocampus 6 weeks after SE. n= 6–8/group. *p<0.05 compared to control by t-test.
Salicylamine, a γ-ketoaldehyde scavenger, crosses the blood-brain barrier
SA, a lipophilic scavenger of γ-Ks has been show to exert protection against oxidant-induced cardiac sodium channel dysfunction at concentrations ranging from 10–500μM (Davies et al., 2006). SA has been shown to cross the mouse blood brain barrier and when supplemented in drinking water, results in tissue concentrations that span the range of concentrations shown to inhibit oxidant-induced damage (Zagol-Ikapitte et al., 2010). However, the ability of SA to cross the rat blood brain barrier is unknown. Therefore, we measured SA concentrations in the plasma brain and plasma of vehicle- and KA-treated rats using an HPLC method coupled to electrochemical detection. SA concentrations in KA-treated rat plasma were 5.7 μM ± 2.3 (mean ± SEM n=10) and the concentration of SA in brain were 8.9 μmoles/kg tissue ± 4.9 (mean ± SEM n=10). In control rats, the plasma concentration of SA was 5.9 μM ± 2.2 (mean ± SEM n=6) and the brain concentration was 6.3 μmoles/kg tissue ± 3.5(mean ± SEM n=6). Treatment with SA did not alter the amount of water consumed by the rat or the animal’s body weight at any point after treatment monitored up to three months after SE. Additionally, as body weight increased, water intake also increased suggesting that SA dose increased with body weight.
Pharmacological scavenging of γ-ketoaldehydes does not alter severity of status epilepticus
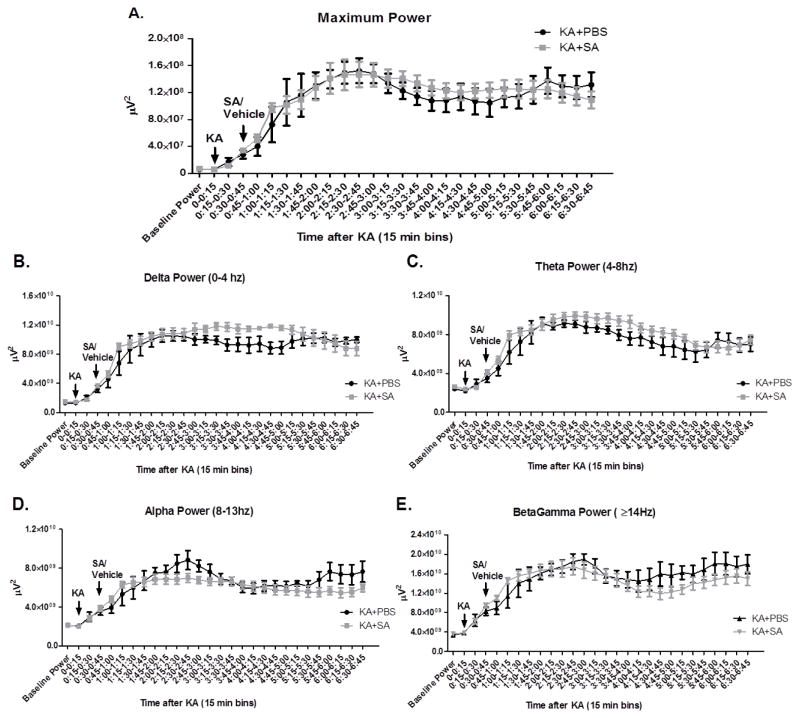
To ensure that the inciting injury, SE was not different between groups treated with and without SA, we sought to determine the effect of treatment with SA on SE. Rats were implanted with bilateral dural electrodes and observed via V-EEG. After a baseline recording, all rats were treated with KA followed 30 minutes later by an acute injection of SA as described above. No difference was detected between groups in average maximal power at any point during SE (treatment effect p=0.83; Figure 3A). Additionally, no differences were detected in any frequency band including delta 0–4 Hz (p=0.23, Figure 3B), theta 4–8 Hz (p=0.33, Figure 3C), alpha 8–13 Hz (p=0.37, Figure 3D), or betagamma ≥14 Hz (p=0.57, Figure 3E). To determine if the length of SE varied between groups, we analyzed time to 20% power, 10% power and 5% power (data not shown). No differences were detected between groups suggesting that length of SE was equivalent between groups. Taken together the data suggest that treatment with SA did not attenuate the overall severity of the initiating injury, SE.
Figure 3. Pharmacological scavenging of gamma-ketoaldehydes did not alter electrographic severity of SE.
(A) Maximal power during SE. (B) Power in Delta frequency range. (C) Power in Theta frequency range. (D) Power in Alpha frequency range and (E) Power in BetaGamma frequency range. Analyzed via two-way repeated measures ANOVA n=5–6/group.
Pharmacological scavenging of γ-ketoaldehydes attenuates SE-induced memory deficits
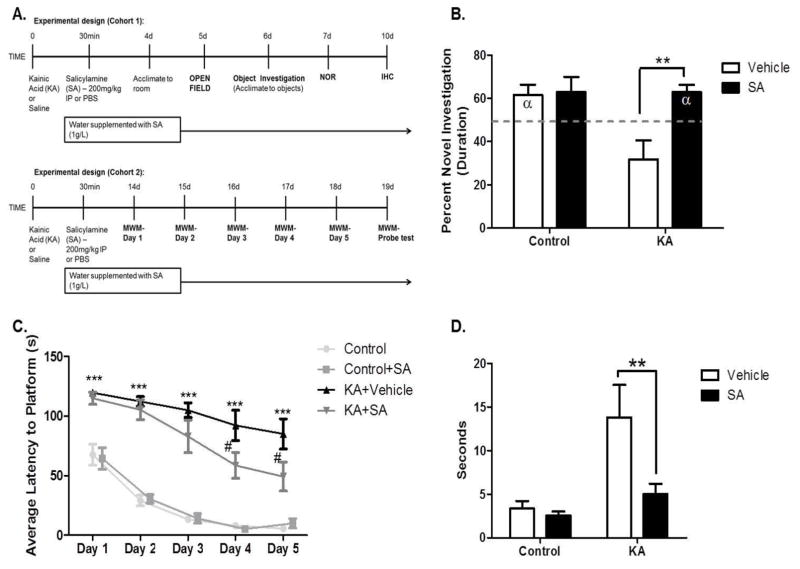
To determine whether increased γ-K protein adducts contribute to cognitive dysfunction associated with epileptogenesis, rats were treated with SA and tested in cognitive paradigms evaluating both recognition and spatial memory (Figure 4A). During acclimations, no differences in locomotion or indices of anxiety-like behavior were detected in the open field task and all rats approached the objects at equivalent rates in an object acclimation task. Compared to saline, treatment with SA significantly improved recognition memory performance in KA-treated rats as measured by the NOR task performed after a 60 minute delay (p<0.01; Figure 4B), to a level not significantly different from controls (p=0.41). To assess hippocampal-associated spatial memory, rats were tested in the MWM. Treatment with SA significantly improved learning on days 4 and 5 of the MWM (p <0.05 and p <0.01 respectively; Figure 4C). During the probe test, performed 24 hours after the last trial, KA-treated rats that received SA displayed significantly shorter latencies to reach the target quadrant than did KA-treated rats that received vehicle (p <0.01; Figure 4D). To assess the ability of SA to improve cognition once spontaneous seizures occurred, rats were treated with SA in drinking water starting 3 weeks after SE. After one week of treatment, epileptic rats were tested in the NOR task followed by the Y-Maze (Figure 5A). KA-treated rats performed significantly worse than controls on the NOR task (p <0.05; Figure 5B), indicative of deficits in recognition memory. Treatment with SA significantly attenuated these deficits (p <0.01; Figure 5B) to a level not significantly different from control (p >0.05). Similarly, in the Y-Maze, KA-treated rats performed significantly worse than controls (p <0.05; Figure 5C), indicative of deficits in spatial memory. Treatment with SA significantly attenuated these deficits (p <0.05; Figure 5C) to a level not significantly different from control (p >0.05). To ensure that effects on cognition were model independent, pilocarpine-treated rats were treated with SA and subjected to NOR and Y-Maze testing (Figure 6A). Pilocarpine-treated animals that received SA performed significantly better on the NOR task than pilocarpine-treated rats that received vehicle (p <0.01; Figure 6B) to a level not significantly different from control (p >0.05). Similarly, pilocarpine-treated rats that received SA performed significantly better than pilocarpine-treated rats that received vehicle on the Y-Maze (p <0.0001, Figure 6C) to a level not significantly different from control (p >0.05). Treatment with SA in both KA and pilocarpine models resulted in Cohen effect sizes d = −0.64 and d = −0.78 respectively, indicating a moderate to high practical significance. The data suggest that pharmacological scavenging of γ-Ks attenuates deficits in both recognition and spatial memory in epileptic rats when treatment begins during SE. Attenuation of deficits in cognition was achieved even when treatment was delayed 3 weeks after SE, after the appearance of chronic, spontaneous seizures. Furthermore, pharmacological scavenging of γ-Ks similarly improved cognition in the pilocarpine model of epileptogenesis, establishing the model independence of the findings. Taken together, the data implicate γ-Ks as a novel therapeutic target to attenuate epilepsy-associated deficits in learning and memory.
Figure 4. Scavenging gamma-ketoaldehydes starting 30 minutes after KA improves recognition and spatial memory.
(A) Experimental timeline. (B) Recognition memory performance on the novel object recognition task after a one hour delay. Discrimination ratios for the NOR task were calculated as follows: (duration sniffing novel object/[duration sniffing novel object + duration sniffing familiar object]). (C) Spatial memory performance in the Morris water maze and followed 24 hours later by the (D) probe test. **p< 0.01, ***p< 0.001, #p< 0.05. Dashed line indicates no preference for either the novel or familiar object i.e. 50% or chance preference, α denotes a significant difference from chance preference indicating a preference for the novel object. n= 7–9/group.
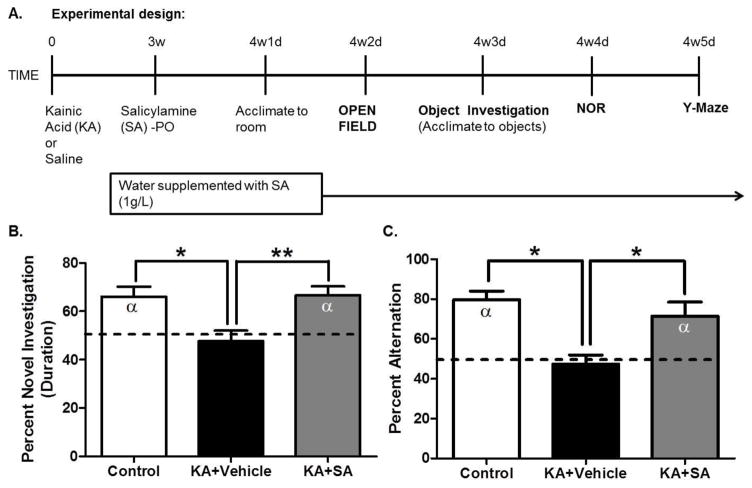
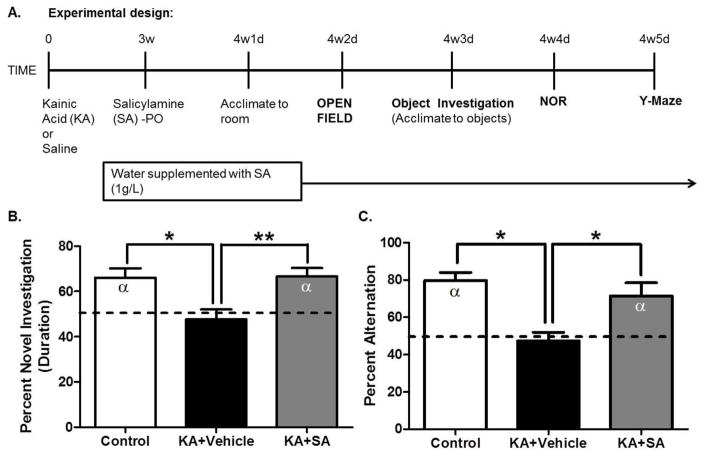
Figure 5. Scavenging gamma-ketoaldehydes starting 3 weeks after SE improves recognition and spatial memory in the KA model of epileptogenesis.
(A) Experimental timeline. (B) Recognition memory performance on the novel object recognition task after a one hour delay. Dashed line indicates no preference for either the novel or familiar object i.e. 50% or chance preference, α denotes a significant difference from chance preference indicating a preference for the novel object. Discrimination ratios for the NOR task were calculated as follows: (duration sniffing novel object/[duration sniffing novel object + duration sniffing familiar object]). (C) Spatial memory performance in the Y-Maze. Dashed line indicates chance alternation. α denotes a significant difference from chance alternation. *p< 0.05. **p< 0.01, n=10–12/group.
Figure 6. Scavenging gamma-ketoaldehydes starting 30 minutes after pilocarpine injection improves recognition and spatial memory in the pilocarpine model of epileptogenesis.
(A) Experimental timeline. (B) Recognition memory performance on the novel object recognition task after a one hour delay. Dashed line indicates no preference for either the novel or familiar object i.e. 50% or chance preference, α denotes a significant difference from chance preference indicating a preference for the novel object. Discrimination ratios for the NOR task were calculated as follows: (duration sniffing novel object/[duration sniffing novel object + duration sniffing familiar object]). (C) Spatial memory performance in the Y-Maze. Dashed line indicates chance alternation. α denotes a significant difference from chance alternation **p< 0.01 ***p< 0.001 compared by a two way ANOVA. n=6–7/group.
Treatment with SA attenuates γ-ketoaldehyde adducts in areas important for learning and memory
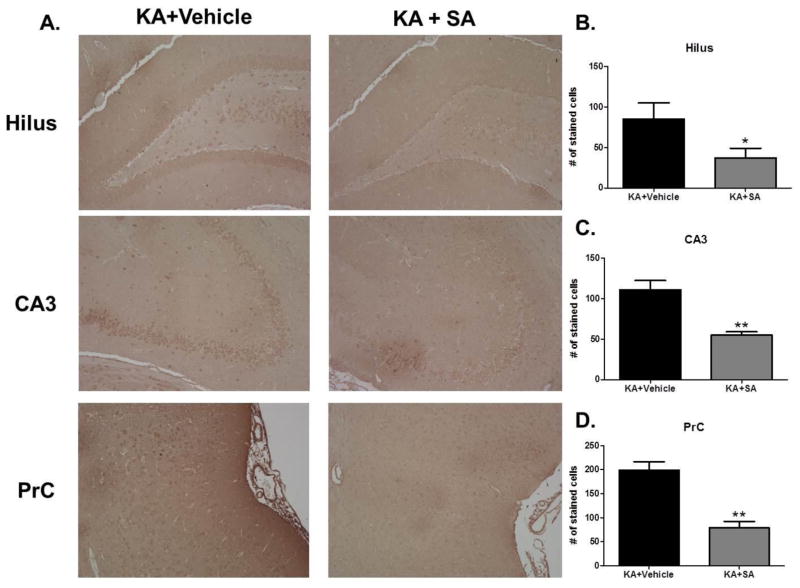
To ensure that differences in learning and memory were indeed due to a reduction in γ-K adducts, immunohistochemistry using an antibody against γ-K adducts was performed on brain sections obtained from animals that underwent behavioral testing detailed in figure 4A. KA-treated rats that received SA had significantly fewer stained cells within the hilar region of the dentate gyrus (p <0.05, Figure 7B), CA3 (p <0.01, Figure 7C), and perirhinal cortex (p <0.01, Figure 7D) compared to KA-treated rats that received vehicle treatment. These data further support the ability of SA to scavenge γ-K adducts in the KA model of epileptogenesis.
Figure 7. Treatment with SA attenuates gamma-ketoaldehydes adducts in brain regions important for learning and memory in the KA model of TLE.
(A) Representative images of gamma-ketoaldehyde staining in various brain regions of KA rats taken 10 days after vehicle or SA treatment initiated 30 minutes after KA injection. Average number of stained cells in (B) dentate hilus (C) CA3 and (D) perirhinal cortex. n =4/group.
Pharmacological scavenging of γ-ketoaldehydes is neuroprotective in vivo
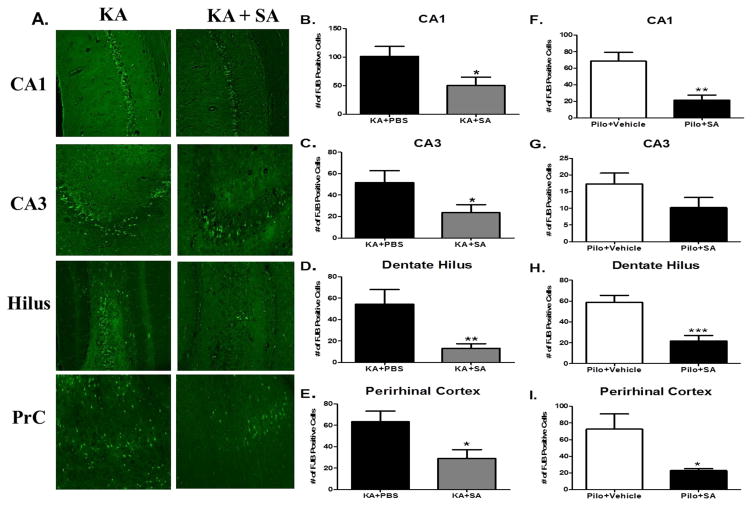
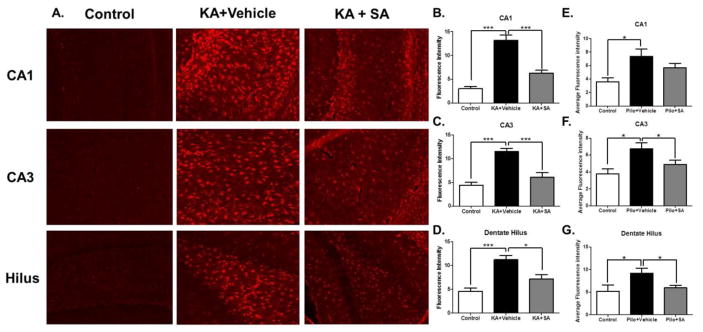
IsoKs are known to form cytotoxic phosphatidylethanolamine adducts in endothelial cells (Sullivan et al., 2010). However, whether γ-Ks can induce cell death in neurons has not been reported. Neuronal death, particularly in the hippocampus, is a common feature of TLE and is thought to contribute to cognitive dysfunction (Pitkänen and Sutula, 2002). To determine if γ-Ks mechanistically contribute to neuronal loss in the areas imperative for learning and memory, FJB labeling of degenerating neurons in the hippocampus and perirhinal cortex was quantified 10 days after SE in both the KA (Figure 8A) and pilocarpine models. Relative to rats in the KA+vehicle group, KA rats treated with SA had significantly fewer FJB labeled neurons in the hippocampal CA1 region (p <0.03; Figure 8B) CA3 region (p <0.04; Figure 8C) dentate hilus (p <0.01; Figure 8D) and perirhinal cortex, (p <0.03; Figure 8E) indicative of neuroprotection. Similarly, pilocarpine-treated rats that received SA had significantly fewer FJB labeled neurons in the hippocampal CA1 region (p <0.01; Figure 8F), dentate hilus (p <0.01; Figure 8H) and perirhinal cortex, (p <0.03; Figure 8I) but not CA3 region (p >0.05; Figure 8G). Taken together, these data indicate that γ-Ks contribute to SE-induced neuronal loss. GFAP labeling of reactive astrocytes in the hippocampus was quantified 10 days after SE in the KA (Figure 9A) and pilo groups. The KA group that received SA had significantly reduced GFAP fluorescence compared to the vehicle-treated KA group in CA1 (p <0.001; Figure 9B), CA3 (p <0.001; Figure 9C), and dentate hilus (p <0.05 Figure 9D) indicative of attenuated astrogliosis. Similarly, the pilocarpine group that received SA had significantly reduced GFAP fluorescence compared to the vehicle-treated pilocarpine group in the CA3 (Figure 9F) and dentate hilus (Figure 9G) but not the CA1 (Figure 9E). Taken together, these data suggest that treatment with SA is neuroprotective.
Figure 8. Pharmacological scavenging of gamma-ketoaldehydes is neuroprotective in both the KA and pilocarpine models of TLE.
(A) Representative images of FJB staining in various brain regions of KA rats taken 10 days after vehicle or SA treatment initiated 30 minutes after KA injection. Average number of FJB positive neurons indicative of neuronal injury in the KA model in (B) CA1 (C) CA3 (D) hilus and (E) perirhinal cortex. Data expressed as average number of FJB positive neurons, n =15/group. Average number of FJB positive neurons indicative of neuronal injury in the pilocarpine model in (F) CA1 (G) CA3 (H) hilus and (I) perirhinal cortex. Data expressed as average number of FJB positive neurons, n =7–8/group. *p< 0.05, **p< 0.01 compared by t-test.
Figure 9. Pharmacological scavenging of gamma-ketoaldehydes attenuates astrogliosis in the KA model of TLE.
(A) Representative images of GFAP staining in brain regions taken 10 days after KA-induced SE. Quantification of the average fluorescence intensity in the KA model in (B) CA1 (C) CA3 and (D) hilus and in the pilo model (E) CA1 (F) CA3 and (G) hilus. *p< 0.05, ***p< 0.001 compared by one-way ANOVA, n =5–7/group.
Pharmacological scavenging of γ-ketoaldehydes does not alter chronic epilepsy
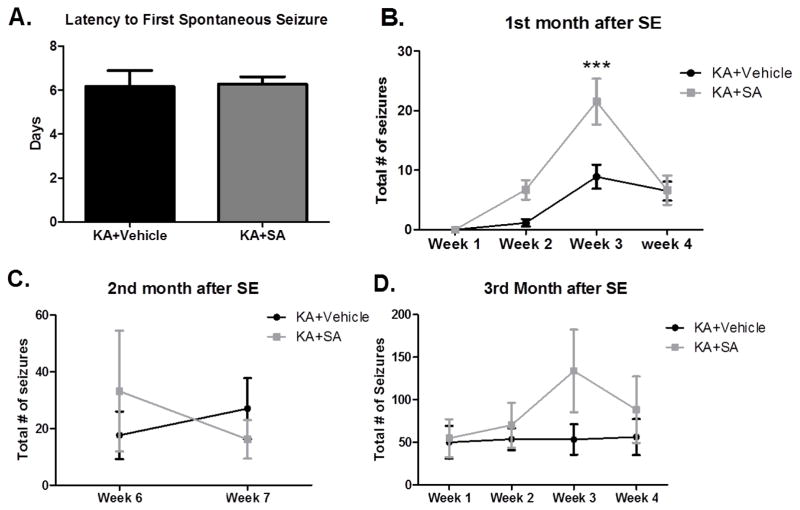
To ensure that differences in learning and memory were not the result of decreased seizure burden in either group, the rats that were monitored for differences during SE and given either vehicle or SA treatments starting 30 minutes after SE remained under V-EEG observation at various points during the first, second and third month after SE. There was no significant difference in the latency to the first spontaneous seizure (p >0.05, Figure 10A). Both groups had the first spontaneous seizure at approximately 6 days after SE. During the first month after SE, KA-treated rats that received SA exhibited significantly more seizures in the 3rd week after SE (p <0.001, Figure 10B). However, this effect was not seen during the second month (p >0.05, Figure 10C), or during the third month after SE (p >0.05, Figure 10D) when the frequency of seizures was equivalent between groups. Additionally, no differences were detected in the severity of the seizures (scored according to the Racine scale). This suggests that differences in learning and memory between groups are not the result of decreased frequency or severity of spontaneous seizures.
Figure 10. Pharmacological scavenging of gamma-ketoaldehydes has transient effects on seizures.
(A) The average latency to the first spontaneous seizure. (B) total frequency of seizures during the first month after SE. (C) total frequency of seizures during week 6 and 7 of the second month after SE and (C) total frequency of seizures during the third month after SE. ***p< 0.001 compared by two-way ANOVA, n =11–12/group.
Discussion
In the current study, we identify γ-K protein adducts as novel mediators of oxidative damage leading to cognitive deficits associated with TLE. We show that γ-K protein adducts are increased starting 24 hours after SE in the hippocampus and the perirhinal cortex, two brain regions imperative for learning and memory. Pharmacological scavenging of γ-Ks attenuated both recognition and spatial memory impairment in two separate models of TLE, without affecting the intensity of the initiating injury, SE or chronic seizure burden. Attenuation of memory deficits was accompanied by attenuation of neuronal loss and gliosis, providing a potential mechanism by which memory is protected. Improvements in memory were also observed when treatment was delayed up to 3 weeks after SE. Taken together the data suggest that γ-K protein adducts are a novel therapeutic target and establish SA as a potential therapy to attenuate cognitive dysfunction associated with epilepsy. The novelty of SA as a potential therapeutic strategy lies in its ability to scavenge reactive aldehydes that mediate oxidative protein damage rather than acting as an antioxidant capable of decreasing steady-state levels of ROS.
The precursors of γ-Ks, F2-IsoPs, have been detected in patients with a variety of pathological conditions including AD, Huntington’s disease, Creutzfeldt-Jakob disease, multiple sclerosis and recently in children with epilepsy (Greco et al., 1999; Grosso et al., 2011; Minghetti et al., 2000; Montine et al., 1999; Montine et al., 1998). IsoFurans, which are produced under conditions of increased oxygen tension, have been found in disease-affected brain regions from Parkinson’s disease patients (Fessel et al., 2003). Importantly, γ-K protein adducts have been identified in post-mortem brain tissue of AD patients and have been shown to correlate with disease severity (Davies et al., 2011; Zagol-Ikapitte et al., 2005). We have previously shown the occurrence of F2-IsoPs and IsoFurans in the KA model of TLE, however whether the highly reactive γ-Ks are produced as a consequence of SE was unknown (Patel et al., 2008; Patel et al., 2001). The current study links the formation of these lipid peroxidation products i.e. F2-IsoPs to the formation of more reactive products i.e. γ-Ks capable of exerting neuronal damage and cognitive dysfunction in models of epilepsy. Detection of γ-Ks in vivo is complicated by their extreme reactivity and proclivity to form protein adducts (Davies et al., 2007). The reaction of γ-Ks to form protein adducts is at least two orders of magnitude faster than other lipid peroxidation products precluding the detection of unadducted γ-Ks in vivo (Brame et al., 1999). In the KA model of TLE we found γ-K protein adducts in all hippocampal subregions, which were significantly increased in the perirhinal cortex 24 hours after SE and in whole hippocampus 6 weeks after SE. This suggests that the formation of γ-K protein adducts is an early and persistent process once an epileptogenic insult has occurred and establishes γ-K protein adducts as a potential therapeutic target.
In post-mortem AD brains, γ-K protein adducts were shown to co-localize to pyramidal neurons, poising them to have deleterious effects on cognition (Davies et al., 2011). Pyramidal neurons, particularly in the hippocampus, possess the machinery required for long-term potentiation and the adduction of key protein mediators by γ-Ks may be sufficient to produce deficits in learning and memory. Indeed, long-term treatment with SA improved working spatial memory in the ApoE4 mouse model of AD, providing evidence that accumulation of γ-K protein adducts in brain regions governing learning and memory, may have functional consequences on cognition (Davies et al., 2011). To determine if SA could be used to investigate the physiological consequences of γ-K protein adducts on cognition in an epilepsy model, we evaluated if treatment with the compound resulted in therapeutic concentrations of SA in the brain and plasma of KA-treated rats. Treatment with SA resulted in brain and plasma concentrations similar to those reported in the original pharmacokinetic study performed in mice (Zagol-Ikapitte et al., 2010). SA levels in both brain and plasma were in the range known to inhibit the formation of γ-K protein adducts and exert effects on oxidant-induced damage in cell culture (Davies et al., 2006). Concentrations within individual rats were variable and potentially reflect both differences in the amounts of medicated water drank and time elapsed between last drink and sacrifice. No differences between groups in the average amount of water intake were detected, however due to a relatively short half-life of SA (~62 minutes in plasma) it is possible that these values may be an underestimation. Importantly, SA inhibited the formation of γ–K adducts in the hippocampus and perirhinal cortex consistent with its mechanism of action.
Our findings suggest that treatment with SA resulted in alterations to the intensity of the epileptogenic insult, SE, as differences in the severity of the epileptogenic insult could account for differences between groups at any point thereafter. Since length and severity of SE were not significantly different between groups, this allowed us to assess cognition when treatment with SA was initiated 30 minutes after KA injection. This dosing model was useful in the demonstration that pharmacological scavenging of γ-Ks improved both recognition (a form of declarative memory) and spatial memory, in two rodent models of epilepsy. Treatment with SA in the KA and the pilocarpine model of epilepsy resulted in moderate to large effect sizes on cognition relative to the vehicle-treated epileptic rats, which indicates a high practical significance. Importantly, in people with TLE, the most common memory impairments are deficits in declarative and spatial memory and the ability of the pharmacological scavenger of γKs to improve these same types of memory in epilepsy models hints at potential translational implications (Helmstaedter C, 2001). Moreover, attenuation of memory deficits was observed even when treatment with SA was initiated after the appearance of chronic seizures, similar to what would occur in a clinical setting.
We have recently shown that a broad spectrum catalytic antioxidant capable of scavenging reactive species i.e. superoxide radicals, hydrogen peroxide, lipid peroxides and peroxynitrite attenuates learning and memory deficits in the pilocarpine model of TLE (Pearson et al., 2015). It should be emphasized that the current study tests downstream oxidative damage with a specific scavenger of γ-Ks (SA) which is not an antioxidant but rather inhibits oxidative damage mediated by reactive ketoaldehydes. This suggests that of all reactive species, γ-Ks are key mediators of oxidative damage and indeed, γ-Ks are unique in their ability as phospholipids to inflict protein damage. Taken together, the data presented here strengthens the hypothesis that the accumulation of γ-K protein adducts is a contributing factor to the development of deficits in learning and memory associated with epilepsy and warrants further investigation into SA as a potential treatment targeted to improve cognition in epilepsy.
Although γ-Ks are known to be highly cytotoxic, the exact mechanism(s) by which γ-Ks cause cell death is not entirely clear. These reactive aldehydes adduct to protein lysine residues and readily induce protein-protein cross linking (Davies et al., 2004a). Studies have revealed potential protein and non-protein targets of IsoKs that are known to induce cell death. Specifically, IsoKs have been shown to facilitate induction of the mitochondrial permeability transition pore and accelerate the release of cytochrome C, both of which promote entry into cell death cascades (Stavrovskaya et al., 2010). The accelerated release of cytochrome C from the mitochondria was attributed to IsoK-modified lysine residues located at the surface of the molecule (Stavrovskaya et al., 2010). Additionally, IsoKs are known to react with phosphatidylethanolamine, to form highly cytotoxic adducts within the membrane (Sullivan et al., 2010). In the current study, protection of cognition by pharmacological scavenging of γ-Ks was accompanied by protection from neuronal loss and astrogliosis in two models of TLE. While the role of neuronal loss in the progression of epileptogenesis is somewhat controversial, there is increasing evidence that neuronal loss and/or processes underlying neuronal loss may contribute to epilepsy-associated cognitive impairment (Pearson et al., 2015; Pitkänen and Sutula, 2002). Indeed, protection of memory was accompanied by at least a 50% reduction in neuronal loss in brain regions governing learning and memory. We observed the greatest neuroprotection in the dentate hilus, the region with the greatest production of IsoK adducts, where up to 75% of seizure-induced neuronal loss was prevented by treatment with SA. While it may seem intuitive that more neurons within brain regions important for cognition will improve learning and memory, it is probably more likely that treatment with SA prevented adduction of key protein mediators necessary for cell death and potentially for cognition as well.
An interesting aspect of this work was the observation of a disassociation between cognitive performance and seizure frequency. While the common belief is that if seizures are well controlled, memory impairments will subside, increasing evidence suggests that these two “hallmarks” of TLE can be manipulated independently of each other (Bender et al., 2013; Pearson et al., 2015). In the current study, rats treated with SA showed more frequent seizures relative to vehicle-treated KA rats during the third week after SE but at no other times during the study. Despite experiencing more seizures the week following cognitive testing, SA-treated rats performed cognitively similarly to control rats. This suggests that seizures and cognitive dysfunction can be separated and potentially develop from different underlying factors. For example, it is possible that γ-Ks adduct to proteins important for long-term potentiation and would therefore affect cognition without persistent effects of seizures. Future studies should evaluate pairing SA with common anticonvulsants to attain optimal control of both seizures and cognitive deficits.
Highlights.
Gamma-ketoaldehyde adducts capable of oxidative damage are increased in TLE
Scavenging aldehyde adducts improves memory in models of TLE
Scavenging aldehyde adducts prevents neuronal loss and gliosis in models of TLE
Acknowledgments
This work was funded by grants NIHRO1NS039587 (M.P.), NIHRO1NS086423 (M.P.) UO1NS083422 (M.P.), F31NS086405 (J.N.P.). The authors would like to thank Dr. Raymond Mernaugh for providing the gamma-ketoaldehyde antibody. We thank Michael Hall and the Neuroscience Machine Shop supported by the Rocky Mountain Neurological Disorders Core Center Grant NIH/NS048154 for manufacture of behavioral testing areas. We would like to acknowledge the Center for NeuroScience animal behavior core where behavioral studies were performed. We thank the University of Colorado Anschutz Medical Campus Rodent In Vivo Neurophysiology Core for providing facilities to acquire and review video-EEG data. We thank Dr. Andrew White for assistance with EEG analysis. We thank Kalynn Schulz for technical assistance with the Y-Maze.
Footnotes
Author contributions: JNP, LJR, and MP designed research. JNP, LPL, and EW acquired and analyzed data. JNP and MP wrote the manuscript with input from all authors.
Disclosures: LJR and Vanderbilt University hold intellectual property rights for salicylamine.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bender AC, Natola H, Holmes GL, Scott RC, Lenck-Santini P-P. Focal Scn1a knockdown induces cognitive impairment without seizures. Neurobiology of disease. 2013;54:297–307. doi: 10.1016/j.nbd.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behaviour. British Journal of Psychology General Section. 1950;41(1–2):68–80. [Google Scholar]
- Berr C, Balansard B, Arnaud J, Roussel A-M, Alpérovitch A, Group EVAS. Cognitive decline is associated with systemic oxidative stress: The EVA Study. Journal of the American Geriatrics Society. 2000;48(10):1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [DOI] [PubMed] [Google Scholar]
- Boyden PADS, Viswanathan PC, Amarnath V, Balser JR, Roberts LJ., II Potential role of Isoketals formed via the Isoprostane pathway of lipid peroxidation in ischemic arrhythmias. Journal of Cardiovasc Phamacol. 2007;50:480–486. doi: 10.1097/FJC.0b013e31815a0564. [DOI] [PubMed] [Google Scholar]
- Brame CJ, Boutaud O, Davies SS, Yang T, Oates JA, Roden D, Roberts LJ. Modification of proteins by isoketal-containing oxidized phospholipids. Journal of Biological Chemistry. 2004;279(14):13447–13451. doi: 10.1074/jbc.M313349200. [DOI] [PubMed] [Google Scholar]
- Brame CJ, Salomon RG, Morrow JD, Roberts LJ. Identification of extremely reactive γ-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. Journal of Biological Chemistry. 1999;274(19):13139–13146. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- Davies SS, Amarnath V, Brame CJ, Boutaud O, Roberts LJ. Measurement of chronic oxidative and inflammatory stress by quantification of isoketal/levuglandin [gamma]-ketoaldehyde protein adducts using liquid chromatography tandem mass spectrometry. Nat Protocols. 2007;2(9):2079–2091. doi: 10.1038/nprot.2007.298. [DOI] [PubMed] [Google Scholar]
- Davies SS, Amarnath V, Montine KS, Bernoud-Hubac N, Boutaud O, Montine TJ, Roberts LJ., II Effects of reactive g-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. The FASEB Journal. 2002 doi: 10.1096/fj.01-0696fje. [DOI] [PubMed] [Google Scholar]
- Davies SS, Amarnath V, Roberts LJ., II Isoketals: highly reactive γ-ketoaldehydes formed from the H2-isoprostane pathway. Chemistry and Physics of Lipids. 2004a;128(1–2):85–99. doi: 10.1016/j.chemphyslip.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, Olson SJ, Montine TJ, Amarnath V, Roberts LJ. Treatment with a γ-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. Journal of Alzheimer’s Disease. 2011;27(1):49–59. doi: 10.3233/JAD-2011-102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA, Roberts LJ., II Pyridoxamine analogs scavenge lipid-derived γ-ketoaldehydes and protect against h(2)o(2)-mediated cytotoxicity. Biochemistry. 2006;45(51):15756–15767. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SS, Talati M, Wang X, Mernaugh RL, Amarnath V, Fessel J, Meyrick BO, Sheller J, Roberts LJ., II Localization of isoketal adducts in vivo using a single-chain antibody. Free Radical Biology and Medicine. 2004b;36(9):1163–1174. doi: 10.1016/j.freeradbiomed.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Fessel JP, Hulette C, Powell S, Roberts LJ, Zhang J. Isofurans, but not F2-isoprostanes, are increased in the substantia nigra of patients with Parkinson’s disease and with dementia with Lewy body disease. Journal of Neurochemistry. 2003;85(3):645–650. doi: 10.1046/j.1471-4159.2003.01709.x. [DOI] [PubMed] [Google Scholar]
- Gilliam F, Hecimovic H, Sheline Y. Psychiatric comorbidity, health, and function in epilepsy. Epilepsy & Behavior. 2003;4:26–30. doi: 10.1016/j.yebeh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Greco A, Minghetti L, Sette G, Fieschi C, Levi G. Cerebrospinal fluid isoprostane shows oxidative stress in patients with multiple sclerosis. Neurology. 1999;53(8):1876. doi: 10.1212/wnl.53.8.1876. [DOI] [PubMed] [Google Scholar]
- Grosso S, Longini M, Rodriguez A, Proietti F, Piccini B, Balestri P, Buonocore G. Oxidative stress in children affected by epileptic encephalopathies. Journal of the Neurological Sciences. 2011;300(1–2):103–106. doi: 10.1016/j.jns.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Kurthen M. Memory and epilepsy: characteristics, course, and influence of drugs and surgery. Curr Opin Neurol. 2001;14:211–216. doi: 10.1097/00019052-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Milne GL, Musiek ES, Morrow JD. F2-Isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers. 2005;10(sup1):10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ., II Isoprostane generation and function. Chemical reviews. 2011;111(10):5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Greco A, Cardone F, Puopolo M, Ladogana A, Almonti S, Cunningham C, Perry VH, Pocchiari M, Levi G. Increased brain synthesis of prostaglandin E2 and F2-isoprostane in human and experimental transmissible spongiform encephalopathies. Journal of Neuropathology & Experimental Neurology. 2000;59(10):866–871. doi: 10.1093/jnen/59.10.866. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Beal MF, Robertson D, Cudkowicz ME, Biaggioni I, O’Donnell H, Zackert WE, Roberts LJ, II, Morrow JD. Cerebrospinal fluid F2-isoprostanes are elevated in Huntington’s disease. Neurology. 1999;52(5):1104. doi: 10.1212/wnl.52.5.1104. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Markesbery WR, Morrow JD, Roberts LJ., II Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Annals of Neurology. 1998;44(3):410–413. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & Memory. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Liang L-P, Hou H, Williams BB, Kmiec M, Swartz HM, Fessel JP, Roberts LJ., II Seizure-induced formation of isofurans: novel products of lipid peroxidation whose formation is positively modulated by oxygen tension. Journal of Neurochemistry. 2008;104(1):264–270. doi: 10.1111/j.1471-4159.2007.04974.x. [DOI] [PubMed] [Google Scholar]
- Patel M, Liang L-P, Roberts LJ., II Enhanced hippocampal F2-isoprostane formation following kainate-induced seizures. Journal of Neurochemistry. 2001;79(5):1065–1069. doi: 10.1046/j.1471-4159.2001.00659.x. [DOI] [PubMed] [Google Scholar]
- Pearson JN, Rowley S, Liang L-P, White AM, Day BJ, Patel M. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiology of Disease. 2015;82:289–297. doi: 10.1016/j.nbd.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JN, Schulz KM, Patel M. Specific alterations in the performance of learning and memory tasks in models of chemoconvulsant-induced status epilepticus. Epilepsy Research. 2014;108(6):1032–1040. doi: 10.1016/j.eplepsyres.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. The Lancet Neurology. 2002;1(3):173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Praticò D, Clark CM, Liun F, Lee VM, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of alzheimer disease. Archives of Neurology. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- Reich EE, Markesbery WR, Roberts LJ, II, Swift LL, Morrow JD, Montine TJ. brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in alzheimer’s disease. The American Journal of Pathology. 2001;158(1):293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Research. 2000;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- Stavrovskaya IG, Baranov SV, Guo X, Davies SS, Roberts LJ, II, Kristal BS. Reactive γ-ketoaldehydes formed via the isoprostane pathway disrupt mitochondrial respiration and calcium homeostasis. Free radical biology & medicine. 2010;49(4):567–579. doi: 10.1016/j.freeradbiomed.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan CB, Matafonova E, Roberts LJ, II, Amarnath V, Davies SS. Isoketals form cytotoxic phosphatidylethanolamine adducts in cells. Journal of Lipid Research. 2010;51(5):999–1009. doi: 10.1194/jlr.M001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiels E, Urban NN, Gonzalez-Burgos GR, Kanterewicz BI, Barrionuevo G, Chu CT, Oury TD, Klann E. Impairment of long-term potentiation and associative memory in mice that overexpress extracellular superoxide dismutase. The Journal of Neuroscience. 2000;20(20):7631–7639. doi: 10.1523/JNEUROSCI.20-20-07631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. Journal of Neuroscience Methods. 2006;152(1–2):255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Zagol-Ikapitte I, Masterson TS, Amarnath V, Montine TJ, Andreasson KI, Boutaud O, Oates JA. Prostaglandin H2-derived adducts of proteins correlate with Alzheimer’s disease severity. Journal of Neurochemistry. 2005;94(4):1140–1145. doi: 10.1111/j.1471-4159.2005.03264.x. [DOI] [PubMed] [Google Scholar]
- Zagol-Ikapitte I, Matafonova E, Amarnath V, Bodine CL, Boutaud O, Tirona RG, Oates JA, Roberts LJ, II, Davies SS. Determination of the pharmacokinetics and oral bioavailability of salicylamine, a potent γ-ketoaldehyde scavenger, by LC/MS/MS. Pharmaceutics. 2010;2(1):18–29. doi: 10.3390/pharmaceutics2010018. [DOI] [PMC free article] [PubMed] [Google Scholar]