Abstract
Background
Evidence for an association between neighborhood disadvantage and smoking is mixed and mainly based on cross-sectional studies. To shed light on the causality of this association we examined whether change in neighborhood socioeconomic disadvantage is associated with within-individual change in smoking behaviors.
Methods
The study population comprised participants of the Finnish Public Sector study who reported a change in their smoking behavior between surveys in 2008/09 and 2012/13. We linked participants’ residential addresses to a total population database on neighborhood disadvantage with 250×250m resolution. The outcome variables were changes in smoking status (being a smoker vs. not) as well as the intensity (heavy/moderate vs. light smoker). We used longitudinal case-crossover design, a method that accounts for time-invariant confounders by design. We adjusted models for time-varying covariates.
Results
Of the 3443 participants, 1714 quit while 967 began to smoke between surveys. Smoking intensity increased among 398 and decreased among 364 participants. The level of neighborhood disadvantage changed for 1078 participants because they moved residence. Increased disadvantage was associated with increased odds of being a smoker (odds ratio (OR) of taking up smoking 1.23 (95% CI 1.04-1.47) per 1 standard deviation (SD) increase in standardized national disadvantage score). OR for being a heavy/moderate (vs. light) smoker was 1.14 (95% CI 0.85-1.52) when disadvantage increased by 1 SD.
Conclusions
These within-individual results link an increase in neighborhood socioeconomic disadvantage, due to move in residence, with subsequent smoking behaviors.
Introduction
Smoking remains a major preventable health risk across the world.1, 2 In industrialized countries, individual-level socioeconomic disadvantage, indexed as low occupational position or low education, is associated with an increased likelihood of smoking.3, 4 Over and above this association, it has been suggested that neighborhood socioeconomic disadvantage may also affect smoking initiation/relapse and daily intensity.5–7 Possible mechanisms for the association include: higher density of tobacco retail outlets in disadvantaged neighborhoods;8 targeting of tobacco advertisements in deprived areas,9 combined with lower enforcement of local ordinances (e.g. laws prohibiting the sale of cigarettes to minors); and higher levels of psychosocial stress in disadvantaged areas, leading to nicotine abuse as a form of self-medication.10, 11
However, evidence for the association between area disadvantage and tobacco use remains mixed,12, 13 and is mostly based on cross-sectional studies. The contradictory findings in the literature may reflect residual confounding and selective retail outlet location.14, 15 For example, tobacco retail outlets are more likely to move into disadvantaged area where there are more residents addicted to tobacco.8, 16 A case–crossover design (also known as within-individual or fixed effect approach), where persons serve as their own controls when exposed to different levels of neighborhood disadvantage, would offer a more convincing demonstration of causality in observational data.17, 18 So far, only two previous studies conducted a case–crossover approach to examine the impacts of neighborhood disadvantage on smoking status within-individuals.19, 20 One study reported that within-individuals, a person was no more likely to smoke when living in a disadvantaged neighborhood compared to living in an advantaged neighborhood.19 In that study, persons who moved to more disadvantaged neighborhoods, compared with those who moved to less disadvantaged neighborhoods, were more likely to smoke in the study wave preceding the move. Thus, social selection rather than social causation seemed to explain the association between neighborhood disadvantage and smoking behavior. In that study, however, neighborhood characteristics were determined at the level of statistical local areas, where the median area size was large; 75 km2. Smaller spatial units may cover local variability in people’s social environments as well as ‘local health-related cultures’ that may contribute to smoking behavior.
In this study, we examined changes in smoking behaviors in relation to changes in neighborhood disadvantage measured using a fine resolution, 250×250m, grid database based on the total population in Finland. We used a case–crossover approach that controls for all unmeasured time-invariant confounders because each participant serves as his/her own control, and report results also by sex, age group, and occupational status.
Methods
Study population
Participants were from the Finnish Public Sector (FPS) study that includes employees of ten towns and six hospital districts in Finland who are in a wide range of occupations from city administrators and doctors to semiskilled cleaners. The FPS study was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa.
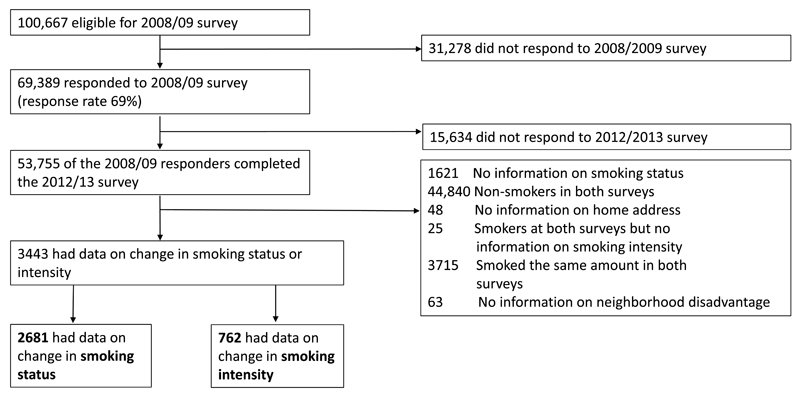
Figure 1 shows a flow chart of the analytic sample selection for the present study. The eligible participants were those eligible for a postal survey in 2008/09 (current employees or participants of earlier surveys who had left the organisations; n=100,667) as indicated by the employers’ registers, with responding being voluntary. We included participants who responded to the year 2008/09 survey (n=69 389, response rate 69%) and again in the 2012/13 survey (n =53 755), among whom the smoking rate was 14%. Coordinates of the residential buildings of each participant at the time of both surveys were obtained from the Population Register Center. The center’s data on nearly three million residences is maintained and checked in close cooperation with municipal building supervision authorities and local register offices.21 Of the residential building locations 90% are estimated to be correct to within 20 m accuracy, and the coverage is the best in the city plan areas (where most participants resided).22 We excluded those who had missing smoking status, were non-smokers in both waves, smoked at both time points with same intensity or had no information on daily smoking intensity, or had no information on the coordinates of residence or area level deprivation.
Figure 1.
Flow chart of the study sample selection.
During the 2008/09 survey, the 3443 included lived in slightly less advantaged areas when compared to the cohort participants not fulfilling the inclusion criteria for the case-crossover analysis (mean disadvantage score -0.23 (SD=0.70) among the included vs. -0.35 (SD=0.70) among the excluded), the included were slightly younger (mean age of the included 45.8 years (SD=10.9) vs. mean of the excluded 53.7 years (SD=11.1)), and they were slightly more often manual workers (15% vs. 11% manual workers).
Outcomes
Smoking behaviors were requested in both surveys with the identical questions: “Do you smoke or have you previously smoked regularly, that is daily or nearly daily?” and “Do you still smoke regularly?”. Those who responded “yes” to both questions were defined as current smokers while those who responded “no” to either question were defined as non-smokers. In the survey, we also requested the daily number of cigarettes smoked: “How many cigarettes do you on average smoke daily?”. The response options were categorized as “none”, “less than 5”, “5 to 9”, “10 to 14”, “15 to 19”, “20 to 24”, “25 to 39” and “over 40”. The daily number of cigarettes smoked was defined as the median number in each category. In the analyses we used smoking intensity defined as: “heavy/moderate” (≥10 cigarettes/day) and “light” (<10 cigarettes/day) smoker.23
Exposure
We calculated an index for neighborhood disadvantage using small-area data on median household income (coded as additive inverse), educational attainment (percentage of people over 18 years old whose highest education level is elementary school), and unemployment rate (unemployed people belonging to the labor force/total labor force). For each of the three variables, we derived a standardized z-score (mean=0, standard deviation=1) based on the total Finnish population, and the disadvantage scores were then calculated by taking the mean value across the three z-scores.24 Higher scores indicate higher disadvantage. We calculated measures of the three area characteristics using population registers from 2008 by Statistics Finland. The information for 250×250 m map grids was based on the total population living within each grid at the time of data collection.25 The average total population in the neighborhoods where the cohort participants lived was 153.5; and there were on average 4.9 participants per grid.26 Missing data for the 250×250 m neighbourhoods (i.e. information on income and education was confidential if 10 cases within a square at the time of demographic data collection, n=193 among smokers and n=58 among heavy/moderate smokers) were replaced using information from a 1×1km grid into which the residence fell.
Covariates
Time-invariant potential effect modifiers, age, and sex, as well as occupational status, were obtained from the employers’ registers. Occupational status was defined by the Statistics Finland’s Classification of Occupations 2001,27 and it was categorized as: non-manual (classes 1-5, e.g., senior officials and managers, technicians, and service and care workers) and manual workers (classes 6-9, e.g., craft and related trades workers and elementary occupations). Occupational status changed only among 1% of the participants during the study period. Time-varying potential confounders included survey year, marital status (married or cohabiting vs. not), chronic diseases (e.g., asthma, diabetes, ischemic heart disease, hypertension, depression) diagnosed by a doctor (yes vs. no), severe financial difficulties during the past year (yes vs. no) and work status (working vs. not).
Statistical analyses
We examined the longitudinal within-individual associations with the case–crossover method.17, 18, 28 This method can be applied in longitudinal data where the participant has changed the outcome status (here, being a smoker and being a heavy/moderate smoker) and exposure. Thus, each participant serves as his/her own control when exposed to different neighborhood environments and the design controls for all time-invariant covariates such as sex and unobserved genetic characteristics. For neighborhood disadvantage we used two measures: continuous disadvantage, and dichotomous disadvantage defined as above vs. below the national mean (standardized mean of the disadvantage score as the cut point). The first was used for the main analyses and the latter to test the robustness of the findings. For each individual, data from the case (smoker or heavy/moderate smoker) time point was compared with that from the control (non-smoker or light smoker) time point using conditional logistic regression models. The analyses were first adjusted for survey year, and then also for the time-varying covariates (marital status, chronic disease, financial difficulties and work status). Sample code (using PROC LOGISTIC in SAS) is provided in the eAppendix 1 of Supplemental Digital Content. We also tested whether disadvantage was associated with continuous change in daily number of smoked cigarettes. We ran stratified analyses by sex because smoking is more common in men than in women,29 by age (<65 vs. ≥65 years) because in Finland smoking is more prevalent and severe illnesses affecting smoking behaviors are less common among 15-64 years olds than among retirees (65-84 years),30 and by occupational status (manual vs. non-manual) because higher smoking rates have been reported for people with low compared to high occupational status.31 To get further insight into the direction of the association we performed sensitivity analyses where we examined the effect of moving to a more disadvantaged area on smoking status separately among those who were non-smokers and smokers before the move. In another sensitivity analysis we included only those whose residence at the time of the survey had been the same for at least one year. This restricts the possibility that the change in neighborhood disadvantage took place after the change in smoking behavior. Results for change in smoking status (a smoker vs. not) and change in intensity (heavy/moderate vs. light smoker) are presented as odds ratios (OR) for one unit (i.e., SD) increase in the standardized national disadvantage score with 95% confidence intervals (CI). For the analyses using the dichotomized disadvantage measure we present ORs (95% CI) for living in the more disadvantaged area (≥national mean) when compared to less disadvantaged area (<national mean). Odds ratios instead of risk ratios are presented because conditional Poisson models are likely to provide identical effect estimates with conditional logistic regression when each individual has one case and one control time point, as in our study.32 We used SAS 9.4 statistical software for all of the analyses (SAS Institute Inc., Cary, NC).
Results
The majority of the participants were women (77%) (Table 1). A total of 2681 smokers reported a change in smoking status between the waves, of whom 1714 quit and 967 started smoking (either initiated or relapsed). Number of continuous smokers who reported a change in their daily smoking intensity (heavy/moderate vs. light smoker) was 762. Of them 398 decreased while 364 increased their daily intensity. In Table 2 we present mean ages and disadvantage scores by case/control status. Neighborhood disadvantage increased in 382 (14%) and decreased in 467 (17%) participants in the group who changed their smoking status. The corresponding numbers of participants were 106 (14%) and 123 (16%), respectively, in the group who changed their daily smoking intensity. In Supplemental Digital Content eTable 1 we show categorical changes in disadvantage.
Table 1.
Descriptive statistics of the dichotomous variables for the case period (the time during which the subject was a smoker or heavy/moderate smoker).
| Smoking status changed cohort (n=2681) | Smoking intensity changed cohort (n=762) | |||||
|---|---|---|---|---|---|---|
| Variables, n (%) | Smokera | Status changedb | Missing | Heavy/moderate smokerc | Status changedb | Missing |
| Time/invariant | ||||||
| Male participant | 641 (24) | - | - | 141 (19) | - | - |
| Occupational status | 20 (0.8) | 6 (0.8) | ||||
| Non-manual | 2290 (85) | - | 604 (79) | - | ||
| Manual | 371 (14) | - | 152 (20) | - | ||
| Time-varying | ||||||
| Married/co-habiting | 1795 (67) | 422 (16) | 39 (1) | 502 (66) | 115 (15) | 12 (2) |
| Chronic disease | 588 (22) | 432 (16) | 55 (2) | 191 (25) | 124 (16) | 19 (2) |
| Severe financial difficulties | 198 (7) | 302 (11) | 98 (4) | 60 (8) | 86 (11) | 26 (3) |
| Working | 2446 (91) | 252 (9) | 5 (0.2) | 667 (88) | 91 (12) | 3 (0.4) |
| Exposure: neighbourhood disadvantage | ||||||
| ≥ National mean | 944 (35) | 375 (14) | - | 301 (40) | 115 (15) | - |
Case time point, i.e., a participant was a smoker
Change between surveys
Case time point, i.e., a participant was heavy/moderate smoker
Table 2.
Descriptive statistics of the continuous variables at case and control time points.
| Variable | Smoking status changed cohort (n=2681) | Smoking intensity changed cohort (n=762) |
|---|---|---|
| mean (SD), range | mean (SD), range | |
| Age | ||
| Casea | 47.0 (11.1), 19 to 76 | 48.9 (10.6), 20 to 72 |
| Control | 48.1 (11.2), 20 to 77 | 49.1 (10.8), 22 to 72 |
| Number of cigarettes smoked | ||
| Casea | 8.7 (6.3), 0 to 45 | 12.8 (2.2), 12 to 32 |
| Control | 0 (0), 0 to 0 | 6.7 (1.2), 0 to 7 |
| Neighborhood disadvantageb | ||
| Casea | -0.23 (0.7), -2.2 to 3.6 | -0.16 (0.7), -1.6 to 2.1 |
| Control | -0.27 (0.7), -2.2 to 3.6 | -0.18 (0.7), -1.7 to 2.1 |
Case = smoker in the smoking status changed cohort and heavy/moderate smoker in the smoking intensity changed cohort
Missing information for 33 participants
The odds of being a smoker (through either initiation or relapse between waves) increased when neighborhood disadvantage increased. The odds ratio for being a smoker (vs. control time when non-smoker) per one unit increase in disadvantage was 1.3 (95% CI 1.1-1.5) in the model adjusted for survey year, and 1.2 (95% CI 1.0-1.5) when additionally adjusted for the time-varying confounders (Table 3). Although no interactions were observed between neighborhood disadvantage and sex, age, or occupational status, in sex-stratified analyses, increase in disadvantage was more strongly associated with being a smoker among men than among women when adjusted for the time-varying confounders (men: OR 1.5, 95% CI 1.0-2.1, vs. women: OR 1.2, 95% CI 0.95-1.4). The association was observed among adults under 65 years of age (OR 1.3, 95% CI 1.1-1.5), but not among those aged ≥65 years (OR 0.89, 95% CI 0.43-1.8). Effect estimates were larger for manual workers than for non-manual workers, but the estimates for manual workers were imprecise (Table 3). Analyses using the dichotomized neighborhood disadvantage provided similar results (see eTable 2). In the sensitivity analyses, moving to more disadvantaged neighborhood decreased the odds of quitting smoking among those 1714 who were smokers before the move with an adjusted OR 0.70 (95% CI 0.56-0.89) per one unit increase in neighborhood disadvantage, but had no clear association with -becoming a smoker (either through initiation or relapse) among those 967 participants who were non-smokers before the move (an adjusted OR 1.1, 95% CI 0.8-1.4). Including only those with at least 1 year of exposure to the survey neighborhood the unadjusted OR for being a smoker was 1.4 (95% C 1.1-1.7) among all participants (n=2138).
Table 3.
Within-individual changes in smoking in relation to increase in neighbourhood disadvantage. Odds ratios for being a smoker when compared to control time point for all participants and by sex, age and occupational status.
| Smoker vs. not | Modela | Modelb | ||||
|---|---|---|---|---|---|---|
| Per 1 unit increase in disadvantage | OR | 95% CI | OR | 95% CI | ||
| All (n=2681) | 1.3 | 1.1 | 1.5 | 1.2 | 1.04 | 1.5 |
| Men (n=641) | 1.4 | 1.0 | 1.9 | 1.5 | 1.0 | 2.1 |
| Women (n=2040) | 1.3 | 1.0 | 1.5 | 1.2 | 0.95 | 1.4 |
| <65 years (n=2437) | 1.4 | 1.2 | 1.6 | 1.3 | 1.08 | 1.6 |
| ≥65 years (n=244) | 0.78 | 0.39 | 1.6 | 0.89 | 0.44 | 1.8 |
| Non-manual workers (n=2290) | 1.3 | 1.1 | 1.6 | 1.2 | 1.02 | 1.5 |
| Manual workers (n=371) | 1.2 | 0.73 | 1.8 | 1.2 | 0.75 | 2.0 |
Model adjusted for survey year
Model adjusted for all time-variant covariates: survey year, marital status, chronic disease, severe financial difficulties, and working status
We also performed a post-hoc analysis examining changes among movers and non-movers for whom we also had neighborhood disadvantage data for 2012. Among the movers (N=840) the variance in the difference in neighborhood disadvantage (between 2008 and 2012) was 2.8 times higher than among the non-movers (0.76 for movers vs. 0.27 among non-movers). Consequently, among the non-movers, there was no association between change in neighborhood disadvantage and change in smoking status (OR 1.0, 95% CI 0.83-1.2 per one unit (i.e., SD) increase in disadvantage).
The adjusted odds for being a heavy/moderate smoker (vs. control time when a light smoker) was 1.1 (95% CI 0.85-1.5) per one-unit increase in neighborhood disadvantage (Table 4). In analyses stratified by sex, age, and occupational status most effect estimates for the change in smoking intensity were above one with wide confidence intervals, although below one for men and ≥65 years old (Table 4). Analyses using the dichotomized neighborhood disadvantage provided similar results (see eTable 3), as did analyses using continuous number of daily smoked cigarettes (data not shown). In the sensitivity analyses including only those with at least one year exposure to the survey neighborhood the unadjusted OR for being a heavy/moderate smoker was 0.91, (95% CI 0.61-1.3) among all participants (n=607).
Table 4.
Within-individual changes in smoking intensity in relation to increase in neighbourhood disadvantage. Odds ratios for being a heavy/moderate smoker when compared to control time point for all participants and by sex, age, and occupational status.
| Heavy/moderate vs. light smoker | Modela | Modelb | ||||
|---|---|---|---|---|---|---|
| Per 1 unit increase in disadvantage | OR | 95% CI | OR | 95% CI | ||
| All (n=762) | 1.1 | 0.86 | 1.5 | 1.1 | 0.85 | 1.5 |
| Men (n=141) | 0.90 | 0.49 | 1.7 | 0.91 | 0.46 | 1.8 |
| Women (n=621) | 1.2 | 0.87 | 1.7 | 1.2 | 0.87 | 1.7 |
| <65 years (n=682) | 1.2 | 0.92 | 1.7 | 1.2 | 0.89 | 1.6 |
| ≥65 years (n=80) | 0.27 | 0.05 | 1.4 | 0.23 | 0.03 | 2.1 |
| Non-manual workers (n=604) | 1.2 | 0.88 | 1.7 | 1.2 | 0.83 | 1.6 |
| Manual workers (n=152) | 0.90 | 0.49 | 1.7 | 1.3 | 0.60 | 2.6 |
Model adjusted for survey year
Model adjusted for all time-variant covariates: survey year, marital status, chronic disease severe financial difficulties, and working status
Discussion
In this longitudinal within-individual study, we observed associations between change in neighborhood disadvantage and change in smoking behavior. We found that an increase in neighborhood disadvantage due to moving to a more disadvantaged residential area increased the odds of being a smoker, but not the odds of being a heavy/moderate (vs. light) smoker. The association for being a smoker seemed to be stronger among men than women, and among middle aged than older adults, but there was no strong evidence of differences by occupational status. That no association was observed among older adults may be related to the higher level of severe illnesses affecting smoking33 in this age group compared to younger adults. In Finland, only about 7% of women and 8% of men aged 65-84 are smokers.30 However, as the heterogeneity tests indicated no interactions by age or sex, the differences in the point estimates may also be due to chance, and no definitive interpretation of the stratified results is possible.
In line with our findings, some cross-sectional studies, mainly from the United States, have reported positive associations between neighborhood disadvantage and likelihood of smoking.5, 6 To the best of our knowledge, only two previous studies have examined within-individual changes in smoking in relation to changes in neighborhood disadvantage.19 In an Australian population the changes observed in smoking status over time were mainly due to between-individual differences, i.e., to unobserved confounding variables. Compared with our study, however, the definition of neighborhoods in that study was coarse as the author had to use statistical local areas, with nearly 6000 inhabitants in each (versus 153 in our study).19, 20 A recent study from New Zealand used smaller neighborhood units and reported that increase in neighborhood disadvantage increased the likelihood of smoking initiation or relapse by 7%.20 More comparable area units in terms of the number of inhabitants; 100 in their study versus 153 in ours, may partly explain the similarity of their findings and ours, particularly when compared to the Australian study.
There are several possible mechanisms behind the observed associations. One mechanism is linked to exposure to local norms, i.e., in disadvantaged neighborhoods individuals may observe others smoking on a more frequent basis, resulting in more permissive attitudes toward smoking.34 More disadvantaged neighborhoods are also less likely to have the collective efficacy to pass indoor smoking restrictions. Such a pattern has been reported in the US,35 where there is as yet no national legislation to restrict smoking in indoor spaces, and where local ordinances vary by locality. This is not likely to be the case in our study, since Finland has passed nationwide legislation to restrict smoking in indoor places. Disadvantaged neighborhoods are more likely to be linked to exposure to social and physical disorder (e.g. crime and noise) which may increase the stress levels of residents and result in increased maladaptive coping behaviors such as smoking.10, 11 Lastly, the availability of tobacco products has been reported to be higher in disadvantaged vs. affluent neighborhoods,8, 36 and in turn higher availability of tobacco has been linked with higher prevalence of smoking37 as well as with reduced likelihood of smoking cessation in cross-sectional38 and longitudinal23 studies. However, a recent study from the US reported that the association between tobacco outlet proximity and smoking cessation may only be seen in poor neighborhoods.39 Indeed, if tobacco outlets are attracted to disadvantaged neighbourhoods due to the presence of consumers and if the presence of consumers is attributable to disadvantage, then it is an effect of disadvantage rather than availability of outlets.
A major strength of our study is the case–crossover study design,18 which strengthens causal inference by allowing for an examination of changes in smoking behaviors associated with changes in neighborhood disadvantage. However, there are also some limitations of our approach. For example, although we controlled for many time-varying confounders, such as changes in marital status, financial difficulties, chronic diseases, and work status, we cannot fully exclude the possibility of confounding by unmeasured time-varying confounders or individual preferences. Whether bias due to unmeasured confounding exists could be tested by substituting the outcome for a “negative control” as suggested by Lipsitch et al.40
The negative control is an alternative outcome that is not supposed to be associated with the exposure (here change in neighborhood disadvantage), but that has the same predictors as the outcome of the main analysis (change in smoking behavior). In our data, however, we were not able to identify a negative control that would have fulfilled these requirements and therefore we could not perform this analysis. Confounding by individual preferences means that times where certain people moved to more affluent neighborhoods could have been the times where they also took the decision to quit smoking, for example, as attributable to a willingness to improve their residential and health capital.
Although selection of the study sample eliminated an important source of confounding by stable subject characteristics, the risk of selection bias increased and precision decreased, both due to the fact that only a small proportion of all the data could be used. Selection bias resulting in ORs that differ from one would be true if subjects selected for the study, i.e. smokers, had fixed or time-varying characteristics consistently different from non-smokers.41 An example of such difference is a severe sudden health event (that was not included in our list of diseases, a severe injury, for example) that was more common in smokers than non-smokers and that would lead to move in residence. Overall, selecting only smokers lead to a smaller analysis sample than what would have been available for between-individual analysis.42 Particularly in the case of the stratified analyses the selection resulted in lower precision and further, indefinite interpretation of the results. However, between-individual association for change in neighborhood disadvantage and being a smoker also indicated that moving from low to high disadvantage area, or remaining in high disadvantage area, increased the odds of being a smoker (eTable 4). As we only knew each individual's smoking behavior at the time of the survey the temporal ordering and proximity of the change in exposure and outcome are unknown. Although the association between disadvantage and being a smoker remained in the sensitivity analysis including those who had not changed their residence (disadvantage) during the year prior to the survey, it is possible that the smoking change preceded the change in disadvantage. Further, smoking may not be the most compliant outcome for a case-crossover study as the onset of smoking initiation or cessation may not be sudden, a feature suggested for an outcome of a case-crossover analysis.28
The follow-up time was also less than five years, but changes in health behaviors may accumulate over longer time periods,43 which may have biased our findings towards the null. In these data, the changes in neighborhood disadvantage occurred in every case as a result of individual residential moves, as opposed to changes in the neighborhood environment per se. However, we think this had little impact on the findings as we believe people are more likely to experience meaningful changes in neighborhoods when they move, rather than when the neighborhoods around them change. This was supported by our post-hoc analysis in which we observed no association between non-movers whose neighborhood disadvantage increased, although the analysis for non-movers might have been under-powered because the neighbourhood environments did not change very much during the study period. The study population was predominantly female, and the participants were mainly of Caucasian ethnicity and employed. These features limit the generalizability of our findings to other populations with different ethnic backgrounds and the unemployed.
In conclusion, our findings are consistent with the hypothesis that neighborhood disadvantage is associated with increased smoking. Whether there are more specific characteristics within the disadvantaged neighborhoods affecting smoking behaviors needs to be investigated in further studies.
Supplementary Material
Source of Funding
This study was supported by Academy of Finland (grant number 264944 to JV and 286294 to SS), Finnish Ministry of Education and Culture; Medical Research Council (grant number K013351 to MK); Economic and Social Research Council (to MK); and NordForsk, the Nordic Programme for Health and Welfare (grant number 75021 to MK).
Footnotes
Conflicts of Interest The authors declare no conflict of interest.
Data sharing: The data are not publicly available. For gaining access, contact the last authors.
References
- 1.WHO. Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huisman M, Kunst AE, Mackenbach JP. Inequalities in the prevalence of smoking in the European Union: comparing education and income. Prev Med. 2005 Jun;40(6):756–64. doi: 10.1016/j.ypmed.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 4.Federico B, Costa G, Kunst AE. Educational inequalities in initiation, cessation, and prevalence of smoking among 3 Italian birth cohorts. Am J Public Health. 2007 May;97(5):838–45. doi: 10.2105/AJPH.2005.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stimpson JP, Ju H, Raji MA, Eschbach K. Neighborhood deprivation and health risk behaviors in NHANES III. Am J Health Behav. 2007;31(2):215–22. doi: 10.5555/ajhb.2007.31.2.215. [DOI] [PubMed] [Google Scholar]
- 6.Karriker-Jaffe KJ. Neighborhood socioeconomic status and substance use by U.S. adults. Drug Alcohol Depend. 2013 Nov 1;133(1):212–21. doi: 10.1016/j.drugalcdep.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuipers MA, Wingen M, Stronks K, Kunst AE. Smoking initiation, continuation and prevalence in deprived urban areas compared to non-deprived urban areas in The Netherlands. Soc Sci Med. 2013 Jun;87:132–7. doi: 10.1016/j.socscimed.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez D, Carlos HA, Adachi-Mejia AM, Berke EM, Sargent JD. Predictors of tobacco outlet density nationwide: a geographic analysis. Tob Control. 2013 Sep;22(5):349–55. doi: 10.1136/tobaccocontrol-2011-050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidenberg AB, Caughey RW, Rees VW, Connolly GN. Storefront cigarette advertising differs by community demographic profile. Am J Health Promot. 2010 Jul-Aug;24(6):e26–31. doi: 10.4278/ajhp.090618-QUAN-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virtanen M, Kivimaki M, Kouvonen A, et al. Average household income, crime, and smoking behaviour in a local area: the Finnish 10-Town study. Soc Sci Med. 2007 May;64(9):1904–13. doi: 10.1016/j.socscimed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.van Lenthe FJ, Mackenbach JP. Neighbourhood and individual socioeconomic inequalities in smoking: the role of physical neighbourhood stressors. J Epidemiol Community Health. 2006 Aug;60(8):699–705. doi: 10.1136/jech.2005.043851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galea S, Ahern J, Tracy M, Vlahov D. Neighborhood income and income distribution and the use of cigarettes, alcohol, and marijuana. Am J Prev Med. 2007 Jun;32(6 Suppl):S195–202. doi: 10.1016/j.amepre.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleischer NL, Thrasher JF, Saenz de Miera Juarez B, et al. Neighbourhood deprivation and smoking and quit behaviour among smokers in Mexico: findings from the ITC Mexico Survey. Tob Control. 2015 Jul;24(Suppl 3):iii56–iii63. doi: 10.1136/tobaccocontrol-2013-051495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakes JM. The (mis)estimation of neighborhood effects: causal inference for a practicable social epidemiology. Soc Sci Med. 2004 May;58(10):1929–52. doi: 10.1016/j.socscimed.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Kawachi I, Subramanian SV. Neighbourhood influences on health. J Epidemiol Community Health. 2007 Jan;61(1):3–4. doi: 10.1136/jech.2005.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaiton MO, Mecredy GC, Cohen JE, Tilson ML. Tobacco retail outlets and vulnerable populations in Ontario, Canada. Int J Environ Res Public Health. 2013 Dec;10(12):7299–309. doi: 10.3390/ijerph10127299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison PD. Fixed Effects Regression Methods for Longitudinal Data Using SAS. SAS, Cary, NC: SAS Institute Inc; 2005. [Google Scholar]
- 18.Gunasekara FI, Richardson K, Carter K, Blakely T. Fixed effects analysis of repeated measures data. Int J Epidemiol. 2014 Feb;43(1):264–9. doi: 10.1093/ije/dyt221. [DOI] [PubMed] [Google Scholar]
- 19.Jokela M. Are neighborhood health associations causal? A 10-year prospective cohort study with repeated measurements. Am J Epidemiol. 2014 Oct 15;180(8):776–84. doi: 10.1093/aje/kwu233. [DOI] [PubMed] [Google Scholar]
- 20.Ivory VC, Blakely T, Richardson K, Thomson G, Carter K. Do changes in neighborhood and household levels of smoking and deprivation result in changes in individual smoking behavior? A large-scale longitudinal study of New Zealand adults. Am J Epidemiol. 2015 Sep 1;182(5):431–40. doi: 10.1093/aje/kwv097. [DOI] [PubMed] [Google Scholar]
- 21.Population Register Centre. [cited Oct 21, 2015];Population Information System. 2013 Available from: http://www.vrk.fi/default.aspx?id=44.
- 22.Väestörekisterikeskus [Population Register Centre] Rakennusten osoite- ja koordinaattitietojen kattavuus väestötietojärjestelmässä [Coverage of address and coordinate data of buildings in the population information system] 2004 [Google Scholar]
- 23.Halonen JI, Kivimaki M, Kouvonen A, et al. Proximity to a tobacco store and smoking cessation: a cohort study. Tob Control. 2014 Mar;23(2):146–51. doi: 10.1136/tobaccocontrol-2012-050726. [DOI] [PubMed] [Google Scholar]
- 24.Halonen JI, Kivimaki M, Pentti J, et al. Quantifying neighbourhood socioeconomic effects in clustering of behaviour-related risk factors: a multilevel analysis. PLoS One. 2012;7(3):e32937. doi: 10.1371/journal.pone.0032937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Statistics Finland. [cited January 1, 2016];Grid Database. 2013 Available from: http://www.tilastokeskus.fi/tup/ruututietokanta/index_en.html.
- 26.Halonen JI, Stenholm S, Pentti J, et al. Childhood Psychosocial Adversity and Adult Neighborhood Disadvantage as Predictors of Cardiovascular Disease: A Cohort Study. Circulation. 2015 Jun 11;132(5):371–9. doi: 10.1161/CIRCULATIONAHA.115.015392. [DOI] [PubMed] [Google Scholar]
- 27.Statistics Finland. [cited June 2, 2015];Classification of Occupations 2001. 2002 Available from: http://www.stat.fi/meta/luokitukset/ammatti/001-2001/index_en.html.
- 28.Maclure M, Mittleman MA. Should we use a case-crossover design? Annu Rev Public Health. 2000;21:193–221. doi: 10.1146/annurev.publhealth.21.1.193. [DOI] [PubMed] [Google Scholar]
- 29.Amos A, Greaves L, Nichter M, Bloch M. Women and tobacco: a call for including gender in tobacco control research, policy and practice. Tob Control. 2012;21(2):236–43. doi: 10.1136/tobaccocontrol-2011-050280. Epub 2011 Dec 13. [DOI] [PubMed] [Google Scholar]
- 30.National Institute for Health and Welfare. Tobacco statistics 2013. Official Statistics of Finland; 2014. [cited June 2, 2015]. Available from: http://urn.fi/URN:NBN:fi-fe2014101445179. [Google Scholar]
- 31.Stringhini S, Dugravot A, Shipley M, et al. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. PLoS Med. 2011 Feb;8(2):e1000419. doi: 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armstrong BG, Gasparrini A, Tobias A. Conditional Poisson models: a flexible alternative to conditional logistic case cross-over analysis. BMC medical research methodology. 2014;14:122. doi: 10.1186/1471-2288-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bednarek M, Gorecka D, Wielgomas J, et al. Smokers with airway obstruction are more likely to quit smoking. Thorax. 2006 Oct;61(10):869–73. doi: 10.1136/thx.2006.059071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nettle D. Large differences in publicly visible health behaviours across two neighbourhoods of the same city. PLoS One. 2011;6(6):e21051. doi: 10.1371/journal.pone.0021051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skeer M, George S, Hamilton WL, Cheng DM, Siegel M. Town-level characteristics and smoking policy adoption in Massachusetts: are local restaurant smoking regulations fostering disparities in health protection? Am J Public Health. 2004 Feb;94(2):286–92. doi: 10.2105/ajph.94.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siahpush M, Jones PR, Singh GK, Timsina LR, Martin J. Association of availability of tobacco products with socio-economic and racial/ethnic characteristics of neighbourhoods. Public Health. 2010 Sep;124(9):525–9. doi: 10.1016/j.puhe.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Peterson NA, Lowe JB, Reid RJ. Tobacco outlet density, cigarette smoking prevalence, and demographics at the county level of analysis. Subst Use Misuse. 2005;40(11):1627–35. doi: 10.1080/10826080500222685. [DOI] [PubMed] [Google Scholar]
- 38.Reitzel LR, Cromley EK, Li Y, et al. The effect of tobacco outlet density and proximity on smoking cessation. Am J Public Health. 2011 Feb;101(2):315–20. doi: 10.2105/AJPH.2010.191676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantrell J, Anesetti-Rothermel A, Pearson JL, Xiao H, Vallone D, Kirchner TR. The impact of the tobacco retail outlet environment on adult cessation and differences by neighborhood poverty. Addiction. 2015 Jan;110(1):152–61. doi: 10.1111/add.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010 May;21(3):383–8. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman JS. Commentary: Why are we biased against bias? Int J Epidemiol. 2008 Jun;37(3):624–6. doi: 10.1093/ije/dyn035. [DOI] [PubMed] [Google Scholar]
- 43.Oakes JM. Invited commentary: repeated measures, selection bias, and effect identification in neighborhood effect studies. Am J Epidemiol. 2014 Oct 15;180(8):785–7. doi: 10.1093/aje/kwu231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.