Abstract
Background
The herpes zoster (HZ) vaccine is recommended for adults age ≥ 60 years without weakened immune systems in the U.S. It is unclear how the risk of HZ varies according to age and disease conditions for younger patients with autoimmune or inflammatory (AI) diseases. We evaluated the age-stratified incidence of HZ associated with AI diseases compared to adults recommended for vaccination by the CDC.
Methods
Using linked commercial and governmentally-insured patients (2007–2010), we assembled seven AI cohorts: systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), gout and two comparison cohorts: diabetes and patients without AI and diabetic conditions. We identified HZ using diagnostic codes. Age-specific incidence rates (IR) were calculated and compared with the IR in patients aged 60–69 and without AI and diabetic conditions.
Results
We identified 8,395 SLE, 7,916 IBD, 50,646 RA, 2,629 PsA, 4,299 PsO, 1,019 AS, 58,934 gout, 214,631 diabetes and 330,727 enrollment periods without AI and diabetic conditions. Highest to lowest, the IRs ranged from 19.9 per 1,000 pys for SLE cohort to 6.8 for gout cohort, versus 5.3 in patients without AI and diabetic conditions. The age-specific IRs of HZ for RA and SLE patients aged ≥40 were 1.5–2 times greater than those observed in healthy adults for whom the vaccine is currently recommended (8.5/1000).
Conclusions
SLE, IBD and RA are associated with higher risks of HZ compared to older adults recommended for vaccination, suggesting that individuals with these conditions as young as age 40 could potentially benefit from vaccination.
INTRODUCTION
Herpes Zoster (HZ), also known as shingles, is a viral disease caused by reactivation of latent varicella-zoster virus in cranial-nerve or dorsal-root ganglia(1). HZ is characterized as a painful, dermatomal, vesicular rash and may be complicated by post-herpetic neuralgia (2, 3). More than one million HZ cases occur in the United States (U.S.) every year. Both the incidence rate (IR) and severity of HZ increase with advancing age, and more than half of all persons in whom HZ develops are older than 60 years (4–6). The Shingles Preventive Study reported that the IR of HZ for healthy people 60–69 years old without autoimmune diseases was 10.8 per 1000 person-years (PYs) in the unvaccinated group (6). Another randomized clinical trial reported the IR of HZ among individuals aged 50–59 years was 6.7 per 1000 PYs (7).
Antiviral medications approved for treatment of HZ, including acyclovir, valacyclovir or famciclovir, can reduce the severity and duration of HZ, but they may not prevent the pain and development of post-herpetic neuralgia, which can persist for years and may be refractory to treatment(8). The live HZ vaccine (Zostavax, Merck & Co., Inc.), the only licensed vaccine for HZ prevention in adults, recommended in 2006 by the U.S. Center for Disease Control (CDC) for adults aged 60 years and older (9). In 2011, the Food and Drug Administration (FDA) approved the use of HZ vaccination in healthy adults aged 50 through 59 years (2)(10). However, perhaps because of the lower absolute risk of HZ in this group, concern regarding the duration of response, and a HZ vaccine supply shortage, the CDC Advisory Committee on Immunization Practices (ACIP) did not recommend the vaccine for adults aged 50 through 59 years(11).
In addition to age, an individual’s immune system is an essential factor in reactivation of the varicella-zoster virus (12). Studies have suggested that HZ events are several times higher among patients with AI conditions such as SLE and RA as compared to patients without autoimmune diseases or inflammatory (AI) conditions (13). Because the HZ vaccine is a live-attenuated vaccine, its use in patients with AI conditions remains the subject of debate even though patients with AI conditions are at an increased risk of HZ (11, 14). Despite a paucity of data, vaccination is not recommended for patients who are on high-dose steroids or on certain medications such as biologic therapies. In 2012, the American College of Rheumatology (ACR) endorsed the ACIP recommendations about appropriate use of HZ vaccination among rheumatoid arthritis (RA) patients age 60 and above. In 2015, the ACR lowered the zoster vaccine recommendation to RA patients age 50 and above (15). No other recommendations have been made about vaccinating individuals with other autoimmune or rheumatic diseases.
Given the appreciable benefits of the vaccine in reducing the burden of zoster-related illness, it is unclear whether the absolute risk for HZ for younger patients with AI conditions might be high enough to warrant vaccination at ages earlier than recommended by the CDC. Accordingly, we evaluated the overall and age-stratified absolute incidence of HZ infection associated with different AI diseases compared to adults without AI conditions. We hypothesized that the absolute rate of HZ in younger patients with AI diseases would be comparable to or greater than the corresponding rate of HZ in healthy adults aged 60 or older without AI, for which zoster vaccination is strongly recommended.
STUDY DESIGN
Data sources
The Department of Health and Human Services Office (HHS) of the Assistant Secretary for Planning and Evaluation (ASPE) and the Centers for Medicare and Medicaid Services (CMS) built a national multi-payer claims database (MPCD) to better support comparative effectiveness research (16). With greater geographic coverage and clinical representativeness than single source data, MPCD database incorporated public and private data, representing beneficiaries with United Healthcare, Medicare, or Medicaid coverage during 2007–2010. The MPCD data contain patients’ demographic and insurance coverage information from enrollment files, claims for inpatient and outpatient services, and prescription medications.
Study design and population
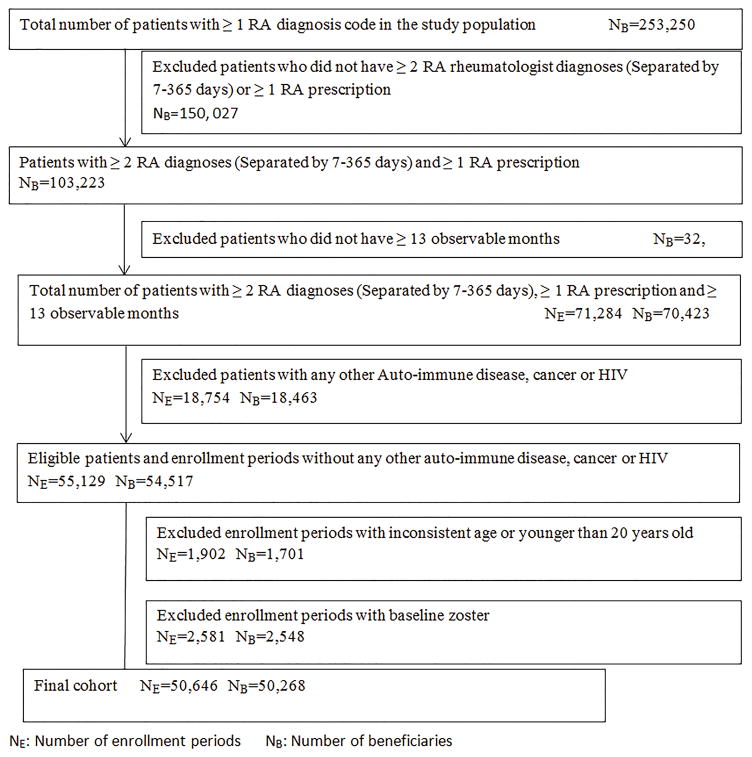
We conducted a retrospective study using MPCD in 2007–2010. After applying inclusion and exclusion criteria, we assembled 7 mutually exclusive AI disease cohorts, including systemic lupus erythematosus (SLE), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), psoriasis (PsO), psoriatic arthritis (PsA), ankylosing spondylitis (AS), and gout. These AI disease cohorts were mutually exclusive to two comparison cohorts: diabetes and adults without AI or diabetic conditions. As the cohort selection diagram shown in Figure 1, we required all patients in the AI disease cohorts or diabetes cohort to meet the following inclusion criteria: 1) at least two physician diagnoses for specific disease that were between 7 and 365 days apart; 2) at least one prescription fill or administration of disease specific treatments(17–20) (See diagnosis and medication list in supplemental table 1); 3) at least13 months continuous medical and pharmacy coverage (i.e. 12 months baseline and ≥ 1 month follow-up); 4) age equal to or older than 20 years old. Thus, the follow-up start date (defined as the index date) was the latest date after which three conditions were met: the second diagnosis of AI disease or diabetes, first date of prescription medication for that condition, or the first date of the 13th month of full health plan coverage.
Figure 1. Cohort selection diagram using RA as an example.
To increase the homogeneity of the patient cohorts, several exclusion criteria were also applied. Since patients with other auto-immune disease, HIV or malignant neoplasms (excluding non-melanoma skin cancer) may experience a higher risk of HZ, we excluded patients with a physician diagnosis of these conditions. For a similar reason, patients with a zoster diagnosis or antiviral prescription at any time prior to the index date were also excluded from the final cohort. Patients in all cohorts were followed until December 31, 2010, experienced HZ, died, or lost coverage. As a small proportion of patients (<1%) had more than one insurance enrollment period during the study period, the analysis allowed inclusion of all enrolled periods and thus to contribute more than one observation to the analysis if all other criteria were met for each enrollment period. Therefore, each observation represented an eligible insurance enrollment period.
As a comparator cohort, we derived a comparator ‘healthy patient’ cohort of individuals who were older than 20, who had 13 months of continuous of medical and pharmacy coverage, and had no any diagnosis code or drugs associated with any of the AI diseases or diabetes. Patients in the comparator cohort without AI or diabetic conditions started their follow-up at the first date of the 13th month of full coverage and follow-up ended at December 31, 2010 or when patients experienced HZ, died, lost coverage or encountered any diagnosis code for an AI disease
Herpes zoster outcome
The outcome was the first HZ event during follow-up. We identified HZ using an International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) inpatient or outpatient diagnosis code (053.x). This algorithm has previously been validated and been shown to have high sensitivity and positive predictive values (>85%) for identifying incident HZ (21, 22).
Factors related to HZ
We examined factors that could confound the associations of age and autoimmune diseases with HZ using data from baseline. We used Current Procedural terminology (CPT) code 90736 or National Drug Codes (NDC) for HZ vaccine to identify patients who had HZ vaccination. Age at the index date was categorized in ten-year increments from 21–30 to older than 70 years old. HZ vaccination status was dichotomous and time varying, meaning that patients could enter the analysis as having not been vaccinated, and subsequently change vaccination status during follow-up. Use of biologics and glucocorticoids were also defined as time varying during follow-up. For each person day, we evaluated whether the patient was on biologic or not based on the days of supply or infusion intervals after identifying injected biologics using pharmacy records and infused drugs (e.g. biologics) using Part B Healthcare Common Procedures Coding System (HCPCS) J–codes (23, 24). Using dispensed glucocorticoid dose 120 days before the index date in pharmacy data, we calculated average daily dose of oral glucocorticoids during baseline and we updated glucocorticoid dose every 4 months during follow-up. We classified daily glucocorticoids into three categories: none, ≤ 5mg/day and >5 mg/day of prednisone (or equivalent). Other factors we examined during baseline included: gender, comorbidities, concurrent medications and long term care residency. Comorbidities included prior outpatient infection, prior hospitalized infection, prior all-cause hospitalization, chronic obstructive pulmonary disease, heart failure, angina, and diabetes. Concurrent medications included methotrexate, non-steroidal anti-inflammatory drugs, narcotics, hypertension medications, antidepressants, and anti-hyperlipidemia medication. We identified patients’ long-term care residency status using prior published algorithms (25).
Statistical analysis
We compared patients’ baseline characteristics across nine cohorts. For each cohort, we calculated the absolute age-specific IR per 1,000 person-years with 95% confidence intervals (CI), and then calculated the incidence rate ratio (IRR) with 95% CI for each age category in each AI disease or diabetes cohort compared to patients aged 60–69 in the cohort without AI or diabetic conditions. Because the FDA approved the HZ vaccine for individuals aged 50 and older, yet the CDC ACIP recommendation is to vaccinate healthy patients ages ≥ 60 (and not people ages 50–59), we therefore derived our non-inferiority bound between 10 year age strata across all diseases., We derived our non-inferiority margin based upon the IRs of HZ reported in the Shingles Preventive Study for the healthy general population aged 60–69 (IR of 10.8 per 1000 PYs) who are recommended by the CDC to be vaccinated and compared it to the IR for patients aged 50–59 years old (not recommended by the CDC to be vaccinated, and with an IR of 6.7 per 1000 PYs) from a similar trial (6, 7). Therefore, we used a non-inferiority margin of 0.62, derived from an incidence rate ratio [IRR] of 6.7 / 10.8 For each 10 year age group-specific IRR, we classified the IR of HZ for each cohort as significantly higher (lower limit of the IRR 95% CI >1.0), comparable (i.e. non-inferior, defined as the lower limit of the IRR 95% CI > 0.62 but ≤ 1.0), or inconclusive (any other result), using the age-specific HZ IR in healthy people aged 60–69 in the data as referent.
Age could confound the comparisons between cohorts if the disease cohort groups had different age distributions. Therefore, we calculated the age standardized IR per 1,000 PYs with 95% CI using the U.S. 2010 census population and as important potentially confounding factors, effects of diabetes and glucocorticoids use among the SLE and RA cohorts were evaluated. A Cox regression model adjusted for age, gender, race, and vaccination was used to determine the hazard ratio (HR) for HZ across cohorts. To avoid adjusting for variables in the causal effect pathways with the AI conditions, comorbidities and concurrent AI medication use measured during baseline were not included in the model.
As a subgroup analysis, we evaluated the magnitude of benefit of HZ vaccination in patients with AI diseases. However, because few patients younger than 60 years old were vaccinated, and we had a reasonable absolute number of vaccinated patients only in the RA cohort, therefore, we included only patients older than 60 with RA in this additional analysis. We calculated the incidence rate of HZ by patients’ time-varying vaccination status and age among patients with RA and patients without AI or diabetic conditions. Cox regression models were constructed to assess the crude and adjusted hazard ratio of HZ associated with the benefit of HZ vaccination on HZ incidence. We performed two levels of adjustment. Initial adjustment included age, sex and race to account for demographic differences between patients with different vaccination status. Subsequent adjustment also included time-varying biologic use and average daily dose of oral glucocorticoids. An interaction between vaccination and glucocorticoid dose was also evaluated in a secondary analysis.
In a sensitivity analysis, we identified HZ using an ICD 9 inpatient diagnosis code alone (053.x) or an outpatient diagnosis code plus a claim for an antiviral medication within 30 days of the code, and then repeated all analyses. All analyses were performed using SAS version 9.3. The study was approved by the University of Alabama Institutional Review Board (IRB) and governed by a Data Use Agreement (DUA) from the data provider.
RESULTS
Our final study population consisted of 330,727 enrollment periods in the cohort without AI or diabetic conditions, 214,631 with diabetes, 8,395 with SLE, 7,916 with IBD, 50,646 with RA, 2,629 with PSA, 4,299 with PsO, 1,019 with AS, and 58,934 with gout. Selection of the final cohort for RA is demonstrated in Figure 1, and similar selection criteria were applied to other cohorts.
Baseline characteristics of the nine cohorts are presented in Table 1. More than 65% of patients in the diabetes, RA, and gout cohorts were older than 60 years old; 90% of SLE and 78% of RA patients were women and 73% of AS patients were men. Overall, baseline comorbidities and medications were similar across the 7 AI conditions. SLE patients had a high proportion of renal diseases and outpatient infections. Compared to the cohort without AI or diabetic conditions, gout patients had a higher rate of diabetes, heart failure, renal disease, hypertension medication, anti-hyperlipidemia medications. As expected, few patients in the diabetes and the cohort without AI or diabetic conditions used oral glucocorticoids or DMARDs, unlike patients in SLE and RA cohorts, among whom 40% of patients took glucocorticoids during baseline.
Table 1.
Baseline characteristics of patients in different auto-immune disease and comparison cohorts
| Subject Characteristics | Disease Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NOAID* | Diabetes | SLE | IBD | RA | PsA | PSO | AS | Gout | |
| Number of Patients | 328,580 | 212,806 | 8,320 | 7,858 | 50,268 | 2,609 | 4,272 | 1,011 | 58,598 |
| Number of Enrollment Periods | 330,727 | 214,631 | 8,395 | 7,916 | 50,646 | 2,629 | 4,299 | 1,019 | 58,934 |
| Duration of Follow up time (years), means | 1.80 | 1.70 | 1.61 | 1.49 | 1.80 | 1.38 | 1.40 | 1.43 | 1.49 |
| Age group, % | |||||||||
| 21–30 | 10.31 | 1.18 | 8.49 | 11.81 | 1.51 | 3.46 | 7.54 | 6.18 | 0.51 |
| 31–40 | 11.79 | 3.44 | 13.91 | 14.55 | 3.80 | 11.79 | 15.19 | 17.47 | 2.57 |
| 41–50 | 14.84 | 9.14 | 20.69 | 16.64 | 9.59 | 22.97 | 21.07 | 26.1 | 6.58 |
| 51–60 | 14.71 | 17.07 | 22.28 | 14.41 | 17.39 | 25.29 | 22.31 | 25.52 | 11.74 |
| 61–70 | 18.12 | 28.86 | 19.56 | 20.45 | 29.97 | 23.85 | 20.68 | 17.17 | 25.08 |
| 71–85+ | 30.22 | 40.31 | 15.07 | 22.13 | 37.74 | 12.63 | 13.21 | 7.56 | 53.51 |
| Zoster Vaccination, % | 0.83 | 1.02 | 0.52 | 1.02 | 1.10 | 0.80 | 0.81 | 0.98 | 1.43 |
| Women, % | 62.83 | 56.7 | 90.95 | 57.16 | 77.95 | 50.97 | 50.15 | 26.89 | 36.95 |
| Race | |||||||||
| White | 51.93 | 63.26 | 55.43 | 74.03 | 72.12 | 71.81 | 67.32 | 70.66 | 67.90 |
| Black | 10.41 | 15.61 | 25.29 | 7.73 | 10.53 | 4.07 | 5.95 | 7.07 | 18.28 |
| Asian | 2.68 | 2.95 | 1.75 | 0.85 | 1.71 | 2.40 | 2.72 | 2.36 | 4.58 |
| Hispanic | 5.78 | 6.13 | 7.08 | 2.60 | 5.39 | 4.07 | 5.86 | 4.51 | 2.45 |
| Other | 1.10 | 1.84 | 1.81 | 0.58 | 1.82 | 0.99 | 1.28 | 1.77 | 1.84 |
| Unknown | 28.10 | 10.21 | 8.65 | 14.21 | 8.43 | 16.66 | 16.86 | 13.64 | 4.96 |
| Comorbidities, % | |||||||||
| Diabetes mellitus | 0.00 | 99.25 | 15.00 | 12.32 | 18.89 | 19.48 | 18.77 | 12.95 | 35.35 |
| COPD** | 5.68 | 12.23 | 9.18 | 7.60 | 12.10 | 5.86 | 6.61 | 4.81 | 13.75 |
| Heart failure | 3.08 | 13.00 | 7.14 | 3.74 | 6.35 | 2.40 | 2.65 | 2.45 | 20.5 |
| Outpatient infection | 27.33 | 40.47 | 44.99 | 37.65 | 38.83 | 34.73 | 35.85 | 32.29 | 38.8 |
| Hospitalized infections | |||||||||
| None | 97.38 | 91.17 | 89.58 | 91.12 | 92.79 | 97 | 96.25 | 96.76 | 87.61 |
| 1–2 | 1.68 | 5.04 | 5.88 | 5.53 | 4.37 | 1.67 | 2.26 | 2.26 | 7.48 |
| ≥3 | 0.94 | 3.79 | 4.54 | 3.35 | 2.84 | 1.33 | 1.49 | 0.98 | 4.91 |
| Medications, % | |||||||||
| Prednisone, mg/day | |||||||||
| None | 96.79 | 95.13 | 56.28 | 72.3 | 61.45 | 83.19 | 90.53 | 80.27 | 82.21 |
| ≤5 | 2.81 | 3.89 | 24.29 | 10.38 | 24.87 | 12.44 | 7.37 | 12.66 | 15.11 |
| >5 | 0.40 | 0.97 | 19.23 | 17.32 | 13.67 | 4.37 | 2.09 | 7.07 | 2.68 |
| Methotrexate | 0.00 | 0.26 | 13.72 | 3.15 | 55.79 | 40.24 | 20.73 | 20.02 | 0.12 |
| Hypertension medications | 19.08 | 69.55 | 49.21 | 29.93 | 42.85 | 35.41 | 33.98 | 29.74 | 71.43 |
| Antidepressants | 16.14 | 28.83 | 43.00 | 32.73 | 30.72 | 32.56 | 30.75 | 31.89 | 20.58 |
| Anti-hyperlipidemia medications and/or physician diagnoses | 17.42 | 64.08 | 27.62 | 23.48 | 33.84 | 32.60 | 30.70 | 25.71 | 54.79 |
| Non-steroidal anti-inflammatory drugs | 13.12 | 19.80 | 31.05 | 15.44 | 38.31 | 37.62 | 23.33 | 47.60 | 41.42 |
| Narcotics | 25.87 | 41.56 | 65.13 | 53.27 | 59.31 | 51.69 | 43.61 | 62.12 | 55.6 |
| All-cause hospitalization, % | 8.58 | 21.20 | 23.78 | 25.00 | 18.65 | 9.17 | 9.63 | 11.58 | 29.81 |
| Long-term care, % | 4.46 | 9.13 | 3.97 | 4.72 | 5.73 | 1.75 | 2.05 | 1.77 | 9.39 |
Individuals without autoimmune and inflammatory and diabetic conditions.
COPD = Chronic obstructive pulmonary diseaseSLE = Systemic lupus erythematosus; IBD = Inflammatory bowel disease; RA = rheumatoid arthritis; PsO = Psoriasis; PsA = Psoriatic arthritis; AS = Ankylosing spondylitis;
Age specific HZ IRs for each cohort are presented in Table 2. The IR of adults aged 61–70 without AI was 8.5 per 1000 person years. Based on the pre-specified non-inferiority margin of an HZ IRR of 0.62, we categorized the age-disease specific IRs into three categories: significantly higher (red), comparable (yellow), or inconclusive or lower (no shading). The age-specific rate of HZ for SLE, IBD and RA patients in their 20s, 30s, and 40s, was comparable or substantially higher than the corresponding rate in adults without AI aged ≥ 60 (see Supplemental table 2).
Table 2.
Incidence rate of herpes zoster per 1000 person years by 10 year age group and auto-immune disease or comparator cohort
| Cohorts | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy* | Diabetes | SLE | IBD | RA | PsA | PsO | AS | Gout | |
| IR | IR | IR | IR | IR | IR | IR | IR | IR | |
| Age group | |||||||||
| 21–30 | 2.7 | 7.8 | 24.6 | 11.6 | 6.6 | N/A | 5.9 | N/A | 2.9 |
| 31–40 | 3.3 | 5.3 | 15.2 | 5.6 | 8.2 | 9.8 | 3.7 | 8.1 | 5.2 |
| 41–50 | 3.9 | 5.3 | 17.5 | 10.4 | 10.0 | 8.5 | 6.4 | 5.1 | 6.1 |
| 51–60 | 5.8 | 8.2 | 20 | 11.7 | 14.6 | 13.2 | 9.7 | 8.3 | 6.9 |
| 61–70 | 8.5 (referent) | 11.0 | 22.7 | 19.0 | 17.1 | 15.9 | 13.3 | 14.3 | 9.5 |
| 71–85+ | 10.6 | 13.0 | 20.9 | 23.8 | 21.3 | 19.4 | 21.2 | 26.3 | 13.3 |
Individuals without autoimmune, inflammatory conditions or diabetes
IR: Incidence per 1000 person years
Compared to healthy older people aged 60–69 (Blue), rates were classified as significantly higher (dark red shading), comparable (light yellow shading) and other (i.e. inconclusive or lower, not shaded).
Based upon the IRs of HZ reported in the Shingles Preventive Study for the healthy general population age 60–69 of 10.8 per 1000 PYs and comparing to the IR for patients age 50–59 years old of 6.7 per 1000 PYs in another randomized study, we selected a non-inferiority margin of 0.62(incidence rate ratio of 6.7 / 10.8 = 0.62).
SLE = Systemic lupus erythematosus; IBD = Inflammatory bowel disease; RA = rheumatoid arthritis; PsO = psoriasis; PsA = Psoriatic arthritis; AS = Ankylosing spondylitis;
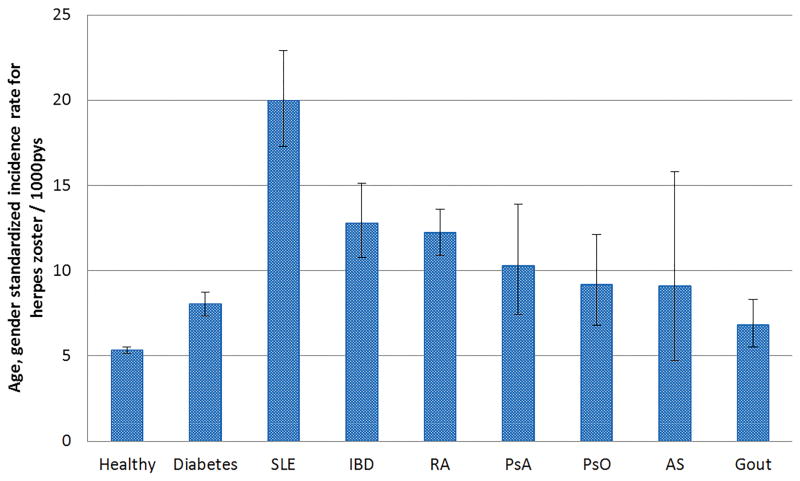
The age standardized IR for each cohort adjusted to 2010 U.S. Census population (>20 years old) is presented in Figure 2. Across the 7 AI disease cohorts, the age-standardized IR ranged from a high of 20.0 per 1000 person years (SLE) to a low of 6.8 (gout). For diabetes, the associated age-standardized rate was 8.0/1,000, and for cohort without AI or diabetic conditions, was 5.3/1000. The age-standardized IRs for female patients were numerically higher than those for male patients in all cohorts (data not shown).
Figure 2. Age-standardized incidence rate for herpes zoster per 1000 pys, standardized to the U.S. 2010 census*.
*among adults age >= 20
SLE = Systemic lupus erythematosus; IBD = Inflammatory bowel disease; RA = rheumatoid arthritis; PsO = psoriasis; PsA = Psoriatic arthritis; AS = Ankylosing spondylitis;
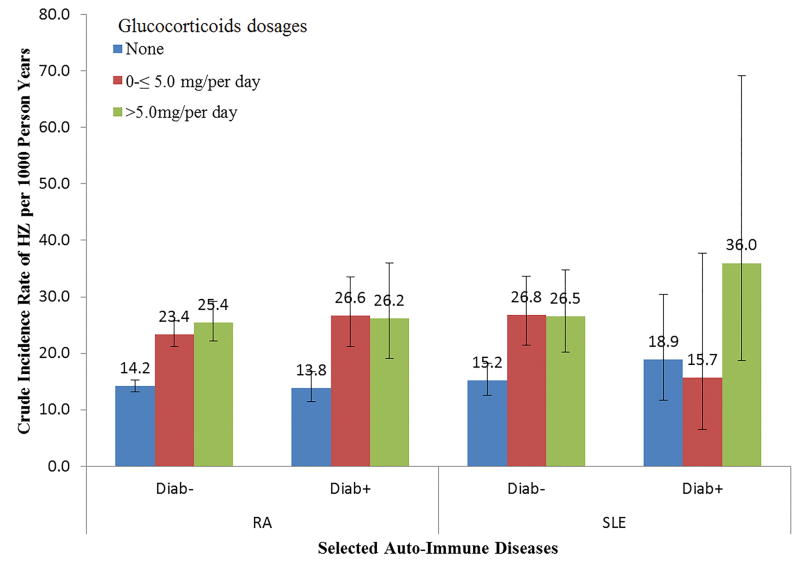
Based on whether patients had diabetes or used glucocorticoids during baseline, IRs of HZ for patients with SLE or RA are presented in Figure 3. Patients using glucocorticoids experienced higher IRs than those patients not using any glucocorticoids. However, diabetes as comorbidity concurrent with RA and SLE did not appreciably elevate the IR of HZ.
Figure 3.
Incidence rate based on different combination of diabetes and glucocorticoids dosages, using RA and SLE as examples
Compared with patients in the cohort without AI or diabetic conditions, the adjusted HRs for the AI and diabetes cohorts were increased significantly (Supplemental Table 3). After controlling for age, sex, race, and vaccination, HZ rates were approximately 1.5-fold (diabetes) to approximately 3-fold (SLE) greater than patients in the cohort without AI or diabetic conditions.
The crude and adjusted HRs associated with HZ vaccination among the RA patients are shown in Table 3. Vaccinated patients were less likely to have subsequent HZ events than those not vaccinated. Both biologic and oral glucocorticoids use were associated with an elevated risk of HZ in the fully-adjusted model 2, and higher glucocorticoid dose was more strongly associated with HZ incidence. There was no significant interaction between vaccination and glucocorticoid use. Patients in the healthy patient cohort also were observed to have a significantly lower rate of HZ associated with vaccination (not shown).
Table 3.
Hazard ratios for herpes zoster (HZ) associated with HZ vaccination and other factors in the RA cohort
| Hazard ratios (95% CI) | |||
|---|---|---|---|
| RA | |||
| Unadjusted | Model 1 | Model 2 | |
| HZ vaccination | |||
| No | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Yes | 0.74 (0.53–1.03) | 0.71 (0.51–0.99) | 0.73 (0.52–1.02) |
| Age, years | |||
| 61–65 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| 66–70 | 1.28 (1.04–1.57) | 1.28 (1.04–1.57) | 1.29 (1.05–1.59) |
| 71–75 | 1.34 (1.09–1.65) | 1.33 (1.08–1.64) | 1.34 (1.08–1.65) |
| 76–80 | 1.66 (1.35–2.04) | 1.64 (1.33–2.02) | 1.64 (1.33–2.03) |
| ≥81 | 1.63 (1.32–2.01) | 1.59 (1.28–1.97) | 1.61 (1.30–2.00) |
| Sex | |||
| Male | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Female | 1.30 (1.13–1.50) | 1.29 (1.11–1.48) | 1.30 (1.12–1.32) |
| Race | |||
| White | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Black | 0.89 (0.73–1.10) | 0.90 (0.73, 1.10) | 0.91 (0.74–1.12) |
| Asian | 1.40 (0.96–2.02) | 1.40 (0.97–2.03) | 1.42 (0.98–2.06) |
| Hispanic | 0.81 (0.59–1.12) | 0.82 (0.59–1.13) | 0.83 (0.60–1.15) |
| Other | 0.92 (0.59–1.44) | 0.96 (0.62–1.49) | 0.96 (0.62–4.50) |
| Unknown | 0.88 (0.69–1.11) | 0.93 (0.73–1.17) | 0.99 (0.78–1.26) |
| Biologic | |||
| No | 1.0 (Ref) | 1.0 (Ref) | |
| Yes | 1.14 (1.00–1.29) | 1.18(1.04, 1.34) | |
| Glucocorticoids | |||
| No | 1.0 (Ref) | 1.0 (Ref) | |
| <5mg/day | 1.65 (1.46–1.87) | 1.63(1.44,1.85) | |
| ≥5mg/day | 2.14 (1.86–2.47) | 2.15(1.87, 2.48) | |
Model 1 is adjusted for age, sex and race. Model 2 adjusted all variables in the table.
Non biologic DMARDs and other comorbidities in table 1 were not significant in the univariate analysis.
Sensitivity analyses using both diagnosis codes and anti-viral medications for identifying HZ cases yielded lower absolute age-specific IRs and similar or higher IRRs compared to the main analysis (Supplemental table 4). HZ rates in IBD patients aged 51–60 or older, RA aged 41–50 or older, gout aged 61–70 or older and PsA aged 71–85+ were significantly higher than the 61–70 year-old patients in the cohort without AI or diabetic conditions.
DISCUSSION
This study found that younger SLE, IBD and RA patients had higher rates of HZ compared to older healthy patients without AI or diabetic conditions aged 60–69. Overall, the risk for HZ associated with the AI conditions was approximately 1.5 to 2.0-fold higher than corresponding rates in healthy individuals, and after accounting for differences in age and sex between various disease groups, HZ incidence was more than 3 fold greater in some diseases (e.g. SLE) compared to healthy younger people. These results suggest that preventive strategies such as vaccination might be considered for younger people in high risk groups, such as patients with RA or SLE. Our results also suggest that the vaccine has comparable efficacy in autoimmune disease patients as it does in an older population (26).
Age is the most important risk factor for HZ, and thus current recommendations on HZ vaccination are based mainly on the age of individuals. Clinical trials of the zoster vaccine reported that the IR of HZ in the unvaccinated group was 10.8/1000 pys for people 60–69 years old and 6.7/1000 pys among individuals aged 50–59 years (6), (7). By way of contrast, the annual incidence of HZ is 1.2/1000 pys in healthy adults 20–40 years old (27–29). The live zoster vaccine has been approved for use in the general population aged 50 years or older in the United States, but the ACIP recommends routine HZ vaccination for persons aged 60 or older. While elevated HZ rates have been observed in patients with auto-immune disease at younger ages (13, 30), few studies have evaluated the disease spectrum in adults younger than 50 years or compared their age specific absolute IRs to the general population. Additionally, concerns have been raised that if patients receive HZ vaccine at young age, there is no sufficient evidence to indicate whether patients would need a booster dose at a later time. Results from our study have shown that the risk of HZ in patients of all ages who have SLE, IBD and RA are comparable to and in many cases exceed the risk seen in older individuals for whom the vaccine is recommended. The reasons for the increased HZ risk are likely multifactorial, and the increased risk may partly reflect exposure to immunosuppressive drugs (including glucocorticoid use), as well as the autoimmune conditions themselves. Our findings are consistent with previous studies in finding an increased risk of HZ associated with increasing doses of glucocorticoids (31) and biologic use (32).
Guidelines of recommendations for HZ vaccination for patients with autoimmune disease are not entirely consistent with one another. In the U.S. and Canada, the HZ vaccine is considered appropriate for patients who use methotrexate, and low to moderate doses of glucocorticoids, but not for patients treated with high dose glucocorticoids and biologics (33) (34). In contrast, national committees on immunization practices in Europe recommend avoiding HZ vaccination in patients receiving any immunosuppressive treatment (35). The European League against Rheumatism recognizes the high disease burden of HZ and recommends HZ vaccination to mildly immunosuppressed patients (36). Furthermore, although the evidence is of very low quality, the recently updated 2015 ACR guideline conditionally recommended giving the HZ vaccine in both early and established RA patients ages ≥ 50 years (15), rather than RA patients ages ≥ 60 years recommended in the 2012 ACR guideline (33). These updated guidelines reflect the continued theoretical concern for vaccine-induced infection associated with a live virus vaccine, especially in biologic-treated patients, although the ACR panel deemed that RA patients as young as 50 had a high enough risk for zoster to consider them appropriate candidates to receive the vaccine (36).
Although there are no completed prospective trials evaluating the clinical safety of HZ vaccination in large cohort with AI, a pilot study in 10 SLE patients did not identify any HZ-related safety problems when patients were vaccinated according to published guidelines(14). An ongoing trial in inflammatory arthritis patients treated with anti-TNF agents has not revealed any safety signals(37). Our findings suggest that the effectiveness of the zoster vaccine for patients with RA is comparable to that in patients without AI or diabetic conditions regardless their age. This finding is consistent with an observational analysis that showed that receipt of the HZ vaccine was not associated with a short-term increase in HZ incidence and in fact, reduced long term HZ risk, in Medicare patients with selected AI conditions, including those exposed to biologics (26). Preliminary results for the live zoster vaccine have been shown that the vaccine is both safe and effective in HIV positive patients with CD4 counts ranging between 200 and 350 (38). Accumulating evidence from observational or trials indicates that routine HZ vaccine administration for patients with autoimmune disease is well tolerated and has the important effect of reducing the burden of comorbid HZ. In addition, our findings are consistent with previous studies in finding an increased risk of HZ associated with increasing doses of glucocorticoids (31, 32, 39).
A major strength of this study is that we examined the age-specific HZ IRs within large cohorts of seven AI diseases and compared these rates to HZ IRs in diabetic and healthy populations. Our study provided cumulative HZ IRs by disease and steroid use when patients were on different combinations of these conditions. However, several features of our study design may impact the interpretation of results. We did not have medical records to confirm the occurrence of HZ, although the definition of HZ using our criterion of an inpatient or outpatient HZ diagnosis has been shown to have a high positive predictive value. The sensitivity analysis using an inpatient or outpatient HZ diagnosis accompanied by anti-viral drug use presumably conferred greater specificity to identify HZ infections and yielded similar results in our age-specific results. Moreover, we found that the HZ IRs in our data were very similar to the HZ rates reported in clinical trials (6), (7) and misclassification of treated HZ was unlikely to be differential by AI condition or drug exposure. Additionally, individual medical charts were not used to identify patients with auto-immune diseases; instead, we applied a validated algorithm with greater than 80% of PPV. Thus, some misclassification of auto-immune diseases is possible. However, these diagnoses are relatively accurate by requiring all patients in the study cohorts to have had at least 2 physician diagnoses and at least prescription fill or administration of disease specific medications(17–20). Finally, we did not apply a lag time after vaccine to exclude vaccine-related HZ because less than 1% of patients received vaccination during the baseline.
CONCLUSION
The high absolute incidence rates of HZ in people younger than 50 with certain autoimmune and inflammatory conditions suggest that it might be appropriate to consider vaccinating these individuals based on comparable absolute risk relative to the healthy people age ≥ 60 currently recommended for vaccination by the CDC. Clinical trials addressing the safety and efficacy of HZ vaccination among patients with these autoimmune and inflammatory conditions are underway and may provide more definitive information on which to base vaccination decisions and inform the need for, as well as safety and effectiveness of, vaccination for younger patients with autoimmune and inflammatory conditions.
Supplementary Material
Acknowledgments
Funding information
Dr. Curtis received support from the Agency for Healthcare Research and Quality (R01 HS018517) and Acturial Research Corporation (On behalf of the Department of Health and Human Services)
Dr. Yun was supported by grant 1 K12 HS021694 from the Agency for Healthcare Research and Quality, Rockville, MD, USA
This work was supported by the Agency for Healthcare Research & Quality (R01 HS018517) and Actuarial Research Corporation (On behalf of the Department of Health and Human Services).
Footnotes
Drs. Yun and Curtis had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest and Financial Disclosures
Disclosures for unrelated work
Yun: Research grants: Amgen
Winthrop: research grants: Pfizer, Inc/consulting fees: Pfizer, UCB, Genentech, Regeneron
Baddley: consulting for Eli Lilly, Pfizer and research grants from BMS
Saag: research grants: Amgen, Ardea, Takeda/Consulting fees: Amgen Ardea, Astra Zeneca Bayer, Genentech, Merck,, Takeda
Singh: research grants: Savient, Takeda; Consulting fees: Savient, Takeda, Allergan, Regeneron,
Curtis: research grants and/or consulting: Abbott, Amgen, BMS, Centocor, Crescendo, CORRONA, Pfizer, Roche/Genetech, UCB
Author contributions
Conception and design: Yun, Yang, Chen, Xie, Curtis
Acquisition of Data: Yun, Yang, Chen, Xie, Curtis
Analysis and interpretation of data: Yun, Yang, Xie, Chen, Curtis
Drafting manuscript: Yun
Critical revision of manuscript for important intellectual content: Yun, Yang, Chen, Xie, Winthrop, Baddley, Saag, Singh, Curtis
Statistical analysis: Yun, Yang, Curtis
Acquisition of Funding: Curtis
Administrative, technical, or material support: Yun, Yang, Chen, Xie, Winthrop, Baddley, Saag, Singh, Curtis
Study supervision: Curtis
References
- 1.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255–63. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RW, Bouhassira D, Kassianos G, Leplege A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37. doi: 10.1186/1741-7015-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray AV, Reisinger KS, Kerzner B, Stek JE, Sausser TA, Xu J, et al. Safety and tolerability of zoster vaccine in adults >/=60 years old. Hum Vaccin. 2011;7(11):1130–6. doi: 10.4161/hv.7.11.17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hope-Simpson RE. The Nature of Herpes Zoster: A Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 5.Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309(23):1434–40. doi: 10.1056/NEJM198312083092306. [DOI] [PubMed] [Google Scholar]
- 6.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 7.Schmader KE, Levin MJ, Gnann JW, Jr, McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54(7):922–8. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin RH, Schmader KE. Treatment and prevention of postherpetic neuralgia. Clin Infect Dis. 2003;36(7):877–82. doi: 10.1086/368196. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2008;57(RR-5) [PubMed] [Google Scholar]
- 10.Merck & Co., Inc. Zostavax (package insert) Whitehouse Station, NJ: Merck & Co., Inc; 2011. [Google Scholar]
- 11.Harpaz R, Ortega-Sanchez IR, Seward JF Advisory Committee on Immunization Practices Centers for Disease C, Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. quiz CE2-4. [PubMed] [Google Scholar]
- 12.Wilson DD. Herpes zoster: A rash demanding careful evaluation. Nurse Pract. 2014;39(5):30–6. doi: 10.1097/01.NPR.0000445781.37062.16. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarty EF, Michaud K, Katz R, Wolfe F. Increased incidence of herpes zoster among patients with systemic lupus erythematosus. Lupus. 2013;22(3):238–44. doi: 10.1177/0961203312470186. [DOI] [PubMed] [Google Scholar]
- 14.Guthridge JM, Cogman A, Merrill JT, Macwana S, Bean KM, Powe T, et al. Herpes zoster vaccination in SLE: a pilot study of immunogenicity. J Rheumatol. 2013;40(11):1875–80. doi: 10.3899/jrheum.130170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh JA, Saag KG, Bridges SL, Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2015 doi: 10.1002/art.39480. [DOI] [PubMed] [Google Scholar]
- 16. [Assessed on 12/11/2015];The ASPE/CMS Multi-Payer Claims Database (MPCD) for Comparative Effectiveness Research Initiative. http://www.brookings.edu/~/media/events/2010/11/18-basic/amol-navathe-slides.pdf.
- 17.Maclean C, Park GS, traina SB, Liu H, Hahn BH, Paulus HE, et al. Positive Predictive Value (PPV) of an administrative data-based algorithm for the identification of patients with RA. Arthritis & Rheumatism. 2001;44(S9):S106. [Google Scholar]
- 18.Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh JA, Holmgren AR, Noorbaloochi S. Accuracy of Veterans Administration databases for a diagnosis of rheumatoid arthritis. Arthritis Rheum. 2004;51(6):952–7. doi: 10.1002/art.20827. [DOI] [PubMed] [Google Scholar]
- 20.Asgari MM, Wu JJ, Gelfand JM, Salman C, Curtis JR, Harrold LR, et al. Validity of diagnostic codes and prevalence of psoriasis and psoriatic arthritis in a managed care population, 1996–2009. Pharmacoepidemiol Drug Saf. 2013;22(8):842–9. doi: 10.1002/pds.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155(15):1605–9. [PubMed] [Google Scholar]
- 22.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect. 2005;133(2):245–53. doi: 10.1017/s095026880400281x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-alpha antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306(21):2331–9. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dixon WG, Symmons DP, Lunt M, Watson KD, Hyrich KL, Silman AJ. Serious infection following anti-tumor necrosis factor alpha therapy in patients with rheumatoid arthritis: lessons from interpreting data from observational studies. Arthritis Rheum. 2007;56(9):2896–904. doi: 10.1002/art.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun H, Kilgore ML, Curtis JR, Delzell E, Gary LC, Saag KG, et al. Identifying types of nursing facility stays using medicare claims data: an algorithm and validation. Health Serv Outcomes Res Method. 2010;10(1–2):100–10. [Google Scholar]
- 26.Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, et al. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308(1):43–9. doi: 10.1001/jama.2012.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell ML, Schopflocher DP, Svenson L, Virani SN. Secular trends in the epidemiology of shingles in Alberta. Epidemiol Infect. 2007;135(6):908–13. doi: 10.1017/S0950268807007893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimland D, Moanna A. Increasing incidence of herpes zoster among Veterans. Clin Infect Dis. 2010;50(7):1000–5. doi: 10.1086/651078. [DOI] [PubMed] [Google Scholar]
- 29.Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20(8):748–53. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology (Oxford) 2006;45(11):1370–5. doi: 10.1093/rheumatology/kel328. [DOI] [PubMed] [Google Scholar]
- 31.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57(8):1431–8. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 32.Winthrop KL, Baddley JW, Chen L, Liu L, Grijalva CG, Delzell E, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA. 2013;309(9):887–95. doi: 10.1001/jama.2013.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64(5):625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Public Health Agency of Canada. Canadian Immunization Guide. 2012 Available from: http://www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php (cited 2014 July 7)
- 35.Papadopoulou D, Sipsas NV. Comparison of national clinical practice guidelines and recommendations on vaccination of adult patients with autoimmune rheumatic diseases. Rheumatol Int. 2014;34(2):151–63. doi: 10.1007/s00296-013-2907-9. [DOI] [PubMed] [Google Scholar]
- 36.van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70(3):414–22. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 37. [Assessed on Apr 21, 2015];Safety and effectiveness study of the live zoster vaccine in anti-TNF users (VERVE) https://clinicaltrials.gov/ct2/show/NCT01967316.
- 38.Gebo KA, Kalyani R, Moore RD, Polydefkis MJ. The incidence of, risk factors for, and sequelae of herpes zoster among HIV patients in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2005;40(2):169–74. doi: 10.1097/01.qai.0000178408.62675.b0. [DOI] [PubMed] [Google Scholar]
- 39.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301(7):737–44. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.