Abstract
The potential hazards and risks associated with well-stimulation in unconventional oil and gas development (hydraulic fracturing, acid fracturing, and matrix acidizing) have been investigated and evaluated and federal and state regulations requiring chemical disclosure for well-stimulation have been implemented as part of an overall risk management strategy for unconventional oil and gas development. Similar evaluations for chemicals used in other routine oil and gas development activities, such as maintenance acidizing, gravel packing, and well drilling, have not been previously conducted, in part due to a lack of reliable information concerning on-field chemical-use. In this study, we compare chemical-use between routine activities and the more closely regulated well-stimulation activities using data collected by the South Coast Air Quality Monitoring District (SCAQMD), which mandates the reporting of both unconventional and routine on-field chemical-use for parts of Southern California. Analysis of this data shows that there is significant overlap in chemical-use between so-called unconventional activities and routine activities conducted for well maintenance, well-completion, or rework. A comparison within the SCAQMD shows a significant overlap between both types and amounts of chemicals used for well-stimulation treatments included under State mandatory-disclosure regulations and routine treatments that are not included under State regulations. A comparison between SCAQMD chemical-use for routine treatments and state-wide chemical-use for hydraulic fracturing also showed close similarity in chemical-use between activities covered under chemical disclosure requirements (e.g. hydraulic fracturing) and many other oil and gas field activities. The results of this study indicate regulations and risk assessments focused exclusively on chemicals used in well-stimulation activities may underestimate potential hazard or risk from overall oil field chemical-use.
Introduction
Scientific, regulatory, and public debates on the environmental and public health dimensions of oil and gas development have been focused on hazardous chemicals used for hydraulic fracturing and other well-stimulation treatments, such as matrix acidizing, that are classified as “unconventional” oil and gas development methods [1–4]. Consequently, new regulations that govern oil and gas development require disclosure of chemical-use during well stimulation activities, but do not require disclosure of chemicals used for any other oil and gas field activities [1,2,4]. However, potentially hazardous chemicals are used throughout the entire oil and gas development process, not just during well stimulations [5–9], so there is interest in examining overall chemical-use on oil and gas fields and comparing chemical-use between regulated “unconventional” development activities and other oil and gas field activities.
Disclosure of chemical-use during well stimulation is considered an important requirement for the protection of human and environmental health, since knowledge of the types and amounts of chemicals used is fundamental to risk assessment [10]. Recent Federal and State regulations mandate chemical disclosures for well stimulations, including hydraulic fracturing and in some cases matrix acidizing and acid fracturing, but reporting chemical-use for other oil and gas field activities, such as well drilling, well completion, well maintenance, and well re-work is not required, unless pressures above the fracture gradient are used [1,2,4,11]. Given the public and scientific concern regarding the use and release of hazardous chemicals during the current oil and gas development boom [12] and the reuse of oil and gas field produced water for beneficial purposes in arid regions [13–15], it is important to evaluate the potential environmental and public health impacts of all chemical additives used in oil and gas development.
Chemicals are used routinely in oil and gas development as part of drilling and cementing of the well casing, repair of formation damage, wellbore clean-outs, scale and corrosion control, and for other production activities. Chemical additives are also used in enhanced oil recovery (EOR) to change fluid properties of oil (e.g. viscosity) and to otherwise increase production of oil within the formation [16]. During well construction, hazardous chemicals may be added to drilling fluids, drilling muds, and cements and are also used to remove debris from wellbores prior to cementing of the annular space between the steel casing and geological formations [9,17]. Chemical additives, including strong acids, are also used for well completion and rework to facilitate hydrocarbon production.
While large numbers and masses of chemical additives are used in routine oil and gas development activities, only a few surveys of routine chemical-use by the oil and gas industry have been conducted [5–8,18]. There is widespread use of potential chemicals of concern, including biocides, quaternary ammonium compounds, and corrosion inhibitors both off-shore and on-shore [5–8,18]. In contrast, several studies examined chemical-use during well stimulation activities, including hydraulic fracturing and matrix acidizing [19–24]. It has been established that chemicals used during well stimulation treatments have environmental pathways of exposure which include accidental spills, reuse of treated produced water, improper zonal isolation of fluids in the subsurface infrastructure and geologies, and discharge of wastewaters to aquatic ecosystems [3,21,24]. It is also known that produced water has similar exposure pathways, so it is of interest to determine overall oil and gas field chemical-use when evaluating the potential environmental and health impacts of oil and gas development.
The reuse of produced water for agricultural purposes is permissible in the western US and produced water is being reused for irrigation, watering livestock, aquifer recharge, and other purposes [13–15,24–26]. In California, produced water from oil fields is used for food crop irrigation, livestock watering, groundwater recharge, and for wetlands and other environmental purposes [15,27]. There are concerns that oil field chemicals or their degradation products will occur in produced water and that these chemicals may pose an unrecognized hazard or risk for produced water beneficial reuse, since potential exposure pathways from beneficial reuse include chemical uptake or deposition on food crops, contamination of regional aquifers through recharge, and the direct contact of farmworkers with produced water [15]. The hazard posed by oil and gas field chemicals would be in addition to other hazards associated with naturally occurring constituents of produced water, such as salts, metals, aromatic hydrocarbons, and naturally occurring radioactive material. The increased interest in reusing produced water [13,28] suggests that the hazards associated with oil and gas field chemicals should be evaluated.
The objective of this study is to assess chemical-use during routine oil and gas development and to compare chemical-use in routine production activities with chemical-use during well stimulation. To our knowledge, only one regulatory agency in the US, the South Coast Air Quality Management District (SCAQMD) in Southern California, requires mandatory disclosure of on-field chemical-use for well drilling, well completion, and well rework activities. These data were used by Abdullah et al. [19] to characterize chemical-use in acidizing. We use these data to compare chemical additive use between well-stimulation (hydraulic fracturing and matrix acidizing treatments) and routine oil field activities to determine similarities and differences in chemical-use. We summarize the chemicals used with respect to frequency of use, masses applied, and toxicity data. Similar data driven approaches have been used previously to evaluate hazards associated with hydraulic fracturing and matrix acidizing [19,21]. The results of our analysis are interpreted in the context of public and scientific concerns about hydraulic fracturing and the beneficial reuse of produced water.
Methods
Chemical-use data reported to the South Coast Air Quality Management District (SCAQMD) in southern California was analyzed in this study [29]. Under SCAQMD Rule 1148.2, which went into effect on June 4, 2013, operators and chemical suppliers are required to submit and make publicly available chemical usage data related to routine oil and gas activities (well drilling, well completion, and well rework) and well stimulation (hydraulic fracturing, matrix acidizing) in the California counties of San Bernardino, Orange, Riverside, and Los Angeles, including the City of Los Angeles [29]. These counties represent the second most productive oil and gas region in the third largest oil producing state in the United States. Chemical-use for enhanced oil recovery (EOR) and activities beyond upstream oil and gas development such as refining, transmission, and storage are not included in the SCAQMD datasets and are not included in this analysis.
Data on chemical type, mass injected, and water volumes used in oil and gas operations were downloaded from the SCAQMD database for the period of June 4, 2013 to September 2, 2015 [29]. The dataset used for this study consists of 51,514 entries from 1,207 oil and gas “events” conducted at 302 unique locations (identified by latitude and longitude). Events were categorized by operators as well drilling, completion, or rework activities. For completion, activities were further categorized as acidizing, gravel packing, hydraulic fracturing, maintenance acidizing, matrix acidizing, or acid fracturing. In order to focus on routine oil and gas activities, we separated well stimulation events (hydraulic fracturing, matrix acidizing and acid fracturing) from other routine events in our dataset. Entries were edited to standardize chemical names and to validate the assigned Chemical Abstracts Services Registry Number (CASRN). Changes to names of proprietary chemicals that could not be identified by CASRN were limited to correcting obvious spelling errors (e.g., aicd to acid, kerosine to kerosene), changing capitalization, and altering punctuation (e.g. removing dashes). Proprietary chemicals with singular and plural names that indicate chemical mixtures (e.g., ionic surfactant vs ionic surfactants) were maintained as separate entries. In cases where duplicate event IDs were reported, data were consolidated into one event ID entry. In cases where multiple chemical information documents were reported for the same event ID, data were individually assessed and duplicates, where apparent, were deleted.
For the chemical additives identified by CASRN, toxicological data were collected from online chemical databases [30–41]. Computational models within the U.S. EPA EPI Suite software (e.g., BIOWIN) were used to fill data gaps when experimental data were unavailable. Rat, mouse, and rabbit acute oral toxicity data and rat and mouse inhalation toxicity data were collected to represent and compare mammalian toxicity among the chemical constituents. To assess acute environmental toxicity, data for water flea (Daphnia magna), fathead minnow (Pimephales promelas), rainbow trout (Oncorhynchus mykiss), and green algae were collected. Mammalian median lethal dose (LD50) and median lethal concentration (LC50) were used to assess mammalian hazard. Median effective concentration (EC50) and LC50 data were used to assess aquatic species hazard. Toxicity ratings were assigned using the United Nations Globally Harmonized System (GHS) of Classification and Labelling of Chemicals [42]. In the GHS system, lower numbers indicate higher toxicity, with a designation of “1” indicating the most toxic category. When multiple GHS values were available for a given chemical, the lowest value was used. Chemicals for which the LD50, LC50, or EC50 exceeded the least toxic GHS category were classified as non-toxic.
Chemical were identified for further hazard assessment based on frequency of use, median mass of chemical-use per event, and available toxicity data. Frequency of use was calculated by dividing the number of events that utilized a given chemical by the total routine oil and gas events reported in the SCAQMD database. The median mass of chemical usage per event represents the median mass for all events containing that chemical. Where chemical mixtures were reported, individual chemical masses were calculated by multiplying the total mixture mass by the maximum individual chemical concentration. When multiple entries for a given chemical were reported for a single event, the chemical masses were summed within that event.
We compared the chemical-use in routine oil and gas activities in the SCAQMD dataset to hydraulic fracturing chemicals disclosed in the state of California via the voluntary FracFocus chemical disclosure registry, as summarized by Stringfellow et al. [21]. This dataset contains records of chemical use for 1,623 individual hydraulic fracturing operations conducted in California between January 30, 2011 and May 19, 2014. Stringfellow et al. [21] identified 338 unique additives based on name and CASRN combinations, of which 228 were reported with a CASRN and 110 were identified by chemical or common name only or had proprietary designations. The additives included chemicals, mineral proppants and carriers, and base fluids consisting of water, salt, and brine solutions. There were 326 unique additive names identified in the database [21].
Results and discussion
Chemical-use in the SCAQMD
In total, 548 chemical additives were used in the SCAQMD between June 2013 and September 2015, with 525 of these being used for routine oil and gas development activities. The most frequently used chemicals include solvents (e.g. methanol), petroleum products (e.g. distillates), and salts (e.g. sodium chloride) that are employed in formulating commercial blends of production chemicals (S1 Table). Also on the list of frequently used chemicals are carboxylic acids (e.g. citric acid and erythorbic acid) used for scale and iron control, biocides, and corrosion inhibitors. For routine acidizing (e.g., acid cleaning for well-maintenance), hydrochloric acid (HCl) and hydrofluoric acid (HF) were used extensively and in large quantities (mean masses of 1,791 and 161 kg per event, respectively). These quantities are consistent with the analysis by Abdullah et al. [19], who reported mean values of 1,908 kg HCl and 175 kg HF per acidizing event (also exclusive of matrix acidizing). Our values may differ due to the different study periods or deletion of duplicate entries by operators. Other additives used in the highest masses include minerals and other chemicals used for gravel packing (e.g. silica), cementing of well casings (e.g. Portland cement and additives), and sealing wells (e.g. bentonite) (S2 Table).
Table 1 is presented as an analysis of chemical use (numbers of chemicals used and masses) by reported activity. There were only a limited number of well-stimulation events in the SCAQMD during this period and no acid fracturing events were reported. Acidizing, maintenance acidizing, well drilling, and gravel packing accounted for the majority of the 1,207 events in the data set (Table 1). Chemical-use for these types of oil and gas field activities is only subject to mandatory reporting in the SCAQMD region.
Table 1. Number of chemicals used and their summed masses per event for oil and gas development (does not include water)a.
| Chemicals per event | Mass per event (kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled Activities | Eventsa | Mean | Median | Min | Max | Mean | Median | Min | Max |
| Acidizing | 256 | 25 | 20 | 1 | 41 | 4,132 | 3,459 | 10 | 24,043 |
| Gravel packing | 169 | 6 | 3 | 1 | 65 | 24,655 | 6,297 | 61 | 710,722 |
| Hydraulic fracturing | 13 | 25 | 23 | 15 | 37 | 129,910 | 142,245 | 4,526 | 243,219 |
| Maintenance acidizing | 390 | 30 | 35 | 2 | 52 | 2,779 | 2,028 | 155 | 15,548 |
| Maintenance acidizing and gravel packing | 3 | 27 | 27 | 27 | 27 | 7,712 | 6,632 | 6,518 | 9,985 |
| Matrix acidizing | 7 | 21 | 20 | 20 | 23 | 4,210 | 3,055 | 1,970 | 10,791 |
| Well completion and rework—type not specified | 43 | 20 | 21 | 1 | 71 | 16,287 | 8,028 | 215 | 100,566 |
| Well drilling | 186 | 46 | 54 | 3 | 72 | 1,828,619 | 97,669 | 96 | 309,284,305 |
| Well drilling with gravel packing | 136 | 57 | 58 | 26 | 66 | 239,305 | 181,098 | 21,552 | 1,233,365 |
aThere are 1,207 events in the data set but four events have only water listed so they are not included in this table (N = 1,203).
Comparison of chemical-use between routine activities and well-stimulation treatments within the SCAQMD
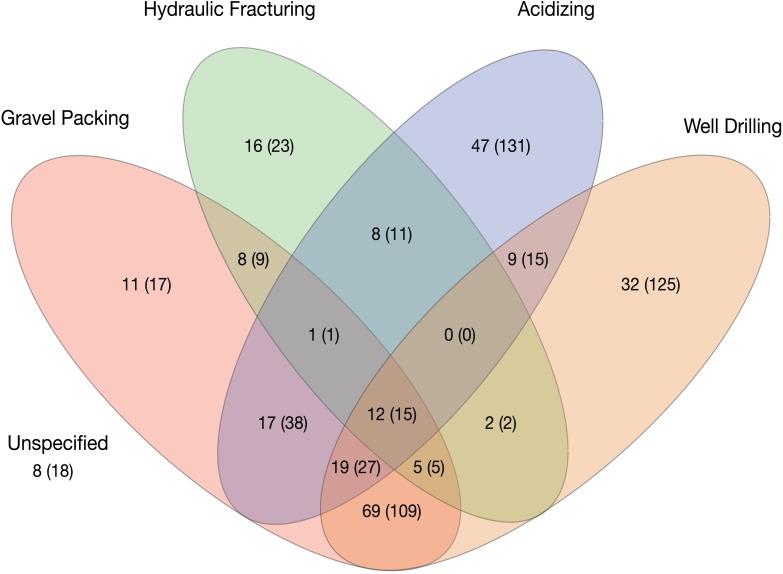
Overall, a large number of constituents were used in both routine activities and well-stimulation activities and chemicals were applied in large masses (Table 1). The masses used in hydraulic fracturing were high because of the large quantities of proppants used. Similarly, well drilling uses large quantities of Portland cement and minerals for well construction. Comparison of the chemicals used for different on-field activities showed significant overlap in the chemicals used for hydraulic fracturing and routine oil and gas development operations (Fig 1). Only 23 (4.2%) chemicals were used exclusively for hydraulic fracturing in the SCAQMD. However, the SCAQMD dataset includes only a small number of hydraulic fracturing operations (13) and the degree of overlap in chemical use between different oil field operations may not be representative of other regions. A comparison of chemical use for routine oil and gas development as reported in the SCAQMD database and chemical use for fracturing in the whole state of California, indicates the degree of overlap is less.
Fig 1. Venn diagram showing number of chemicals used in oil and gas production.
The first number represents chemicals with CASRN and the number in parentheses represents the total number of reported chemicals. Does not include base fluids. Acidizing includes matrix acidizing, acidizing, and maintenance acidizing.
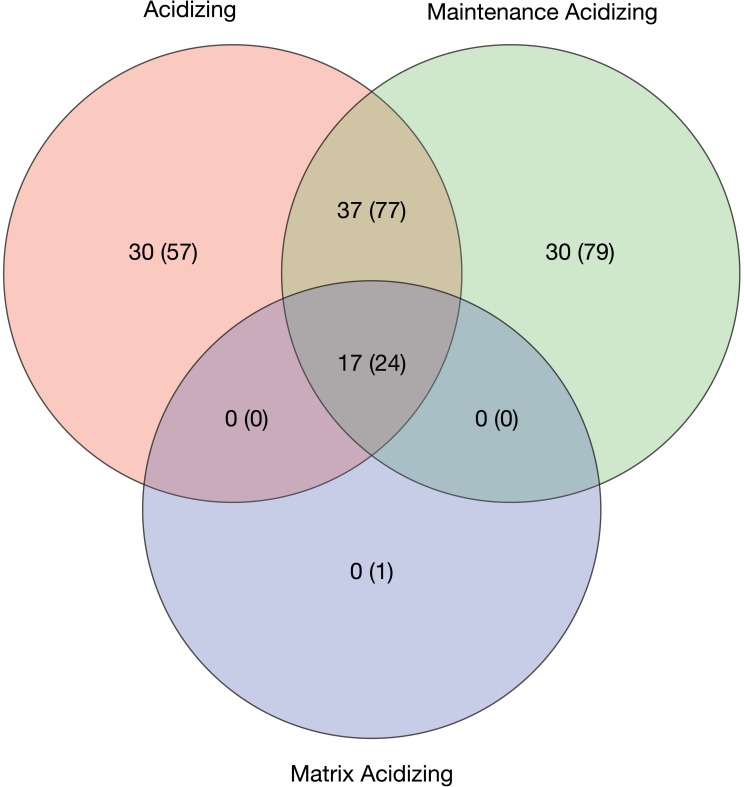
Examining different types of acidizing within the SCAQMD, the median numbers of chemicals used in routine acidizing (20 for acidizing and 35 for maintenance acidizing) were similar in number to the median value of 20 used in matrix acidizing (Table 1). An analysis of chemicals used for acid treatments shows that there is considerable overlap in the chemicals used for the different applications of acid (Fig 2). The one compound used exclusively for matrix acidizing was identified only as “DDBSA salt,” presumably a dodecylbenzenesulfonic acid salt, but without a corresponding CASRN, this identification is tentative. Maintenance acidizing used a lower median mass of chemicals (2,028 kg) than treatments reported as acidizing (3,459 kg) or matrix acidizing (3,055 kg). These quantities demonstrate that additives usage in other acidizing is not appreciably different than what is used in matrix acidizing (classified as well stimulation).
Fig 2. Venn diagram showing number of chemicals used for acidizing operations (routine and well stimulation).
The first number represents chemicals with CASRN and the number in parentheses represents the total number of reported chemicals. Does not include base fluids.
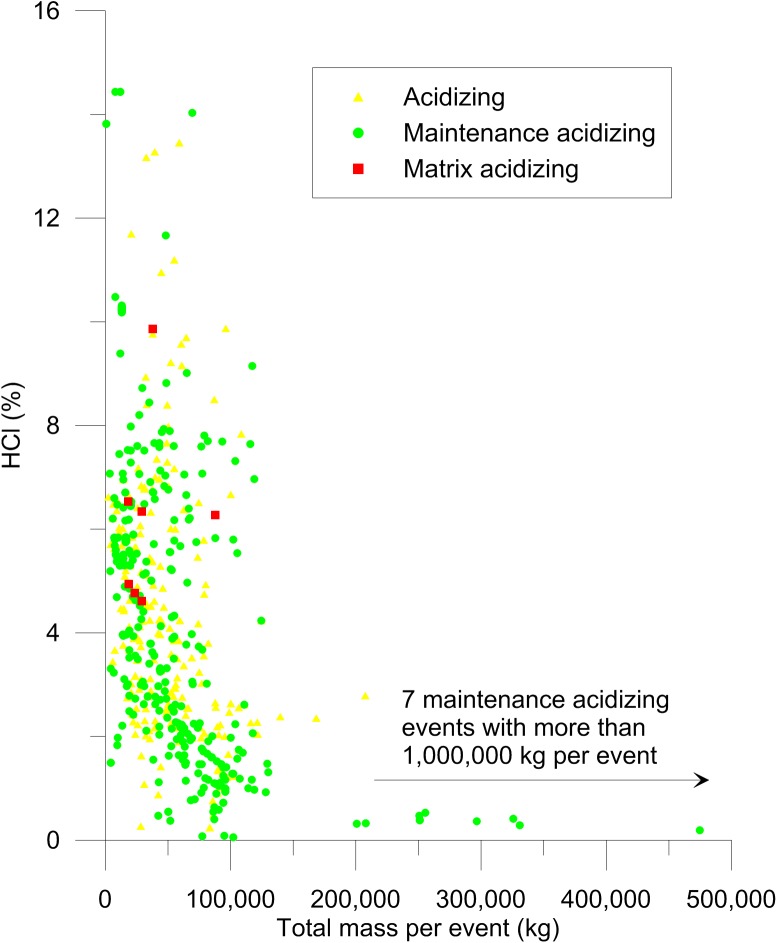
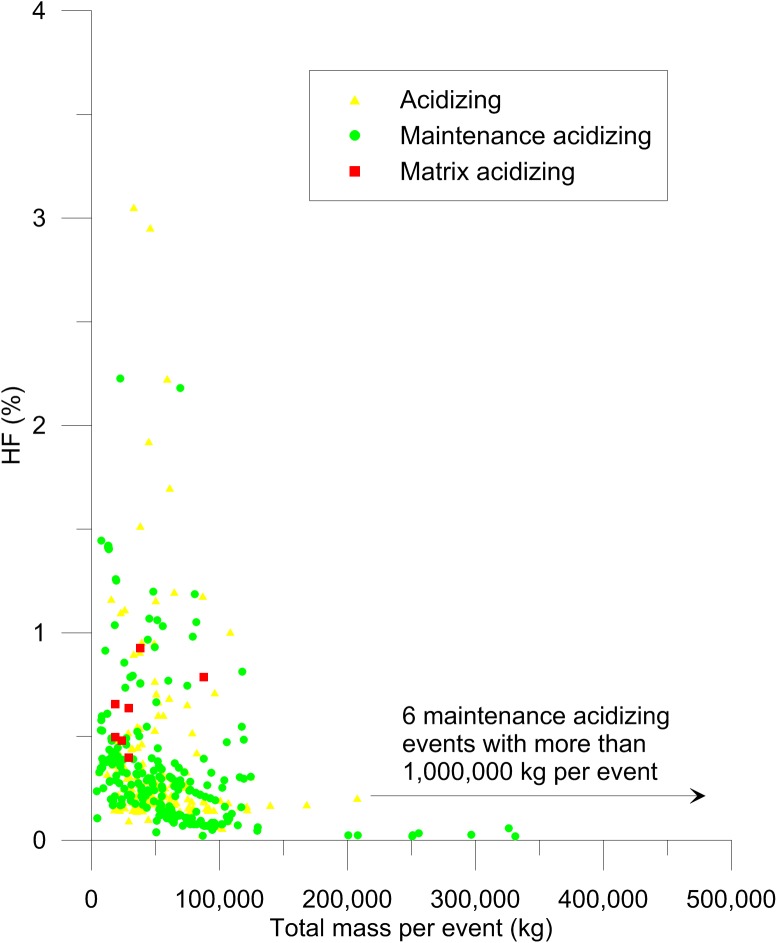
Concentrations of hydrochloric acid (HCl) and hydrofluoric acid (HF) used in all types of acidizing events were similar, as were the total masses of additives used (Figs 3 and 4). Hydrochloric acid concentrations ranged from approximately 0–15% (Fig 3) while HF concentrations were approximately 0–3% (Fig 4). In California, the distinction between routine acidizing and acid stimulation (matrix acidizing and acid fracturing) is based on calculation of the acid threshold volume that is determined based on wellbore volume and formation porosity [1]. The acid threshold volume cannot be calculated without site-specific information that is not reported to the publically available SCAQMD database. However, it is apparent that large quantities of acid and high concentrations are being used in all types of acidizing events. Since there is clear overlap in concentrations and amounts of acid used for events reported as matrix acidizing, which are potentially regulated by state law, and routine maintenance acidizing (Figs 3 and 4), these results suggest that regulations focused only on disclosures of chemicals used in well stimulation events may not be sufficiently protective of public or environmental health.
Fig 3. Concentrations of hydrochloric acid (HCl) used in acidizing.
Sixteen events where water was not reported were excluded because the concentrations could not be calculated.
Fig 4. Concentrations of hydrofluoric acid (HF) used in acidizing.
Sixteen events where water was not reported were excluded because the concentrations could not be calculated.
Comparison of chemical-use between routine oil and gas development activities in the SCAQMD and hydraulic fracturing throughout California
The number of chemicals used in routine oil and gas development activities in the SCAQMD is as high or higher than the number of chemicals used for hydraulic fracturing throughout the State of California [21]. In Stringfellow et al. [21], 338 unique chemical additives were identified as used in hydraulic fracturing fluids in California, with 228 of these identified by CASRN. These data were reported voluntarily by industry, but are believed to be representative of hydraulic fracturing as practiced in California [21,24,43,44]. Here, we identified 525 additives used in routine oil and gas production, with 249 identified by CASRN. In Stringfellow et al. [21], there was a median of 23 components per hydraulic fracturing treatment, inclusive of base fluids and proppants. In the SCAQMD, the number of additives per event varied by activity (Table 1). The median number of chemical additives was as low as three for gravel packing and the median number of chemical additives used in well drilling was much higher (54).
In the SCAQMD, the median mass used per hydraulic fracturing event was high (142,245 kg), but when water and quartz sand proppants were removed, the median mass of chemical additives was 6,725 kg. This is approximately three times higher than the value of 2,057 kg obtained by Stringfellow et al. [21], who analyzed voluntarily reported data from the whole state of California. This difference may be attributed to differences in regional reservoir geology between the SCAQMD and the rest of California [44] and corresponding hydraulic fracturing practices: most of the data analyzed by Stringfellow et al. [21] was reported from Kern County, CA while the data here originated primarily from Orange and Los Angeles Counties.
Of the 249 chemicals identified by CASRN that are used for routine oil and gas development in the SCAQMD (Table 2), 124 (24%) were identified by Stringfellow et al. [21] as being used for hydraulic fracturing in California, further demonstrating overlap in chemical usage between hydraulic fracturing and routine activities. Further examination of the types of chemicals used in routine oil and gas development activities and in hydraulic fracturing yields both similarities and differences. As an example, ten biocides were identified in the hydraulic fracturing data set reported by Stringfellow et al. [21] while only six were identified here as used in routine activities. The biocides were used in 63% of routine activities conducted in the SCAQMD compared to 93% of hydraulic fracturing treatments [21]. In routine use, the most commonly used biocides were formaldehyde, used in 677 (57%) events, and glutaraldehyde, used in 274 (23%) events. In the hydraulic fracturing treatments, isothiazolones were used in 73% of treatments [21,24]. This demonstrates that biocides are used extensively in different types of oil and gas production activities.
Table 2. Data availability for chemicals used in routine oil and gas development.
| Number of chemicals | Proportion of all chemicals | CASRN | Mass data | Toxicity data |
|---|---|---|---|---|
| 151 | 30% | Available | Available | Availablea |
| 1 | 0% | Available | Unavailable | Availablea |
| 97 | 18% | Available | Available | Unavailablea |
| 43 | 8% | Unavailable | Available | Unavailable |
| 233 | 44% | Unavailable | Unavailable | Unavailable |
aDoes not include EPI Suite computational estimates for green algae ecotoxicity
Corrosion inhibitors were used more extensively in routine operations than in hydraulic fracturing treatments. Ten corrosion inhibitors were identified in both the current data set and in the hydraulic fracturing data set [21], although the numbers are likely higher since many chemicals used as corrosion inhibitors also have other functions in oil and gas production (e.g. surfactants). In routine operations in the SCAQMD, corrosion inhibitors were used in 894 events (75% of all events), but they were only used in 6% of the hydraulic fracturing treatments [21]. The prevalent use of corrosion inhibitors in the SCAQMD is not surprising given the common use of strong acids in well maintenance and completion activities.
The substantial overlap between chemicals used in hydraulic fracturing fluids and those used in routine oil and gas development processes clearly demonstrate that the regulatory focus on reporting chemical-use for well-stimulation activities (e.g. hydraulic fracturing) to the exclusion of routine maintenance activities (e.g. wellbore cleaning) does not fully address potential environmental and public health concerns from on field chemical-use, particularly in the context of beneficial reuse of produced water for agriculture [15]. A more complete understanding of chemical usage–including type; toxicity and environmental profile; and mass, timing, frequency used–in routine oil and gas development is needed to support decision making with respect to beneficial reuse of produced water and this study contributes to filling this data gap.
Comparison of chemical-use between routine oil and gas development activities in the SCAQMD and other oil and gas fields throughout the U.S. and World
It is difficult to determine with certainty if chemical use on oil fields in the SCAQMD is representative of chemical-use on oil fields throughout the U.S. or the world, since data on chemical-use is rarely collected by governments or published by industry. Hudgins analyzed and published chemical-use data provided voluntarily by off-shore operations in the Gulf of Mexico [7] and the North Sea [8]. Comparison of the Hudgins’ studies with chemicals used in the SCAQMD shows that chemicals are used for common purposes, such as microbial control, scale control, and cleaning, at all locations [7,8]. Hudgins’ studies did not identify chemicals by CASRN, but some chemicals were identified sufficiently by name to allow positive identification of 47 chemicals from the North Sea study [8] and 25 chemicals from the Gulf of Mexico study [7]. Thirty-five chemicals could be positively identified as being used in both the North Sea and in the SCAQMD and 15 were positively identified as being used in both the Gulf of Mexico and the SCAQMD. Overall, these results, combined with a review of industrial literature, patents, and other sources, suggests that many of the chemicals used on the SCAQMD, or closely related compounds, would be found on oil fields worldwide [5–8,19–22,45].
Analysis of chemical hazards using data science approaches
One of the important requirements of regulations directed at oil and gas development and production is the disclosure of the types and amounts of chemicals used on-field [1,2,4,11,46]. Chemical disclosure is widely recognized as a fundamental prerequisite for the open and transparent analysis of the hazards and risks associated with chemicals [2,4,10,27,45,46]. Previous studies have shown that many oil and gas field chemicals are not expected to have negative environmental or health impacts, but that some compounds, including surfactants, biocides, and corrosion inhibitors may be harmful to the environment, and that in many cases there is insufficient information to confidentially evaluate the potential environmental impact of chemicals that are used in significant amounts on oil and gas fields [19–24,47,48].
A preliminary hazard assessment for oil field chemicals being used in the SCAQMD was conducted using data science methods applied against hydraulic fracturing chemicals [20,21]. As shown in Table 2, 52% of the chemicals used in the SCAQMD were reported without a CASRN and could therefore not be evaluated using a data science approach, which requires CASRN to match compounds with corresponding environmental and toxicity information. Of the 53 chemicals used most frequently (top 10%), 18 were reported without a corresponding CASRN. The top 10% of the chemicals used in the highest median masses per event also did not always have associated CASRN (S2 Table). For example, the fourth most commonly used additive is a proprietary chemical identified only as “polyoxyalkylenes,” which could be any one of potentially hundreds of chemicals or chemical formulations. Compounds reported by CASRN mostly had corresponding mass-usage information, important for risk analysis, but 97 did not have toxicity profiles in the public databases used in this study (Table 2; S3 Table). Altogether, 70% of the chemical additives reported in the SCAQMD could not be fully evaluated using data-based hazard analysis approaches [20,21,47], suggesting that current reporting requirements may need to be strengthened, if the regulatory objective includes generating data needed for risk assessments.
Analysis of chemicals by mammalian toxicity revealed that five chemicals were classified as GHS Category 2 contaminants based on acute mammalian oral exposure and 13 were classified as GHS Category 1 or 2 for acute mammalian inhalation toxicity (Table 3). These results are similar to results found by Stringfellow et al. [21] for hydraulic fracturing operations. Several of the most toxic chemicals identified are biocides: 5-chloro-2-methyl-3(2H)-isothiazolone, DBNPA (2,2-dibromo-3-nitrilopropionamide), formaldehyde, and glutaraldehyde. Corrosion inhibitors are also represented on the list of most toxic chemical additives: propargyl alcohol and thioglycolic acid (Table 3). Mammalian toxicity data were unavailable for 105 (42%) of the 249 chemicals with CASRN.
Table 3. Chemicals used in routine oil and gas development that are classified by the United Nations Globally Harmonized System (GHS) Categories 1 and 2 for acute mammalian toxicitya.
| Chemical name | CASRN | Oral toxicity ratings | Inhalation toxicity ratings | Frequency of use (% events) | Median mass per event (kg) | |||
|---|---|---|---|---|---|---|---|---|
| Rat | Mouse | Rabbit | Rat | Mouse | ||||
| 2-Butoxyethanol (Ethylene glycol butyl ether) | 111-76-2 | 4 | 4 | 3 | 2 | - | 26.5% | 545 |
| 5-Chloro-2-methyl-3(2H)-isothiazolone | 26172-55-4 | 4 | - | - | 2 | - | 0.1% | 5.2 |
| DBNPA (2,2-dibromo-3-nitrilopropionamide) | 10222-01-2 | 3 | - | 3 | 1 | - | 0.3% | 4.1 |
| Ethylene oxide | 75-21-8 | 3 | 3 | - | 2 | 3 | 1.0% | <0.1 |
| Ferric chloride | 7705-08-0 | 2 | 4 | - | - | - | 0.5% | 30 |
| Formaldehyde | 50-00-0 | 2 | 2 | - | 2 | 2 | 57.0% | <0.1 |
| Glutaraldehyde | 111-30-8 | 3 | 3 | - | 1 | - | 23.1% | 75 |
| Glycolic acid | 79-14-1 | 4 | 4 | - | 1 | - | 0.1% | 89 |
| Hydrofluoric acid | 7664-39-3 | - | - | - | 2 | 2 | 43.6% | 96 |
| Lithium hydroxide | 1310-65-2 | 3 | 4 | - | 2 | - | 0.2% | 22 |
| Petroleum distillates | 64741-44-2 | - | - | - | 2 | - | 0.1% | 138,679 |
| Propargyl alcohol | 107-19-7 | 2 | 2 | - | 3 | - | 53.8% | 3.7 |
| Sulfuric acid | 7664-93-9 | 5 | - | - | 2 | - | 2.1% | <0.1 |
| Tetrasodium ethylenediaminetetraacetate | 64-02-8 | 4 | 2 | - | - | - | 0.3% | <0.1 |
| Thioglycolic acid | 68-11-1 | 3 | 3 | 3 | 1 | - | 0.1% | 98 |
| Toluene | 108-88-3 | 4 | - | - | >4 | 2 | 1.4% | 6.7 |
| Zinc sulfate | 7733-02-0 | 3 | 2 | 4 | - | - | 0.2% | 50 |
aOnly chemicals with valid CASRN could be evaluated.
Analysis of ecotoxicity characteristics of the chemicals revealed that 58 chemical additives were classified as GHS Category 1 or 2 (Table 4). Twenty-six of these classifications were determined using computational estimates from the U.S. EPA Ecological Structure Activity Relationships (ECOSAR) software for green algae ecotoxicity, available through EPI Suite. The remainder of the ecotoxicity determinations were made using experimental data. A wide range of chemicals were identified as being toxic to aquatic organisms. The list includes acids, hydrocarbons, biocides, corrosion inhibitors, surfactants, and other industrial chemicals (e.g. tall oil). Experimental ecotoxicity data were unavailable for 146 (59%) of the 249 chemicals with CASRN; when ECOSAR estimates were included, ecotoxicity data were unavailable for 129 (52%) chemicals with CASRN.
Table 4. Chemicals used in routine oil and gas development that are classified by the United Nations Globally Harmonized System (GHS) in Categories 1 and 2 for ecotoxicitya.
| Chemical name | CASRN | Water Fleab | Fathead Minnowc | Rainbow Troutd | Green Algaee | Frequency of use (% events) | Median mass per event (kg) |
|---|---|---|---|---|---|---|---|
| 1,2,3-Trimethylbenzene | 526-73-8 | - | - | - | 2 | 0.3% | 1.0 |
| 1,2,4-Trimethylbenzene | 95-63-6 | 2 | 2 | - | 2 | 5.7% | 1.6 |
| 1,3,5-Trimethylbenzene | 108-67-8 | 2 | - | - | 2 | 0.3% | 2.3 |
| 2-Mercaptoethyl alcohol | 60-24-2 | 2 | - | - | 2 | 0.7% | 2.5 |
| 2-Methyl-3(2H)-isothiazolone | 2682-20-4 | 1 | - | 1 | 1 | 0.2% | 2.6 |
| 5-Chloro-2-methyl-3(2H)-isothiazolone | 26172-55-4 | 1 | - | 1 | 1 | 0.1% | 5.2 |
| Acrylamide | 79-06-1 | 3 | >3 | >3 | 1 | 0.8% | <0.1 |
| Alcohols, C10-14, ethoxylated | 66455-15-0 | - | - | - | 1 | 0.6% | 64 |
| Aluminum | 7429-90-5 | - | - | 1 | - | 16.5% | 9.1 |
| Ammonium chloride | 12125-02-9 | >3 | 2 | >3 | - | 48.4% | 454 |
| Benzene, c10-c16 alkyl derivatives | 68648-87-3 | - | - | - | 1 | 0.9% | <0.1 |
| Benzene, tetrapropylene- | 25265-78-5 | - | - | - | 1 | 0.1% | 2.7 |
| Benzoisothiazolinone | 2634-33-5 | 1 | - | 1 | 1 | 0.1% | <0.1 |
| Bis(isopropyl)naphthalene | 38640-62-9 | - | - | - | 1 | 2.0% | 1.8 |
| Canola oil | 120962-03-0 | - | - | - | 1 | 0.3% | 92 |
| Cocamidopropyl betaine | 61789-40-0 | 2 | - | - | >3 | 0.7% | <0.1 |
| Cyclohexasiloxane, 2,2,4,4,6,6,8,8,10,10,12,12-dodecamethyl- | 540-97-6 | - | - | - | 1 | 0.3% | <0.1 |
| Cyclopentasiloxane, 2,2,4,4,6,6,8,8,10,10-decamethyl- | 541-02-6 | - | - | - | 1 | 0.3% | <0.1 |
| DBNPA (2,2-dibromo-3-nitrilopropionamide) | 10222-01-2 | 1 | 1 | 1 | 1 | 0.3% | 4.1 |
| Dodecylbenzene | 123-01-3 | - | - | - | 1 | 0.1% | 5.4 |
| Dodecylbenzene sulfonic acid | 27176-87-0 | 2 | - | 2 | 3 | 1.4% | <0.1 |
| Ethanesulfonic acid, 2-[methyl[(9z)-1-oxo-9-octadecen-1-yl]amino]-, sodium salt (1:1) | 137-20-2 | - | - | - | 2 | 0.6% | 53 |
| Ethoxylated C14-15 alcohols | 68951-67-7 | 1 | 1 | 1 | 1 | 1.3% | 2.4 |
| Ethoxylated hexanol | 68439-45-2 | 2 | - | 2 | >3 | 0.3% | 16 |
| Ethylbenzene | 100-41-4 | 2 | 2 | 2 | 2 | 31.3% | 2.9 |
| Fatty acids, tall-oil | 61790-12-3 | >3 | - | - | 1 | 0.4% | 7.1 |
| Fatty acids, tall-oil, reaction products with triethanolamine | 67784-78-5 | - | - | - | 2 | 1.3% | <0.1 |
| Ferric chloride | 7705-08-0 | 2 | 3 | - | - | 0.5% | 30 |
| Glutaraldehyde | 111-30-8 | 1 | 2 | 2 | 2 | 23.1% | 75 |
| Glyoxal | 107-22-2 | >3 | >3 | 0 | 2 | 23.0% | 3.6 |
| Hydrochloric acid | 7647-01-0 | 1 | - | 2 | - | 54.8% | 1,311 |
| Hydrotreated light petroleum distillate | 64742-47-8 | - | 3 | 2 | 1 | 32.9% | 17 |
| Isopropylbenzene | 98-82-8 | 3 | 2 | 2 | 2 | 29.5% | 0.3 |
| Lecithins | 8002-43-5 | - | - | - | 1 | 0.3% | 1.4 |
| Lithium hypochlorite | 13840-33-0 | 1 | - | 1 | - | 0.2% | 129 |
| Naphtha (petroleum), heavy catalytic reformed | 64741-68-0 | - | - | - | 2 | 0.2% | 18 |
| Naphthalene | 91-20-3 | 1 | 1 | 1 | 2 | 48.4% | 0.3 |
| Octamethylcyclotetrasiloxane | 556-67-2 | - | - | - | 1 | 0.3% | <0.1 |
| Petroleum distillate-mineral oil grade | 8002-05-9 | 1 | - | - | 1 | 0.1% | 30 |
| Petroleum distillates | 64741-44-2 | - | - | - | 1 | 0.1% | 138,679 |
| Petroleum distillates | 64742-46-7 | - | - | - | 1 | 0.1% | 138,679 |
| Poly(oxy-1,2-ethandiyl), a-(nonylphenyl)-w-hydroxy- | 9016-45-9 | 2 | - | 2 | 2 | 13.2% | 4.6 |
| Polyethylene glycol monostearate | 9004-99-3 | - | - | - | 1 | 1.3% | <0.1 |
| Polypropylene | 9003-07-0 | - | - | - | 1 | 1.1% | 56 |
| Polysiloxanes, di-Me | 63148-62-9 | 3 | - | - | 1 | 1.6% | <0.1 |
| Propargyl alcohol | 107-19-7 | - | 2 | - | >3 | 53.8% | 3.7 |
| Quinoline | 91-22-5 | 3 | 1 | - | 3 | 18.8% | 0.1 |
| Sodium chloroacetate | 3926-62-3 | >3 | - | - | 1 | 0.3% | <0.1 |
| Sodium hypochlorite | 7681-52-9 | 1 | 1 | 1 | >3 | 0.2% | 2.3 |
| Sodium silicate | 1344-09-8 | 1 | - | - | - | 0.7% | 72 |
| Solvent naphtha, petroleum, heavy arom. | 64742-94-5 | 1 | 3 | 2 | 2 | 39.0% | 1.8 |
| Solvent naphtha, petroleum, light arom. | 64742-95-6 | 2 | - | 2 | 2 | 5.8% | 1.7 |
| Sorbitan monostearate | 1338-41-6 | - | - | - | 1 | 1.3% | <0.1 |
| Stearic acid | 57-11-4 | - | - | - | 1 | 12.1% | 150 |
| Sulfonic acids, c14-16-alkane hydroxy and c14-16-alkene, sodium salts | 68439-57-6 | 2 | - | - | 3 | 0.1% | 5.4 |
| Tall oil | 8002-26-4 | - | - | - | 1 | 0.8% | 13 |
| Xylenes | 1330-20-7 | - | 3 | 2 | 2 | 32.0% | 1.5 |
| Zinc sulfate | 7733-02-0 | 1 | 1 | 1 | - | 0.2% | 50 |
aOnly chemicals with valid CASRN could be evaluated.
bDaphnia magna
cPimephales promelas
dOncorhynchus mykiss
ecomputational estimates from EPI Suite.
Although a complete risk assessment is beyond the scope of this study, evaluation of the frequency of chemical use and the mass of chemical used can provide context for the potential risk associated with the use of hazardous chemicals. Of the 17 chemicals with high mammalian toxicity only four of these were used in more than 25% of events (Table 3). Quantities of the most toxic chemicals used varied. Seven of the toxic chemicals were used in median quantities of less than 10 kg per treatment, while nine were used in larger amounts. Glutaraldehyde (used in 23% of events) was applied with a median quantity per treatment of 75 kg. While formaldehyde was used more frequently (57% of events), the median quantity added was less than 1 kg per treatment. The complexity of toxicity information, paired with data on frequency of use and quantities applied (Table 3), suggest that while hazard assessments such as this as useful for characterizing chemical-use, more detailed risk assessments are needed.
Nine of the most toxic chemicals from an aquatic perspective were used in more than 25% of events (Table 4). The most frequently used chemicals on the list were hydrochloric acid, propargyl alcohol, ammonium chloride, and naphthalene, used in 48% of events or more. Propargyl alcohol and naphthalene were used in small quantities (median masses of less than 5 kg per treatment) although hydrochloric acid and ammonium chloride were used in much higher amounts (median masses of 1,311 and 454 kg per treatment). The higher number of chemical additives posing ecotoxicity issues and the frequent use of these chemicals, suggests that the ecosystem risks need to be fully evaluated in produced water reuse projects.
Evaluation of chemical hazards using regulatory lists
To further investigate the potential hazards associated with chemicals used in routine oil and gas development activities, six regulatory lists were referenced (S4 Table). The result of the comparison with these regulatory lists was that twenty-two of the chemicals were on the California Toxic Air Contaminant List [41], 12 were on the California Proposition 65 List [40], 10 were on the U.S. EPA Drinking Water Standards and Health Advisories List [49], six were present on the U.S. EPA Contaminant Candidate List 4 [50], three were on the European Chemicals Agency Substance of Very High Concern Candidate List [51], and two were on the OSPAR List of Substances of Possible Concern [45]. These results demonstrate that some of the chemicals used in routine oil and gas development activities are chemicals of concern, as identified by multiple state, federal, and international environmental agencies due to their toxicities. However, the actual risk proposed by these chemicals would need to be determined in the context of their use and potential release into the environment.
It should be noted that comparison with regulatory lists also indicate that many of the chemicals used in the SCAQMD are expected to present little or no human health or ecotoxicity hazard, even if discharged into the environment. Of the chemicals reported with CASRN, 56 are on the OSPAR list of chemicals not expected to pose environmental harm [22]. These chemicals include inert minerals (e.g. silica, graphite, mica, diatomaceous earth), common salts (e.g. calcium carbonate, calcium chloride, sodium carbonate, etc.), chemicals that rapidly degrade in the environment (e.g. acetic acid, ethylene glycol, 1-butanol), and food additives (e.g. xanthan gum, guar gum, sodium erythorbate, starch).
Conclusions
In this study we compared routine oil and gas field chemical use, which is not typically subject to disclosure regulations, with chemical use for hydraulic fracturing and other well stimulation techniques that are subject to regulation mandating chemical disclosure. Our results indicate that there is substantial overlap between the chemicals used in well stimulation and those used in routine oil and gas development activities. Similarities were observed in the numbers of chemicals used, the masses in which they were applied, the frequency of use, and their toxicological profiles. Our analysis shows that hydraulic fracturing is just one of many applications of hazardous chemicals on oil and gas fields and suggests that limiting disclosure requirements for oil and gas field chemical-use to hydraulic fracturing and other well-stimulation events may not be fully protective of human and environmental health, especially in the context of beneficial reuse of produced water for irrigation, wildlife, livestock watering, and groundwater recharge.
Supporting information
Total number of events is 1,187.
(PDF)
Total number of events is 1,187.
(PDF)
(PDF)
(PDF)
Acknowledgments
This material is based upon work supported by the Department of Energy under Award Number DE-IA0000018 (CERC-WET). This study was also supported by grants from The Broad Reach Fund and Laboratory Directed Research and Development (LDRD) funding from Berkeley Lab, provided by the Director, Office of Science, of the U.S. Department of Energy under Contract No. DE-AC02-05CH1123.
Data Availability
Data are available from the South Coast Air Quality Monitoring District (http://www.aqmd.gov/). Additional data in Research Gate: DOI: 10.13140/RG.2.2.19128.55041.
Funding Statement
This material is based upon work supported by the Department of Energy under Award Number DE-IA0000018. This study was supported in part by grants from The Broad Reach Fund and Laboratory Directed Research and Development (LDRD) funding from Berkeley Lab, provided by the Director, Office of Science, of the U.S. Department of Energy under Contract No. DE-AC02-05CH1123.
References
- 1.California Department of Conservation. SB 4 Well Stimulation Treatment Regulations, Final Text of Regulations, Chapter 4. Development, Regulation, and Conservation of Oil and Gas Resources, Subchapter 2. Environmental Protection. December 30; Sacramento, CA: Division of Oil, Gas, and Geothermal Resources; 2014.
- 2.Richardson N, Gottlieb M, Krupnick A, Wiseman H. The State of State Shale Gas Regulation. Washington, D.C.: Resources for the Future; 2013 June.
- 3.Brantley SL, Yoxtheimer D, Arjmand S, Grieve P, Vidic R, Pollak J, et al. Water resource impacts during unconventional shale gas development: The Pennsylvania experience. Int J Coal Geol. 2014;126:140–56. [Google Scholar]
- 4.Bureau of Land Management. Oil and Gas; Hydraulic Fracturing on Federal and Indian Lands, Final Rule, 43 CFR Part 3160, RIN 1004–AE26. Washington, DC 2012.
- 5.Fink J. Petroleum Engineer's Guide to Oil Field Chemicals and Fluids. 2nd ed. Burlington, MA: Elsevier Science; 2015. [Google Scholar]
- 6.Kelland M. Production Chemicals for the Oil and Gas Industry. 2nd ed. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- 7.Hudgins CM. Chemical treatments and usage in offshore oil and gas production systems. J Pet Technol. 1992;44(5):604–11. [Google Scholar]
- 8.Hudgins CM. Chemical use in North Sea oil and gas E&P. J Pet Technol. 1994;46(1):67–74. [Google Scholar]
- 9.Economides MJ, Hill AD, Ehlig-Economides C, Zhu D. Petroleum Production Systems, 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2013. [Google Scholar]
- 10.National Research Council. Science and Decisions: Advancing Risk Assessment Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 11.California Department of Conservation. Well Stimulation Treatment (WST) Sacramento, CA: Division of Oil, Gas, and Geothermal Resources; 2016 [cited 2016 July 29]. Available from: http://www.conservation.ca.gov/dog/Pages/WST.aspx.
- 12.Doman L. United States remains largest producer of petroleum and natural gas hydrocarbons Washington, D.C.: U. S. Energy Information Administration; 2016. May 23. [Google Scholar]
- 13.Guerra K, Dahm K, Dundorf S. Oil and Gas Produced Water Management and Beneficial Use in the Western United States, S&T Report No. 157. Denver, CO: U.S. Department of the Interior, Bureau of Reclamation; 2011.
- 14.Veil J. U.S. Produced Water Volumes and Management Practices in 2012. Oklahoma City, OK: Ground Water Protection Council, 2015. April. [Google Scholar]
- 15.Heberger M, Donnelly K. Oil, Food, and Water: Challenges and Opportunities for California Agriculture. Oakland, CA: Pacific Institute; 2015 December.
- 16.Muggeridge A, Cockin A, Webb K, Frampton H, Collins I, Moulds T, et al. Recovery rates, enhanced oil recovery and technological limits. Phil Trans R Soc A. 2014;372:20120320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson EB. Well cementing fundamentals. Oilfield Rev. 2012;24(2):59–60. [Google Scholar]
- 18.OSPAR Commission. OSPAR Report on Discharges, Spills and Emissions from Offshore Oil and Gas Installations in 2013. London, UK; 2015. Available from: http://www.ospar.org/documents?v=33826
- 19.Abdullah K, Malloy T, Stenstrom MK, Suffet IHM. Toxicity of acidization fluids used in California oil exploration. Toxicol Environ Chem. 2017;99(1):78–94. [Google Scholar]
- 20.Stringfellow WT, Domen JK, Camarillo MK, Sandelin WL, Borglin S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J Hazard Mater. 2014;275:37–54. doi: 10.1016/j.jhazmat.2014.04.040 [DOI] [PubMed] [Google Scholar]
- 21.Stringfellow WT, Camarillo MK, Domen JK, Sandelin WL, Varadharajan C, Jordan PD, et al. Identifying chemicals of concern in hydraulic fracturing fluids used for oil production. Environ Pollut. 2017;220:413–20. doi: 10.1016/j.envpol.2016.09.082 [DOI] [PubMed] [Google Scholar]
- 22.OSPAR Commission. OSPAR List of Substances Used and Discharged Offshore which Are Considered to Pose Little or No Risk to the Environment (PLONOR). London, UK: OSPAR Commission; 2013 [updated June; cited 2015 Nov]. Available from: http://www.ospar.org/documents?d=32939.
- 23.Shonkoff S, Maddalena R, Hays J, Stringfellow W, Wettstein Z, Harrison R, et al. Chapter 6: Potential Impacts of Well Stimulation on Human Health in California An Independent Scientific Assessment of Well Stimulation in California, Volume II: Generic and Potential Environmental Impacts of Well Stimulation Treatments. Sacramento, CA: California Council on Science and Technology; 2015. [Google Scholar]
- 24.Stringfellow WT, Cooley H, Varadharajan C, Heberger M, Reagan M, Domen JK, et al. Chapter 2: Impacts of Well Stimulation on Water Resources An Independent Scientific Assessment of Well Stimulation in California, Volume II: Generic and Potential Environmental Impacts of Well Stimulation Treatments. Sacramento, CA: California Council on Science and Technology; 2015. [Google Scholar]
- 25.Pattanayek M, DeShields B. Risk-Based Screening Levels for the Protection of Livestock Exposed to Petroleum Hydrocarbons, Publication Number 4733. Washington, D.C.: American Petroleum Institute; 2004 July.
- 26.Vengosh A, Jackson RB, Warner N, Darrah TH, Kondash A. A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol. 2014;48(15): 8334–48. doi: 10.1021/es405118y [DOI] [PubMed] [Google Scholar]
- 27.Long JCS, Birkholzer JT, Feinstein LC. Summary Report: An Examination of Hydraulic Fracturing and Acid Stimulations in the Oil and Gas Industry An Independent Scientific Assessment of Well Stimulation in California. Sacramento, CA: California Council on Science and Technology; 2015. [Google Scholar]
- 28.Shaffer DL, Chavez LHA, Ben-Sasson M, Castrillon SRV, Yip NY, Elimelech M. Desalination and Reuse of High-Salinity Shale Gas Produced Water: Drivers, Technologies, and Future Directions. Environ Sci Technol. 2013;47(17):9569–83. doi: 10.1021/es401966e [DOI] [PubMed] [Google Scholar]
- 29.South Coast Air Quality Management District (SCAQMD). Notification and Reporting Requirements for Oil and Gas Wells and Chemical Suppliers Rule 1148.2: South Coast Air Quality Management District; 2013 [cited 2016 March 15]. Available from: http://www.aqmd.gov/home/regulations/compliance/1148-2.
- 30.Hazardous Substances Data Bank: A TOXNET Database [Internet]. Bethesda, MD: U.S. National Library of Medicine, National Institute of Health; 2015 [cited 2015 Nov]. Available from: http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.
- 31.ChemIDplus Advanced: A TOXNET Database [Internet]. Bethesda, MD: U.S. National Library of Medicine, National Institute of Health; 2015 [cited 2015 Nov]. Available from: http://chem.sis.nlm.nih.gov/chemidplus/.
- 32.SciFinder [Internet]. Colombus, OH: American Chemical Society; 2014 [cited 2015 Nov]. Available from: https://scifinder.cas.org.
- 33.SRC I Physprop Database [Internet]. Syracuse, NY: SRC, Inc.; 2013 [cited 2015 Nov]. Available from: http://esc.syrres.com/fatepointer/search.asp.
- 34.World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans [Internet]. International Agency for Research on Cancer (IARC); 2015 [updated October 24; cited 2015 Nov]. Available from: http://monographs.iarc.fr/ENG/Classification/ClassificationsCASOrder.pdf.
- 35.Chemical Risk Information Platform (CHRIP) [Internet]. Tokyo, Japan: National Institute of Technology and Evaluation; 2015 [cited 2015 Nov]. Available from: http://www.nite.go.jp/en/chem/chrip/chrip_search/systemTop.
- 36.U.S. Environmental Protection Agency. EPI Suite™-Estimation Program, version 4.11,. Washington, D.C.: U.S. Environmental Protection Agency; 2012. Available from: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface.
- 37.Aggregated Computational Toxicology Resource (ACToR) Database [Internet]. Washington, D.C.: U.S. Environmental Protection Agency; 2015 [cited 2015 Nov]. Available from: http://actor.epa.gov/actor/faces/ACToRHome.jsp.
- 38.ECOTOXicology Database (ECOTOX) Release 4.0 [Internet], Washington, D.C.: U.S. Environmental Protection Agency; 2015 [cited 2015 Nov]. Available from: http://cfpub.epa.gov/ecotox/quick_query.htm.
- 39.European Chemicals Agency. International Uniform Chemical Information Database (IUCLID) Year 2000 Edition [CD- ROM]. 2000.
- 40.Office of Environmental Health Hazard Assessment. Current Proposition 65 List. Sacramento, CA; 2015 [cited 2015 Nov]. Available from: http://oehha.ca.gov/prop65/prop65_list/Newlist.html.
- 41.California Air Resources Board. Toxic Air Contaminant (TAC) Identification List. Sacramento, CA; 2010 [cited 2015 Nov]. Available from: http://www.arb.ca.gov/toxics/cattable.htm.
- 42.United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 4th Revised Edition. New York and Geneva; 2013. Report No.: ST/SG/AC.10/30/Rev.5.
- 43.Long J, Birkholzer J, Feinstein L. Summary Report: An Examination of Hydraulic Fracturing and Acid Stimulations in the Oil and Gas Industry Sacramento, CA: California Council on Science and Technology; 2015. [Google Scholar]
- 44.Long JCS, Feinstein LC, Birkholzer J, Jordan P, Houseworth J, Dobson PF, et al. An Independent Scientific Assessment of Well Stimulation in California, Volume I: Well Stimulation Technologies and their Past, Present, and Potential Future Use in California. Sacramento, CA: California Council on Science and Technology; 2015.
- 45.OSPAR Commission. List of Substances of Possible Concern. London, UK: OSPAR Commission; 2015 [cited 2015 Nov]. Available from: http://www.ospar.org/work-areas/hasec/chemicals/possible-concern/list.
- 46.OSPAR Commission. Common Interpretation on which Chemicals are Covered and not Covered by the Harmonised Mandatory Control System under OSPAR Decision 2000/2. London, UK: OSPAR Commission; 2002 [cited 2015 Nov]. Available from: www.ospar.org/documents?d=32731
- 47.Camarillo MK, Domen JK, Stringfellow WT. Physical-chemical evaluation of hydraulic fracturing chemicals in the context of produced water treatment. J Environ Manage. 2016;183: 164–74. doi: 10.1016/j.jenvman.2016.08.065 [DOI] [PubMed] [Google Scholar]
- 48.Kahrilas GA, Blotevogel J, Stewart PS, Borch T. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity. Environ Sci Technol. 2015;49(1): 16–32. doi: 10.1021/es503724k [DOI] [PubMed] [Google Scholar]
- 49.U.S. Environmental Protection Agency. 2012 Edition of the Drinking Water Standards and Health Advisories. Washington, D.C.; 2012 [cited 2015 Nov]. Report No.: EPA 822-S-12-001. Available from: http://water.epa.gov/action/advisories/drinking/upload/dwstandards2012.pdf.
- 50.U.S. Environmental Protection Agency. Contaminant Candidate List (CCL) 4 and Regulatory Determination. Washington, D.C.; 2015 [cited 2015 Nov]. Available from: http://www2.epa.gov/ccl/chemical-contaminants-ccl-4.
- 51.European Chemicals Agency. Candidate List of Substances of Very High Concern for Authorisation. Helsinki, Finland; 2015 [cited 2015 Nov]. Available from: http://echa.europa.eu/candidate-list-table.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total number of events is 1,187.
(PDF)
Total number of events is 1,187.
(PDF)
(PDF)
(PDF)
Data Availability Statement
Data are available from the South Coast Air Quality Monitoring District (http://www.aqmd.gov/). Additional data in Research Gate: DOI: 10.13140/RG.2.2.19128.55041.