Abstract
Spinal anaesthesia is the most preffered anesthesia technique for total hip replacement, and its complications range from low entity (insignificant) to life threatening. The incidence of neurologic complications after neuraxial anaesthesia is not perfectly clear, although there are several described cases of spinal cord ischaemia. We present a case of unilateral T8–T11 spinal cord ischaemia following L2–L3 spinal anaesthesia for total hip replacement. Magnetic resonance imaging showed a hyperintense T8–T11 signal alteration on the leftside of paramedian spinal cord. A temporal epidemiologic linkage between the damage and the surgery seems to be present. The injury occurred without anatomical proximity between the injury site and the spinal needle entry site. This may be due to multiple contributing factors, each of them is probably not enough to determine the damage by itself; however, acting simultaneously, they could have been responsible for the complication. The result was unpredictable and unavoidable and was caused by unforeseeable circumstances and not by inadequate medical practice.
Keywords: Spinal cord injuries, spinal anaesthesia, arthroplasty, hip replacement, spinal cord ischaemia, ischaemic myelopathy
Introduction
Hip surgery is a commonly performed orthopaedic procedure. Spinal anaesthesia (SA) is the most preffered anesthesia tecnique for total hip replacement (THR). This is because it is associated with reduced mortality when compared to general anaesthesia tecnique (1). SA-related complications range from insignificant to life threatening. A large survey in France revealed that the incidence of serious spinal (and epidural) complications were relatively low (2). In this survey, among 40640 performed procedures, the most frequent serious complication was cardiac arrest (n=26), followed by radiculopathy (n=19), death (n=6) and cauda equina syndrome (n=5). No cases of seizures and paraplegia were reported (n=0). Non-life-threatening complications include moderate hypotension, bradycardia, high neural blockade, urinary retention, inadequate anaesthesia, intravascular injection, subdural injection, backache, post-dural puncture headache, haematoma and infection (3).
The incidence of neurological complications after neuraxial anaesthesia is not perfectly clear (0–0.08%) (2,4). Furthermore, spinal cord (SC) ischaemia is a rare event when compared to cerebral ischaemia. In the literature, cases of SC ischaemia after SA with and without hypotension have been described (5,6). Additionally, cases unrelated to SA due to hypotension only (7), after CABG surgery (8) or even cases of spontaneous SC ischaemia have been described (9).
In this report, we present a case of unilateral T8–T11 SC ischaemia following lumbar SA for THR.
Case Presentation
A 79-year-old man (weight: 72 kg; height: 165 cm) was admitted to the hospital for THR. His comorbidities were osteoarthritis, diabetes, grade I hypertension, dyslipidaemia and previous stroke with temporary left hemiparesis. Blood test results and chest X-rays showed no alterations; an electrocardiogram (ECG) showed left anterior fascicular block and right bundle branch block. Informed consent was obtained, and standard monitoring was applied (SpO2, NIBP, ECG and diuresis). Midazolam (5 mg) for premedication and cefazolin (2 g) for antibiotic prophylaxis were administered. An L2–L3 SA was performed with a 27 G Sprotte needle by injecting 15 mg of 0.5% isobaric levobupivacaine after the patient was positioned to left lateral decubitus. After 15 min, a T10 level anaesthesia was obtained, and tranexamic acid (2 g) was administered for reducing the amount of bleeding. THR was performed in 60 min; no complications were recorded. His blood pressure remained above 100/60 mmHg and his heart rate around 70 bpm. The patient was transferred to the recovery room with stable vital signs and was then discharged to a ward with an Aldrete score of 10; he was able to move all four limbs on command.
During the first post-operative day, he was unable to move his left lower limb, despite the preservation of tactile sensation. Osteotendinous reflexes and urination were absent. Magnetic resonance imaging (MRI) showed a hyperintense T8–T11 signal alteration on the leftside of paramedian SC (Figure 1). Electromyography confirmed severe subacute axonal damage due to active denervation in the left sciatic territory and a less severe neurologic damage of the left quadriceps muscle. On the right side, iatrogenic damage of chronic S1 root was also described.
Figure 1.
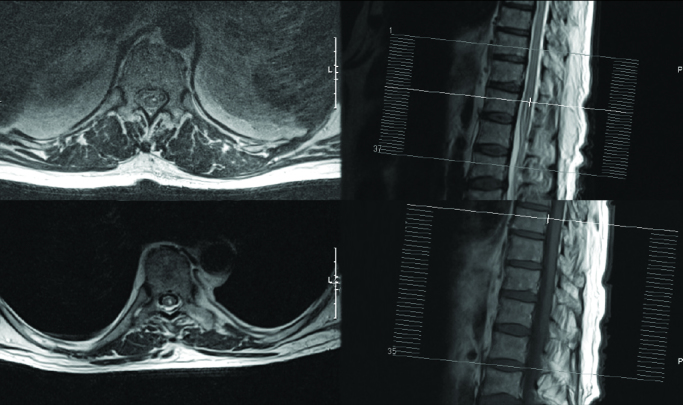
Upper pictures: MRI, 1st post-operative day. Bottom pictures: MRI, 13th post-operative day
MRI: magnetic resonance imaging
Discussion
Despite high-grade surgery and the low complication rate in SA, some concepts have to be clarified. Firstly, the patient had no major contraindications for THR. Regarding the chosen anaesthetic technique, recent works have suggested regional anaesthesia as the main choice for femur and hip fracture surgeries (3,10). Moreover, the spinal anesthesia procedure was performed without any complication or issue. A temporal epidemiologic linkage between the neural damage and the THR seems to be present.
Other factors may be related to SC injury. Hypotension is one of the most common SA adverse effects, although it is not the most serious. This is due to the sympathetic blockade, and it is associated with bradycardia if high thoracic levels are involved. Both have a rapid onset (3–5 min after puncture) and potentially last longer than muscular blockade. There is no pressure cut-off for SC ischaemia, but the risk increases if the systolic pressure falls below 60–80 mmHg (11).
Mild hypotension could also be harmful for a patient who is hypertensive for long. We remind the reader that the patient was affected by grade I hypertension only and that the systolic pressure did not fall below 100 mmHg, while the heart rate remained around 70 bpm, which excluded hypotension as the only cause of SC ischaemia.
Anterior spinal artery syndrome is an entity that occurs due to the occlusion of the anterior spinal artery tract. The artery of Adamkiewicz is its major contributor, along with multiple branches coming from the vertebral, cervical, intercostal, lumbar and sacral arteries. If any occlusion occurs, manifestations are usually bilateral paraparesis, loss of reflexes, loss of pain and temperature sensation and loss of sphincter control with vibration and position sense sparing (12). If the artery of Adamkiewicz is occluded, ischaemia to the lower two-thirds of the SC will occur. Even though there is a wide inter-variability in SC blood supply, any other segmental occlusion will most likely be evident with bilateral signs and symptoms. Although extremely rare, unilateral muscle paralysis due to unilateral SC ischaemia might also be observed (13).
The patient had unilateral damage limited to T8–T11, so an occlusion of the artery of Adamkiewicz as well as the anterior spinal artery has been excluded.
Needle injury, hyperlordosis, large-volume neuraxial anaesthesia and prolonged lateral decubitus may impair SC perfusion or cause damage. Needle injury was excluded because the needle used was atraumatic and was one of the smallest available for adults and because the technique was performed at L2–L3, while the damage occurred at T8–T11.
Hyperlordosis was not applied to the patient. To perform SA, only 3 mL was injected into the intrathecal space, excluding ‘large volume’ as a cause. The only cause that cannot be excluded was prolonged left lateral decubitus, which was also the injured side. There is an increased risk of vessel puncture during SA if it is performed on the right lateral decubitus. However, no information is present in the literature about SC injuries after SA which was performed on the left lateral decubitus.
The role of tranexamic acid remains controversial; in the literature, there is no evidence of increased vascular thrombosis in treated patients compared to non-treated ones (14–16).
Axonal direct local anaesthetic (LA) toxicity may be suspected; however, there is not any study regarding this issue except for the ones that have been performed on rats. (17). Direct LA toxicity is, however, unlikely because the LA used was isobaric and no migration from L2–L3 to T8–T11 was expected.
The presence of possible causes listed above can lead to the hypothesis of medical responsibility for damage genesis. Each of the patient’s potential causal factors is not enough to determine the injury by themselves. Furthermore, the complication that occurred was unique and unpredictable, and the necessary conditions to determine the injury would not have been taken into consideration.
Conclusion
There are no previous similar reports in the literature; it (the injury) occurred without anatomical proximity between the injured site and the spinal needle entry site. The damage may have been due to multiple contributing factors acting simultaneously, each of them probably was not enough to determine damage by themselves; however, all acting simultaneously, they could have been responsible for the complication. The result was unpredictable and unavoidable and was caused by unforeseeable circumstances and not by inadequate medical practice.
Footnotes
Informed Consent: Written informed consent was obtained from patient who participated in this case.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.G., A.F.E., V.A.M.; Design - C.F., V.A.M., D.B.R.; Supervision - A.F.E., V.A.M., T.V.; Resources - C.F., D.B.R., A.F.E., T.V., V.A.M., R.G.; Materials - C.F., D.B.R., A.F.E., T.V., V.A.M., R.G.; Data Collection and/or Processing - V.A.M., T.V.; Analysis and/or Interpretation - V.A.M., T.V.; Literature Search - C.F., V.A.M., D.B.R., T.V.; Writing Manuscript - C.F., D.B.R., V.A.M.; Critical Review - C.F., D.B.R., A.F.E., T.V., V.A.M., R.G.; Other - C.F., D.B.R., A.F.E., T.V., V.A.M., R.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Hunt LP, Ben-Shlomo Y, Clark EM, Dieppe P, Judge A, MacGregor AJ, et al. 90-day mortality after 409,096 total hip replacements for osteoarthritis, from the National Joint Registry for England and Wales: a retrospective analysis. Lancet. 2013;382:1097–104. doi: 10.1016/S0140-6736(13)61749-3. https://doi.org/10.1016/S0140-6736(13)61749-3. [DOI] [PubMed] [Google Scholar]
- 2.Auroy Y, Narchi P, Messiah A, Litt L, Rouvier B, Samii K. Serious complications related to regional anesthesia: results of a prospective survey in France. Anesthesiology. 1997;87:479–86. doi: 10.1097/00000542-199709000-00005. https://doi.org/10.1097/00000542-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Guay J, Parker MJ, Gajendragadkar PR, Kopp S. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst Rev. 2016;2:CD000521. doi: 10.1002/14651858.CD000521.pub3. https://doi.org/10.1002/14651858.cd000521.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DC, Bridenbaugh LD. Spinal (subarachnoid) block. A review of 11,574 cases. JAMA. 1966;195:907–12. doi: 10.1001/jama.195.11.907. https://doi.org/10.1001/jama.1966.03100110075019. [DOI] [PubMed] [Google Scholar]
- 5.Zaphiratos V, McKeen DM, Macaulay B, George RB. Persistent paralysis after spinal anesthesia for cesarean delivery. J Clin Anesth. 2015;27:68–72. doi: 10.1016/j.jclinane.2014.08.003. https://doi.org/10.1016/j.jclinane.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Vermin B, van Poorten F, Stienstra R. Paraplegia following a transurethral prostate resection: the anterior spinal artery syndrome. Ned Tijdschr Geneeskd. 2009;153:A72. [PubMed] [Google Scholar]
- 7.Vongveeranonchai N, Zawahreh M, Strbian D, Sundararajan S. Evaluation of a patient with spinal cord infarction after a hypotensive episode. Stroke. 2014;45:203–5. doi: 10.1161/STROKEAHA.114.006490. https://doi.org/10.1161/STROKEAHA.114.006490. [DOI] [PubMed] [Google Scholar]
- 8.Sevuk U, Kaya S, Ayaz F, Aktas U. Paraplegia due to spinal cord infarction after coronary artery bypass graft surgery. J Card Surg. 2016;31:51–6. doi: 10.1111/jocs.12666. https://doi.org/10.1111/jocs.12666. [DOI] [PubMed] [Google Scholar]
- 9.Bekci T, Yucel S, Aslan K, Gunbey HP, Incesu L. “Snake eye” appearance on a teenage girl with spontaneous spinal ischemia. Spine J. 2015;15:e45. doi: 10.1016/j.spinee.2015.06.002. https://doi.org/10.1016/j.spinee.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Guideline on the management of hip fracture in older people. Scottish Intercollegiate Guidelines Network (SIGN); 2009. [Google Scholar]
- 11.Neal JM. Anatomy and pathophysiology of spinal cord injury associated with regional anesthesia and pain medicine. Reg Anesth Pain Med. 2008;33:423–34. doi: 10.1016/j.rapm.2006.10.014. https://doi.org/10.1016/j.rapm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Novy J. Spinal cord syndromes. Front Neurol Neurosci. 2012;30:195–8. doi: 10.1159/000333682. https://doi.org/10.1159/000333682. [DOI] [PubMed] [Google Scholar]
- 13.Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006;63:1113–20. doi: 10.1001/archneur.63.8.1113. https://doi.org/10.1001/archneur.63.8.1113. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. https://doi.org/10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829. https://doi.org/10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskal JT, Capps SG. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics. 2016:1–10. doi: 10.3928/01477447-20160526-02. https://doi.org/10.3928/01477447-20160526-02. [DOI] [PubMed] [Google Scholar]
- 17.Takenami T, Wang G, Nara Y, Fukushima S, Yagishita S, Hiruma H, et al. Intrathecally administered ropivacaine is less neurotoxic than procaine, bupivacaine, and levobupivacaine in a rat spinal model. Can J Anaesth. 2012;59:456–65. doi: 10.1007/s12630-012-9685-9. https://doi.org/10.1007/s12630-012-9685-9. [DOI] [PubMed] [Google Scholar]


