Abstract
Objective
The aim of the present study is to compare the effect of 20% mannitol and 3% NaCl on blood coagulation in vitro using rotational thromboelastometry (ROTEM).
Methods
Twenty-millilitre blood samples were obtained from 15 volunteers. In each group, 2 mL blood samples were collected into both polypropylene tubes and EDTA tubes for ROTEM and hemogram analysis. After sampling, blood samples were diluted with test solutions. Group C (control): Only blood, Group M (mannitol): 7% vol 20% mannitol concentration in the blood, Group hypertonic saline (HS): 7% vol 3% hypertonic saline (NaCl) in the blood, Group M/H (mannitol and hydroxyethyl starch solutions [HES]): 6% vol 20% mannitol concentration and 8% vol HES in the blood and Group HS/H (hypertonic saline and HES): 6% vol 3% hypertonic saline concentration and 8% vol HES in the blood. The following thromboelastometric parameters were measured automatically: clotting time (CT) and clot formation time (CFT) with intrinsic activation by tissue factor (InTEM), CT, CFT and maximum clot firmness (MCF) with extrinsic activation by tissue factor (ExTEM) and MCF with FibTEM.
Results
The ExTEM CT value was found to be significantly longer in the M/H group than in the controls. The ExTEM CFT median and percentile values were: group C: 85 s (70–95 s), group M: 115 s (94–128 s), group HS: 102 s (84–114 s), group M/H: 128 s (110–144 s) and group HS/H: 118 s (107–132 s). In all the groups, FibTEM MCF values were significantly lower than the control and also there was a significant difference between groups M and HS according to FibTEM MCF values.
Conclusion
Whole-blood coagulation disorder induced by these solutions is mainly dependent on fibrinogen and fibrin interaction. However, 3% HS has much less negative effect on coagulation.
Keywords: 20% mannitol, 3% NaCl, coagulation, neuroanaesthesia
Introduction
Mannitol and hypertonic saline (HS) are commonly used to reduce brain oedema and intracranial pressure and to minimise brain volume in neurosurgical practise (1). Mannitol is recommended as a first-choice hyperosmotic agent (2). However, clinical trials in recent years have shown that HS is at least as effective as, if not better than, mannitol in the treatment of intracranial hypertension (3, 4). Twenty per cent mannitol (1,098 mOsm L−1) and 3% HS (1,024 mOsm L−1) are commonly used hyperosmotic fluids in our clinic, and many centres have found almost the same osmolarity at the same volume (5). Previous in vitro and clinical studies demonstrated that both fluids cause coagulation impairment (clot strength, disturbs fibrin formation and platelet function) and that the degree of coagulopathy increases linearly with the amount of fluid used (6, 7). However, the studies evaluated these hypertonic solutions separately and not with each other. In the literature, only one study has compared the effect of mannitol and HS on coagulation (1). Luostarinen et al. (1) investigated the effects of both fluids at doses higher than used intraoperatively and did not consider the effects of colloid addition. In cranial surgeries, mannitol 1 gr kg−1 (or less) and equivalent HS is used as an anti-oedematous therapy. Colloid use can be necessary during neurosurgery because of bleeding and hypotension. Rapidly degradable hydroxyethyl starch solutions (HES 130/0.4) also have negative effects on coagulation (8). In the present study, we simulated giving 5 mL kg−1 (1 gr kg−1) 20% mannitol and 3% HS to a healthy 70–80 kg patient in an in vitro environment for the first time.
The primary aim of the present study was to compare the effect of 20% mannitol with 3% HS on blood coagulation in vitro using rotational thromboelastometry (ROTEM). Additionally, we investigated the effect of adding HES 130/0.4 to these hypertonic solutions on blood coagulation.
Methods
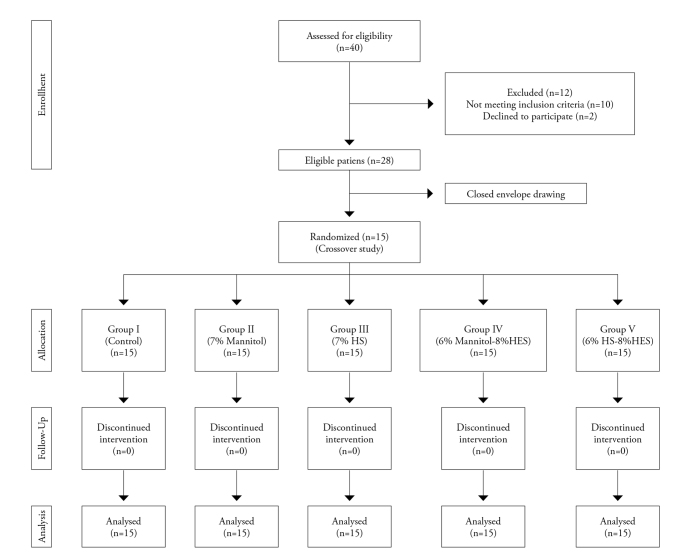
Ethical approval for this study (Ethics Committee No. 2014/1619) was provided by the Ethic Committee on 24 October 2014. The trial was conducted in accordance with guidelines of the Helsinki Declaration on Human Subjects and was registered at anzctr.org.au (ACTRN12615000005550). Written informed consent was obtained from all volunteers. Forty healthy volunteers aged between 18 and 65 years were evaluated for inclusion in the study. Twenty-eight appropriate volunteers were found, and of these 28, 15 were chosen using closed-envelope selection. Inclusion criteria for the study were no history of chronic or acute diseases (e.g. coagulation disorder, renal or hepatic failure); no intake of any medication, in particular acetylsalicylic acid or nonsteroidal anti-inflammatory agents within 1 week of the study; no alcohol or drug abuse and smoking; and haematocrit (Hct), activated partial thromboplastin time (aPTT), international normalised ratio (INR) and platelet values within the normal range (Figure 1). The demographic characteristics of the volunteers who were included in the study were recorded.
Figure 1.
Flow chart of the study
Randomisation and blinding
Blood samples were taken from 15 volunteers by the first researcher in a random order that was determined in a draw. Test solutions were added to the blood samples by the first researcher and the samples were coded. The second researcher made the thromboelastometric analysis, but he/she did not know to which group and volunteer the blood samples belonged. At the end of the research, the two researchers compared their data.
Trial method
Twenty-millilitre blood samples were obtained from an antecubital vein using an 18-G venous cannula (Vasofix™, B. Braun, Melsungen, Germany) from each participant. Four-millilitre blood samples were separated for each group. In each group, 2 mL of blood was collected into polypropylene tubes (Vacuette, Greiner Bio-one, Austria) containing 3.2% buffered citrate, giving a volume ratio of 1:10 for ROTEM analysis, and 2 mL blood was collected into EDTA tubes containing 1.2 mg anhydrous EDTA per 1 mL blood (Vacuette, Greiner Bio-one, Austria) for haemogram analysis. Transferpette® (BRAND, Wertheim, Germany) Single-channel microliter, electronic pipette was used to make the mixtures. Immediately after sampling, all blood samples were diluted with the test solutions (Figure 2). In the present study, the groups were created by simulating giving 375 cc 20% mannitol (1 gr kg−1) or 375 cc 3% NaCl or/and 500 cc HES to a 75 kg adult patient. The total blood volume was considered as 5,250 mL (75 mL kg−1). Accordingly, the groups were assigned as follows:
Figure 2.

Composition of dilutions
Group C (control): only blood
Group M (Mannitol): 7% volume ratio of 20% mannitol in blood
Group HS: 7% volume ratio of 3% HS (NaCl) in blood
Group M/H (mannitol and HES): 6% volume ratio of 20% mannitol and 8% volume ratio of HES 130/0.4 in blood
Group HS/H (HS and HES): 6% volume ratio of 3% HS and 8% volume ratio of HES 130/0.4 in blood.
Thromboelastometric coagulation analysis was performed within 2 h of blood withdrawal (ROTEM; Pentapharm Co., Munich, Germany) in a random order for all samples. The following tests were performed in accordance with the manufacturer’s instructions: intrinsic activation by tissue factor (InTEM), extrinsic activation by tissue factor (ExTEM) and extrinsic activation by tissue factor (FibTEM) with the addition of cytochalasin D to inhibit platelet function and display fibrin polymerisation only. Coagulation was allowed to proceed for 30 min. The following thromboelastometric parameters were measured automatically: clotting time (CT) and clot formation time (CFT) with InTEM; CT, CFT and maximum clot firmness (MCF) with ExTEM and MCF with FibTEM. Moreover, the samples in the EDTA tubes were mixed with the test solutions, and haemoglobin (Hb) concentration, haematocrit value (Hct) and platelet count (Pc) were determined for each group using a Cell-Dyn 610 Hematology Analyzer (Sequoia-Turner Corporation, Mountain View, CA, USA).
Statistical analysis
Previous studies showed that whole-blood coagulation disorder induced by mannitol and HS is mainly dependent on the final fibrinogen–fibrin interaction (1). Therefore, our primary outcome was the comparison of the effects of mannitol and HS on FibTEM MCF values. The number of volunteers was based on earlier data (9). In a crossover study, at least 12 volunteers would be needed to discover greater than 1 standard deviation (SD) difference in FibTEM MCF between the HS and mannitol groups with an α error of 0.05 and power of 80%. We also made a post hoc analysis according to the FibTEM MCF values (12.1±2.3 for the mannitol and 14.7±2 for HS groups); our study power was 89% with α error of 0.05. Sample size analysis was made using G Power version 3.1.7. Distribution of data was evaluated using the kurtosis and skewness test. Data are presented as mean±SD for normally distributed quantitative data and as median and percentage (25%–75%) where the quantitative data were not normally distributed. Qualitative data are presented as the number and percentage of cases. The statistical analyses performed were: the Mann–Whitney U test and the Kruskal–Wallis test for non-normally distributed data and one-way ANOVA and Tukey test for normally distributed data. p<0.05 was considered statistically significant. All statistical analyses were conducted using Statistical Package for the Social Sciences for Windows version 15.0 (SPSS Inc; Chicago, IL, USA).
Results
Nine male and six female volunteers were enrolled in the study. The volunteers’ mean age and body mass index (BMI) was 26.6±3.7 years and 24.2±2.9 kg m2−1, respectively. All voluntaries’ baseline (Group C) Hb, Hct and Pc values were within the normal range. In the groups M/H and HS/H, there was a significant decrease in Hb, Hct and Pc values compared with baseline values (Group C) because of the haemodilution (Table 1).
Table 1.
Hemodilution data
| Hb (g dL−1) | Hct (%) | Pc (109/L) | |
|---|---|---|---|
| Group C | 13.2±1.2 (100%) | 36.9±3.1 (100%) | 231.3±31.4 (100%) |
| Group M | 12.6±1 (95%) | 35.5±2.8 (96%) | 208.2±30.5 (90%) |
| Group HS | 12.7±0.9 (96%) | 35.6±2.7 (96%) | 209.6±29.9 (90%) |
| Group M/H | 11.2±1.1 (84%)*¥ | 31.5±3.1 (85%)*¥ | 193.5±30 (83%)*¥ |
| Group HS/H | 11.3±1.1 (85%)*¥ | 31.7±3.2 (85%)*¥ | 194.6±30.1 (83%)*¥ |
All values are normally distributed. Data are mean±SD and percentage of undiluted value.
ANOVA test and Tukey test were used for comparison.
p<0.001 compared with control.
p<0.05 compared with groups M and HS.
Hb: haemoglobin; Hct: hematocrite; Pc: platelet count.
Intrinsic and extrinsic coagulation pathways data
Computed tomography and CFT values were similar in all groups measured using InTEM (p>0.05). The CT value was only found higher in group M/H than in group C measured using ExTEM; the difference was significant (p=0.02). Median and percentiles (25%–75%) for CFT values measured using ExTEM for groups C, M, HS, M/H and HS/H were 85 s (70–95 s), 115 s (94–128 s), 102 s (84–114 s), 128 s (110–144 s) and 118 s (107–132 s), respectively (Figure 3).
Figure 3.

a, b. CT (a) and CFT (b) values in ExTEM. A, Values were normally distributed. ANOVA test and Tukey test were used for comparison. *: p<0.05 compared with the control group. b, Values were not normally distributed. The Kruskal–Wallis test was used for comparison. *: p<0.05 compared with the control group. ¶p<0.001 compared with the control group. ¥p<0.05 compared with group HS
Platelet function and fibrinogen--fibrin interaction data
Maximum clot firmness values measured using ExTEM in groups M/H and HS/H were significantly lower than in the control group (Figure 4). MCF values measured using FibTEM were determined to be significantly less in groups M, HS, M/H and HS/H than in the control group (per cent less than the control: 30%, 13%, 50% and 46%, respectively) (Figure 4). Also, the MCF FibTEM value was significantly lower in group M than in group HS (p=0.026). The numbers of the cases of abnormal values in all groups are specified in Table 2.
Figure 4.

ExTEM and FibTEM MCF values. Values were normally distributed. ANOVA and Tukey test used for comparison. *: p<0.05 compared with the control group. ¶: p<0.001 compared with the control group. ¥: p<0.05 compared with the group HS. β: p<0.001 compared with groups M and HS
Table 2.
Distribution of abnormal values
| InTEM CT |
InTEM CFT |
ExTEM CT |
ExTEM CFT |
ExTEM MCF |
FibTEM MCF |
|
|---|---|---|---|---|---|---|
| Group C | 0 | 0 | 0 | 0 | 0 | 0 |
| Group M | 0 | 0 | 0 | 1 | 0 | 2 |
| Group HS | 0 | 0 | 0 | 0 | 0 | 0 |
| Group M/H | 0 | 0 | 1 | 3 | 1 | 6 |
| Group HS/H | 0 | 0 | 0 | 1 | 1 | 3 |
Data are number of volunteers.
CT: clotting time; CFT: clot formation time; MCF: maximum clot firmness
Discussion
The present study showed that:
20% mannitol, 3% HS and the combination with HES impairs blood coagulation in vitro,
fluids mostly affected the fibrinogen--fibrin interaction and,
3% HS with 7% vol hemodilution has no significant effect on intrinsic and extrinsic coagulation pathways and impairs fibrinogen--fibrin interaction less than 20% mannitol with 7% vol hemodilution in vitro.
Traditional laboratory coagulation tests (e.g. Pc, prothrombin time, aPTT) are not enough to determine coagulation disorders (10). Contrary to this, ROTEM analysis as a point-of-care test can evaluate the nature of coagulation disturbance in whole-blood samples. In the present study, three different activators (InTEM, ExTEM and FibTEM) were used to examine the intrinsic and extrinsic coagulation pathways, platelet function and fibrinogen--fibrin interaction. Previous studies showed that HES, 20% mannitol and 3% HS caused coagulation impairment (7, 11, 12). When we searched the literature, we found two deficiencies:
Previous studies investigated 10% and more dilutions of blood using 20% mannitol and 3% HS in separate studies. In the intraoperative period, maximum 1 gr kg−1 mannitol or equivalent 3% HS is used, and this dose creates dilutions of 7% and less.
Only one study compared mannitol and HS in vitro together. However, Luostarinen et al. (1) simulated higher doses than would normally be used for anti-oedematous therapy in the intraoperative period.
In the present study, we have shown that the combination of 20% mannitol and 3% HS does not affect the intrinsic coagulation pathway at doses used intraoperatively for anti-oedematous therapy according to data obtained from the InTEM analysis. In the extrinsic coagulation pathway, which we examined using ExTEM, we found that 20% mannitol significantly decreased CFT values compared with the control group. There was no significant difference between 3% HS and 20% mannitol, but we observed that 3% HS had a slight effect on the extrinsic coagulation pathway. Additionally, adding HES to this fluid severely increased the negative impact on the extrinsic coagulation pathway by increasing hemodilution. In previous studies, no InTEM analysis has been performed but data gained using ExTEM analysis were similar to that in the present study (1, 6, 13). We found disruption of platelet function only when mannitol or HS was combined with HES solution.
We also determined that our study solutions had main effects on the fibrinogen--fibrin interaction. We observed that the negative effect of 3% HS on functional fibrinogen--fibrin interaction was less than with 20% mannitol. All of the fluids that we have used in the study impair coagulation, depending on their molecular properties (especially the high osmolarity) and through haemodilution (12). These results indicated that whole-blood coagulation disorder induced by mannitol and HS is mainly dependent on the final fibrinogen--fibrin interaction, which results in a fibrin-poor clot. Also, HES solutions impair fibrinogen--fibrin interaction in vitro (9). According to that, mannitol or HS in combination with HES in vitro induces more whole-blood coagulation disorder. This complicates the control of intraoperative bleeding and increases the risk of postoperative haematoma. In previous studies, no difference was found between the anti-oedematous effects of 20% mannitol and 3% HS (3, 4). For that reason, any one of these hypertonic fluids can be used depending on the choice of the physician. Our study is the first to compare the effects of these two fluids on coagulation at doses used in the intraoperative period. According to the data of this study, 3% HS caused less blood coagulation disorder.
Similar to our study, Luostarinen et al. (1) showed that HS is more problematic than mannitol with respect to coagulation. This study also showed that the use of 3% HS could be a better choice in patients with abnormal bleeding tendency and/or patients who are under a high risk of bleeding surgery. Synthetic colloids could be used in patients with severe bleeding. Our study results support that HES combined with HS is safer than HES combined with mannitol. When we compared the data of our study with other studies that used much higher doses, the amount of hyperosmolar fluid is proportional to the severity of blood coagulation disorder (1, 6, 10, 13).
Study limitations
In vitro studies provide for better standardisation. However, we can only show the effects of mannitol and HS on coagulation that occurs immediately after administration. The administration of mannitol or HS is associated with fluid shifts between different compartments of the body, drawing water from the intracellular compartment into the intravascular compartment and promoting diuresis. This study does not take into account this dynamic process. Because of that, the present study should be supported by clinical trials.
Conclusion
Our results indicate that 3% HS is more suitable than 20% mannitol during cranial surgery in terms of coagulation. Especially for surgeries in which severe bleeding is expected (e.g. intracranial haemangioma, large tumours and tumours associated with vessels), we recommend using 3% HS as an anti-oedematous therapy and, if possible, to decrease the amount of hyperosmotic fluid. Moreover, we should keep in mind that HES used with mannitol or 3% HS may cause more severe coagulation problems.
Acknowledgements
The authors are grateful to Mr. David Chapman who edited the language. Thank you to Istanbul University Scientific Research Projects Unit (BAP) for financial support.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of İstanbul University İstanbul School of Medicine (No: 2014/1619).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – A.A.; Design – A.A., B.Ş.; Supervision – İ.Ö.A. Resources – A.T., P.A.S., L.R.D.; Materials – A.A.; Data Collection and/or Processing – B.Ş., A.A.; Analysis and/or Interpretation – A.A., P.A.S., A.T., B.Ş., İ.Ö.A.; Literature Search – B.Ş., A.A., İ.Ö.A.; Writing Manuscript – A.A., B.Ş., İ.Ö.A.; Critical Review – İ.Ö.A.; Other – A.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: It was supported by İstanbul University Scientific Research Projects Unit (BAP).
References
- 1.Luostarinen T, Niiya T, Schramko A, Rosenberg P, Niemi T. Comparison of hypertonic saline and mannitol on whole blood coagulation in vitro assessed by thromboelastometry. Neurocrit Care. 2011;14:238–43. doi: 10.1007/s12028-010-9475-6. https://doi.org/10.1007/s12028-010-9475-6. [DOI] [PubMed] [Google Scholar]
- 2.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. J Neurotrauma. 2000;17:463–9. doi: 10.1089/neu.2000.17.463. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S, Schwab S, Bertram M, Aschoff A, Hacke W. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke. 1998;29:1550–5. doi: 10.1161/01.str.29.8.1550. https://doi.org/10.1161/01.STR.29.8.1550. [DOI] [PubMed] [Google Scholar]
- 4.Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients: A randomized clinical trial. Crit Care. 2005;9:530–40. doi: 10.1186/cc3767. https://doi.org/10.1186/cc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107:697–704. doi: 10.1097/01.anes.0000286980.92759.94. https://doi.org/10.1097/01.anes.0000286980.92759.94. [DOI] [PubMed] [Google Scholar]
- 6.Lindroos AC, Schramko A, Tanskanen P, Niemi T. Effect of the combination of mannitol and ringer acetate or hydroxyethyl starch on whole blood coagulation in vitro. J Neurosurg Anesthesiol. 2010;22:16–20. doi: 10.1097/ANA.0b013e3181bd4ede. https://doi.org/10.1097/ANA.0b013e3181bd4ede. [DOI] [PubMed] [Google Scholar]
- 7.Wilder DM, Reid TJ, Bakaltcheva IB. Hypertonic resuscitation and blood coagulation: in vitro comparison of several hypertonic solutions for their action on platelets and plasma coagulation. Thromb Res. 2002;107:255–61. doi: 10.1016/s0049-3848(02)00335-3. https://doi.org/10.1016/S0049-3848(02)00335-3. [DOI] [PubMed] [Google Scholar]
- 8.Schramko AA, Suojaranta-Ylinen RT, Kuitunen AH, Kukkonen SI, Niemi TT. Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesth Analg. 2009;108:30–6. doi: 10.1213/ane.0b013e31818c1282. https://doi.org/10.1213/ane.0b013e31818c1282. [DOI] [PubMed] [Google Scholar]
- 9.Niemi TT, Kuitunen AH. Artificial colloids impair haemostasis. An in vitro study using thromboelastometry coagulation analysis. Acta Anaesthesiol Scand. 2005;49:373–8. doi: 10.1111/j.1399-6576.2005.00619.x. https://doi.org/10.1111/j.1399-6576.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 10.Bischof D, Dalbert S, Zollinger A, Ganter MT, Hofer CK. Thrombelastography in the surgical patient. Minerva Anestesiol. 2010;76:131–7. [PubMed] [Google Scholar]
- 11.Casutt M, Kristoffy A, Schuepfer G, Spahn DR, Konrad C. Effects on coagulation of balanced (130/0.42) and non-balanced (130/0.4) hydroxyethyl starch or gelatin compared with balanced Ringer’s solution: an in vitro study using two different viscoelastic coagulation tests ROTEMTM and SONOCLOTTM. Br J Anaesth. 2010;105:273–81. doi: 10.1093/bja/aeq173. https://doi.org/10.1093/bja/aeq173. [DOI] [PubMed] [Google Scholar]
- 12.Hanke AA, Maschler S, Schöchl H, Flöricke F, Görlinger K, Zanger K, et al. In vitro impairment of whole blood coagulation and platelet function by hypertonic saline hydroxyethyl starch. Scand J Trauma Resusc Emerg Med. 2011;19:12. doi: 10.1186/1757-7241-19-12. https://doi.org/10.1186/1757-7241-19-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan TS, Tan KH, Ng HP, Loh MW. The effects of hypertonic saline solution (7.5%) on coagulation and fibrinolysis: an in vitro assessment using thromboelastography. Anaesthesia. 2002;57:644–8. doi: 10.1046/j.1365-2044.2002.02603.x. https://doi.org/10.1046/j.1365-2044.2002.02603.x. [DOI] [PubMed] [Google Scholar]