Abstract
Purpose
Soluble cytotoxic T-lymphocyte antigen 4 (sCTLA-4), one of the isoforms of CTLA-4, was discovered to be critical in downregulating the negative signal of CTLA-4 in T-cell responses. Contrary to the classical immunosuppressive effect of CTLA-4, its immunoregulatory function might be complicated. However, the clinical significance of sCTLA-4 to immune regulation and the variation in cancer therapy have not been elucidated. We postulated that the level of sCTLA-4 might affect the outcome of cancer prognosis.
Patients and methods
Serum concentrations of sCTLA-4 before and after therapy in 141 locally advanced and advanced cancer patients were measured and survival analyses was performed. Hazard ratio and 95% confidence interval for overall survival (OS) were calculated. Cutoffs were determined by median across the sCTLA-4 level of entire patients.
Results
High expression of sCTLA-4 after therapy indicated significant longer OS and progression-free survival (PFS) (all P<0.01). Among all subgroups, sCTLA-4 levels after therapies were found to be significantly higher than that of 1 day before, which was also negatively correlated with tumor node metastasis stage and lymph node metastasis (all P<0.05). Multivariate analysis revealed that sCTLA-4 level was a strong independent prognostic factor for OS and PFS (all P<0.05).
Conclusion
Our data demonstrated the favorable prognostic significance of sCTLA-4 and may lead to the development of new immunotherapy options for cancer patients.
Keywords: soluble cytotoxic T-lymphocyte antigen 4, immunotherapy, survival, cancer therapy, cancer prognosis
Introduction
Tumor-derived immune dysregulation is a key obstacle for antitumor therapies.1 Cancer cells utilize the immune checkpoints to avoid the attack from immune system. The immunosuppressive microenvironment derived from cancer cells consists of cytokines and immune checkpoint molecules that can block antitumor immunity.2,3 One of these immune checkpoint molecules is cytotoxic T-lymphocyte antigen 4 (CTLA-4, cluster of differentiation [CD] 152). CTLA-4 is an inhibitory receptor that works as a “switch-off” for T-cell-targeting tumor antigens.4 Several research works have reported that CTLA-4 was found to be correlated with poor survival in cancer patients. Recent preclinical and clinical studies have proved the value of anti-CTLA-4 drug including ipilimumab, which can be successful for cancer immunotherapy.5–7
As negative feedback to maintain immune self-tolerance and homeostasis, different CTLA-4 isoforms reduce T-cell activation through either intrinsic or extrinsic regulation of T-cell activity. Generally, CTLA-4 is known as an immunoregulatory receptor, and it can also be generated as a naturally secreted soluble form. Soluble CTLA-4 (sCTLA-4), produced by alternatively spliced messenger RNA (mRNA), was identified some years after the original description of CTLA-4 and has been detected in lymph nodes, spleen, CD4, and CD8 subsets of T cells, B lymphocytes,8 and in monocytes9 but not in a wide variety of nonlymphoid tissues. High levels of sCTLA-4 were detected in patients with some autoimmune diseases, such as systemic lupus erythematosus,10 type 1 diabetes,11 and myasthenia gravis.12 In addition, markedly increased plasma levels of sCTLA-4 were observed in celiac patients and were associated with mucosal injury. However, the contribution of sCTLA-4 to immune regulation and the variation during cancer therapies have been less well studied. Contrary to the classical immunosuppressive effect of CTLA-4, several studies showed that sCTLA-4 also functions in downregulating the negative signal of CTLA-4 in T-cell responses, which indicated that the immunoregulatory functions of sCTLA-4 might be complicated. Saverino et al13 demonstrated that sCTLA-4 was capable of interfering with CD80/CD86:CTLA-4 interactions, thereby blocking the negative signal imparted via the membrane-bound form of CTLA-4 in the later phases of T-cell responses.
Studies investigated the effect of anti-CTLA-4 therapy combined with traditional therapies including radiotherapy (RT). Dewan et al observed a growth delay in irradiated tumor of cancer mouse models when combined with anti-CTLA-4 immunotherapy.14 Demaria et al also indicated that the combination of local RT with CTLA-4 blockade was a promising new immunotherapeutic strategy against poorly immunogenic metastatic cancers.15 However, studies evaluating the efficiency in combination of chemotherapy and anti-CTLA-4 therapy are limited. Despite the clinical relevance of CTLA-4 receptor, the functional roles of sCTLA-4 in cancer have not been thoroughly clarified. The purpose of our article is to examine the expression and clinical significance of sCTLA-4 that might vary in advanced cancer and investigate its alterations during antitumor therapy including RT and chemotherapy.
Patients and methods
Patients
This is a retrospective study to investigate the serum levels of CTLA-4 of 141 patients, who were diagnosed with malignant tumors in Shandong Provincial Qianfoshan Hospital between January 2012 and October 2015. This study was performed complying with the 1975 Declaration of Helsinki and was approved by Local Ethics Committee of Qianfoshan Hospital. To be included in the study, patients were required to 1) be adults (≥18 years old) diagnosed with unresectable metastatic or locally advanced lung cancer or esophageal cancer; 2) have received at least 1 clinical treatment, including RT, chemotherapy, and/or chemoradiotherapy (CRT) as first-line therapy; and 3) patients’ entire disease period and follow-up data can be obtained by a review of medical records and telephone. RT was performed using 1.8–2.2 Gy fraction dose, five times a week and with a total dose of 56–66 Gy/5–7 weeks (median; 60 Gy). The major chemotherapy protocol was cisplatin/etoposide for lung cancer and docetaxel/cisplatin for esophageal cancer. Each patient signed informed consent approved by our Institutional Committee on Human Rights in Research. Characteristics of cancer patients are listed in Table 1.
Table 1.
Characteristics of cancer patients (N=141)
| Features | No of patients (%)
|
|||
|---|---|---|---|---|
| RT | Chemotherapy | CRT | Total | |
| Overall | 57 (40.43) | 46 (32.62) | 38 (26.95) | 141 |
| Gender | ||||
| Female | 22 (15.60) | 19 (13.48) | 16 (11.35) | 57 (40.43) |
| Male | 35 (24.82) | 27 (19.15) | 22 (15.60) | 84 (59.57) |
| Age (years) | ||||
| ≤65 | 28 (19.86) | 23 (16.31) | 23 (16.31) | 74 (52.48) |
| .65 | 29 (20.57) | 23 (16.31) | 15 (10.64) | 67 (47.52) |
| Tobacco smoking | ||||
| Ever | 18 (12.77) | 13 (9.22) | 15 (10.64) | 46 (32.62) |
| Never | 39 (27.66) | 33 (23.40) | 23 (16.31) | 95 (67.38) |
| Alcohol drinking | ||||
| Ever | 34 (24.11) | 25 (17.73) | 26 (18.44) | 85 (60.28) |
| Never | 23 (16.31) | 21 (14.89) | 12 (8.51) | 56 (39.72) |
| Type | ||||
| Lung cancer | 16 (11.35) | 18 (12.77) | 23 (16.31) | 57 (40.43) |
| Esophageal cancer | 25 (17.73) | 7 (4.96) | 9 (6.38) | 41 (29.08) |
| Liver cancer | 9 (6.38) | 4 (2.84) | 3 (2.13) | 16 (11.35) |
| Ovarian cancer | 4 (2.83) | 6 (4.26) | 9 (6.38) | 19 (13.48) |
| Cervical cancer | 3 (2.13) | 3 (2.13) | 2 (1.42) | 8 (5.67) |
| Stage | ||||
| II–III | 23 (16.31) | 14 (9.93) | 24 (17.02) | 61 (43.26) |
| IV | 34 (24.11) | 32 (22.70) | 14 (9.93) | 80 (56.74) |
| Lymph node metastasis | ||||
| Yes | 23 (16.31) | 13 (9.22) | 18 (12.77) | 54 (38.30) |
| No | 34 (24.11) | 33 (23.40) | 20 (14.18) | 87 (61.70) |
| Distant metastasis | ||||
| Yes | 27 (19.15) | 10 (7.09) | 21 (14.89) | 58 (41.13) |
| No | 30 (21.28) | 36 (2.53) | 17 (12.06) | 83 (58.87) |
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy.
Tumor grade and clinical stage were determined by imaging techniques including computed tomography (CT) and/or magnetic resonance imaging (MRI) according to the seventh edition of the American Joint Committee on Cancer tumor node metastasis (TNM) classification system.16 Progression was determined by physicians based on radio-graphic evidence indicating progression of tumor lesions or occurrence of new lesions, physical examinations indicating worsening performance status, or cancer-related symptoms (eg, increased pain, fever, and weight loss). Overall survival (OS) was defined from the beginning of radiotherapy to death or last follow-up. Progression-free survival (PFS) was defined as the time from radiotherapy until disease progression or last follow-up.
Detection of sCTLA-4 by ELISA
Blood samples were provided by the 141 patients both 1 day before and after therapies within 3 days and were processed within 2 hours. After centrifuged at 1,000 × g for 10 minutes at 4°C, packaged serum was stored at −80°C until analysis. The serum levels of CTLA-4 were measured by using a solid phase sandwich enzyme-linked-immunosorbent serologic assay (ELISA) kit (Guchen Biotech, Shanghai, People’s Republic of China). All samples were tested in duplicate following the manufacturer’s instructions. The deviation between duplicates is <10% for any reported value. The detection range for sCTLA-4 is 30–960 pg/mL. Blood immune cell counts at the same time with the blood samples were collected from medical records.
Statistical analysis
SPSS 19.0 software (SPSS Inc., Chicago, IL, USA) was used to perform data analysis. Patients were separated into 3 groups according to therapy: RT, chemotherapy, and CRT groups. Granulocyte-macrophage colony-stimulating factor level was expressed as mean ± standard deviation (SD). The median value of sCTLA-4 level was defined as the cutoff value. Survival curves and analysis were performed using the Kaplan–Meier method and the log-rank test. To determine the independent prognostic factor, multivariate analysis was carried out using the Cox proportional hazard model; hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for each factor. Serum CTLA-4 levels before and after therapy were compared using the Mann–Whitney U test. Spearman’s rho analyses were used to determine the relationship between sCTLA-4 level and clinicopathologic features, including age, gender, stage, cancer type, tobacco smoking, alcohol drinking, lymph node metastasis, distant metastasis, and blood immune cell count variation. P<0.05 was considered as statistically significant.
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Serum levels of sCTLA-4
We performed ELISA for the detection of sCTLA-4 level derived from 141 patients. The number of patients diagnosed of lung, esophageal, liver, ovarian, and cervical cancers were 57, 41, 16, 19, and 8, respectively. There were 61 patients with stage II–III cancer and 80 with stage IV cancer. The median follow-up period was 12 months (range: 1–44). sCTLA-4 was categorized according to the median value of 66 pg/mL, calculated by the sCTLA-4 levels after cancer therapy. SCTLA-4 levels after therapy were 108±29 pg/mL for the RT group, 96±16 pg/mL for the chemotherapy group, 110±28 pg/mL for the CRT group, and 105±24 pg/mL for the whole patient group, no statistically significant differences of sCTLA-4 level were observed among these 4 groups (all P>0.05).
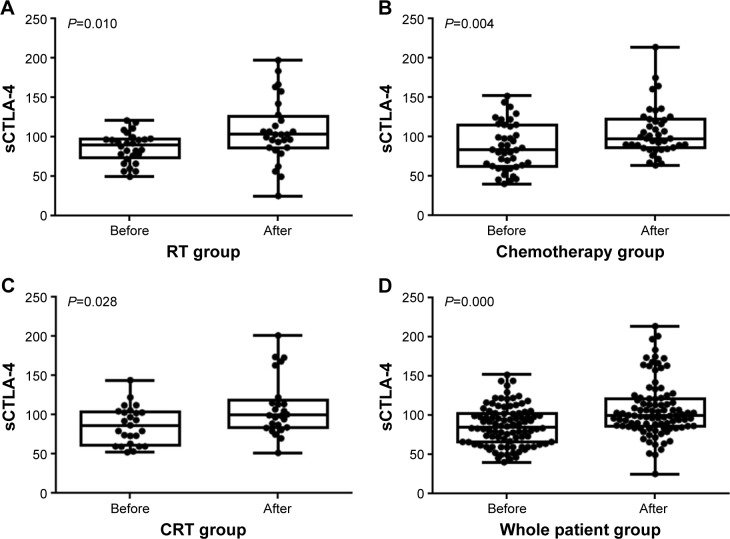
In addition, we also detect sCTLA-4 level before therapies to discover the variation of sCTLA-4 according to cancer treatments. sCTLA-4 levels before therapy were 87±15 pg/mL for the RT group, 77±23 pg/mL for the chemotherapy group, 87±20 pg/mL for the CRT group, and 84±19 pg/mL for the whole patient group. Results showed that compared to sCTLA-4 level detected before therapies, sCTLA-4 level during therapies was significantly higher among all these 4 subgroups, including the RT (P=0.010), chemotherapy (P=0.004), CRT (P=0.028), and the whole patient groups (P=0.028; Figure 1).
Figure 1.
Level of sCTLA-4 in all 4 groups.
Notes: Compared with the level of sCTLA-4 1 day before therapies, sCTLA-4 levels during therapies were significantly higher among all these 4 subgroups including the (A) RT, (B) chemotherapy, (C) CRT, and (D) whole patient group (all P<0.05).
Abbreviations: CRT, chemoradiotherapy; RT, radiotherapy; sCTLA-4, soluble cytotoxic T-lymphocyte antigen 4.
Association of sCTLA-4 level with survival
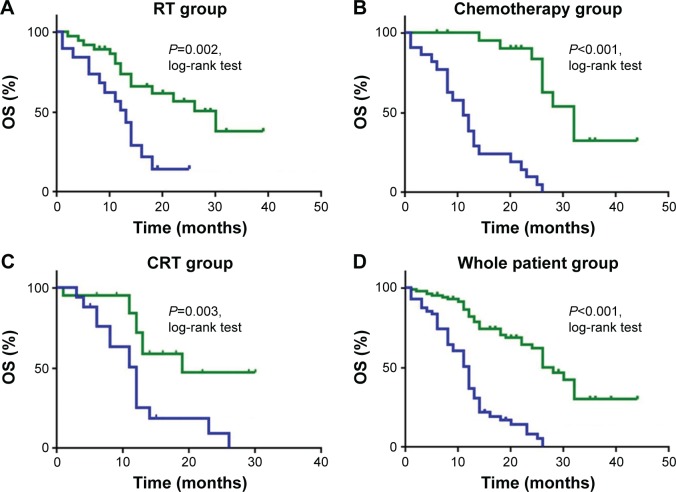
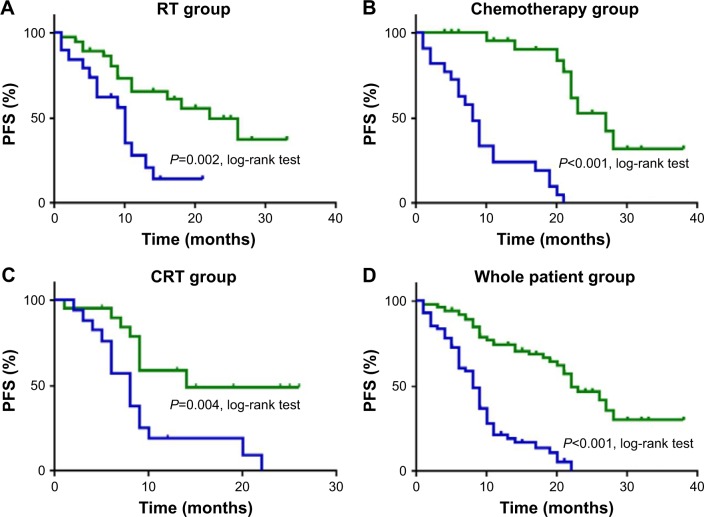
The median OS was 12 months for the whole group, 12 months for the RT group, 12 months for the chemotherapy group, and 18 months for the CRT group. The median PFS was 9 months for the whole group, 9 months for the RT group, 8 months for the chemotherapy group, and 13.5 months for the CRT group. In all groups, high sCTLA-4 level was significantly associated with longer OS and PFS (all P<0.05, log-rank test). Multivariate analysis suggested that high sCTLA-4 level after therapies was an independent prognostic factor in all 4 groups including RT (HR: 0.385, 95% CI: 0.149–0.996), chemotherapy (HR: 0.06, 95% CI: 0.007–0.517), CRT (HR: 0.311, 95% CI: 0.121–0.802), and the whole patient groups (HR: 0.287, 95% CI: 0.166–0.493).
Disease stage was also an independent prognostic factor for all 4 groups including RT (HR: 0.138, 95% CI: 0.034–0.554, P=0.005), chemotherapy (HR: 0.120, 95% CI: 0.028–0.523, P=0.0056), CRT (HR: 0.204, 95% CI: 0.056–0.740, P=0.016), and the whole patient groups (HR: 0.228, 95% CI: 0.125–0.417, P=0.000) in multivariate analysis. However, the upregulated lymphocyte level showed independent prognostic role in the RT group (HR: 0.209, 95% CI: 0.063–0.693, P=0.010). Patient age, gender, and treatment strategy were not associated with prognosis in all 4 groups. Detailed information is listed in Table 2 and Figures 2 and 3.
Table 2.
Details of the Cox proportional hazard model for all 4 groups
| Factor | RT group
|
Chemotherapy group
|
CRT group
|
Whole patient group
|
||||
|---|---|---|---|---|---|---|---|---|
| P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | |
| Age | 0.591 | 0.787 (0.327–1.889) | 0.031 | 0.292 (0.095–0.893) | 0.897 | 0.943 (0.388–2.291) | 0.644 | 0.897 (0.567–1.420) |
| Gender | 0.624 | 1.295 (0.461–3.643) | 0.717 | 0.823 (0.287–2.363) | 0.995 | 1.003 (0.373–2.702) | 0.966 | 0.989 (0.595–1.644) |
| Lymphocyte metastasis | 0.346 | 0.629 (0.239–1.651) | 0.719 | 1.389 (0.231–8.352) | 0.038 | 0.353 (0.132–0.944) | 0.009 | 0.481 (0.277–0.835) |
| Distant metastasis | 0.568 | 1.292 (0.536–3.119) | 0.280 | 0.312 (0.038–2.579) | 0.248 | 1.221 (0.453–3.293) | 0.974 | 1.008 (0.608–1.672) |
| TNM stage | 0.007* | 0.190 (0.057–0.633) | 0.013* | 0.163 (0.039–0.681) | 0.026* | 0.121 (0.019–0.773) | 0.000* | 0.222 (0.122–0.402) |
| sCTLA-4 | 0.049* | 0.385 (0.149–0.996) | 0.016* | 0.311 (0.121–0.802) | 0.010* | 0.061 (0.007–0.517) | 0.000* | 0.287 (0.166–0.493) |
| WBC change | 0.537 | 1.522 (0.402–5.761) | 0.892 | 0.922 (0.285–2.984) | 0.186 | 0.489 (0.169–1.413) | 0.998 | 0.999 (0.568–1.758) |
| NEU change | 0.777 | 0.865 (0.315–2.371) | 0.679 | 1.252 (0.433–3.620) | 0.705 | 0.851 (0.369–1.963) | 0.729 | 0.922 (0.580–1.464) |
| LYM change | 0.209 | 0.550 (0.216–1.398) | 0.066 | 3.219 (0.924–11.215) | 0.584 | 0.732 (0.239–2.239) | 0.115 | 0.681 (0.422–1.098) |
| MON change | 0.325 | 0.480 (0.111–2.068) | 0.148 | 0.362 (0.091–1.433) | 0.161 | 2.688 (0.674–10.715) | 0.827 | 0.938 (0.528–1.666) |
Notes: HR and 95% CI were listed. TNM stage and high sCTLA-4 level were independent prognostic factors.
P<0.05.
Abbreviations: CI, confidence interval; CRT, chemoradiotherapy; HR, hazard ratio; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; RT, radiotherapy; sCTLA-4, soluble cytotoxic T-lymphocyte antigen 4; TNM, tumor node metastisis; WBC, white blood cell.
Figure 2.
Survival curves for OS in all 4 groups.
Notes: Survival analysis was according to sCTLA-4 levels during therapy, with green indicating upregulated sCTLA-4 levels and blue denoting downregulated sCTLA-4 levels. Patients in the (A) RT, (B) chemotherapy, (C) CRT, and (D) whole patient groups with upregulated sCTLA-4 levels had longer OS than patients with downregulated sCTLA-4 levels (all P<0.05, log-rank test).
Abbreviations: CRT, chemoradiotherapy; OS, overall survival; RT, radiotherapy; sCTLA-4, soluble cytotoxic T-lymphocyte antigen 4.
Figure 3.
Survival curves for PFS in all 4 groups.
Notes: Survival analysis was according to sCTLA-4 levels during therapy, with green indicating upregulated sCTLA-4 levels and blue denoting downregulated sCTLA-4 levels. Patients in the (A) RT, (B) chemotherapy, (C) CRT, and (D) whole patient groups with upregulated sCTLA-4 levels had longer PFS than patients with downregulated sCTLA-4 levels (all P<0.05, log-rank test).
Abbreviations: CRT, chemoradiotherapy; PFS, progression-free survival; RT, radiotherapy; sCTLA-4, soluble cytotoxic T-lymphocyte antigen 4.
Correlations between sCTLA-4 and clinicopathological features
The results demonstrated that sCTLA-4 level was negatively correlated with TNM stage (correlation coefficient =−0.405, P<0.001) and lymph node metastasis (correlation coefficient =−0.181, P=0.031). Other features including age, gender, white blood cell (WBC) variation, neutrophil variation, lymphocyte variation, and monocyte variation showed no correlations with sCTLA-4 level. Detailed information is listed in Table 3.
Table 3.
Spearman’s rho analyses for consistency between sCTLA-4 and clinicopathological factors
| Clinicopathological factor | Correlation coefficient | P-value |
|---|---|---|
| Age | −0.083 | 0.325 |
| Gender | −0.088 | 0.302 |
| TNM stage | −0.405 | 0.000* |
| Therapy | −0.079 | 0.350 |
| Distant metastasis | −0.137 | 0.106 |
| Lymph node metastasis | −0.181 | 0.031* |
| WBC change | −0.161 | 0.056 |
| NEU change | 0.093 | 0.271 |
| LYM change | −0.032 | 0.710 |
| MON change | −0.107 | 0.205 |
Notes: Of all the clincopathological factors evaluated in this research, TNM stage and lymph node metastasis were correlated with sCTLA-4 level in whole patient group.
P<0.05.
Abbreviations: CI, confidence interval; HR, hazard ratio; LYM, lymphocyte; MON, monocyte; NEU, neutrophil; sCTLA-4, soluble cytotoxic T-lymphocyte antigen 4; TNM, tumor node metastasis; WBC, white blood cell.
Discussion
S-CTLA-4 is characterized by a cytoplasmic tail shorter than that of the full-length form of the CTLA-4 antigen. As sCTLA-4 lacks the cysteine residue at position 120, it is expressed as a monomer.10,17 However, the biological significance of sCTLA-4 has not been fully elucidated yet, especially in cancer. In this study with 141 numbers of patients, we tested blood samples before and during therapy to examine sCTLA-4 level and demonstrated that significantly higher sCTLA-4 level was found in serum sample collected after therapy than that before therapy. Higher expression of sCTLA-4 level is significantly associated with better OS and PFS in multivariate analysis. This result is distinct from the immune checkpoint blockade effect of CTLA-4 to unleash the suppressed immunity in tumors and to allow host cytotoxic T cells to attack the tumor cells. This showed that the immunoregulatory function of sCTLA-4 is quite complicated. Multivariate analysis suggested that during and after cancer therapy, including RT, chemotherapy and CRT, sCTLA-4 level was an independent prognostic factor. In all groups, including RT, chemotherapy, and CRT groups, high expression of sCTLA-4 level was significantly associated with longer OS and PFS. Therefore, this provided us a theory that fluctuation of sCTLA-4 level after cancer therapies may serve as a biomarker to predict long-term survival.
CTLA-4 is a CD28 homolog and shares 2 ligands, B7–1 (CD80) and B7–2 (CD86), with CD28. The affinity and avidity of CTLA-4 for B7 are stronger than CD28. By binding to B7 on the antigen-presenting cells, CTLA-4 blocks their interaction with the CD28 costimulatory molecule and results in a negative signal, which is responsible for the inactivation of T cell, inhibition of cell proliferation, and suppression of cytokine (interleukin [IL]-2, interferon [IFN]-γ) production and cell cycle progression. CTLA-4 is also constitutively expressed by regulatory T cells, which seem to require it for their immunosuppressive function. In addition, CTLA-4 can also exhibit direct inhibitory effects to exert its inhibitory function on T cells. The mRNA encoding sCTLA-4 consists of exon 1 encoding the leader peptide, exon 2 the ligand-binding domain, and exon 4 the cytoplasmic tail, but it lacks exon 3 encoding the transmembrane domain.18,19 The MYP-PPY motif located in the extracellular domain of sCTLA-4 is critical for B7 molecule binding.20 Thus, sCTLA-4 is capable to bind CD80/CD86 and participate in the B7/CTLA-4/CD28 signaling pathway of T-cell regulation. However, similar with the full-length CTLA-4, sCTLA-4 is also capable to bind CD80/CD86 and to participate in the B7/CTLA-4/CD28 signaling pathway of T-cell regulation, which would lead to its immunosuppressive function.21 In addition, sCTLA-4 lacking the transmembrane domain disabled sCTLA-4 in downregulating immune functions, thereby blocking the negative signal of CTLA-4 after interaction with CD80/CD86 in the later phases of T-cell responses. Consequently, the inconsistency effect of sCTLA-4 potentiates it to be correlated with favorable prognosis.
To find out the influences of cancer therapies on sCTLA-4, we also collected serum samples before treatment to detect sCTLA-4 level. Results showed that compared to sCTLA-4 level detected before therapies, sCTLA-4 level during therapies was significantly upregulated, which could be envisaged that elevated sCTLA-4 exhibits its effect in blocking the immunoregulatory function of CTLA-4 in tumors and to allow host cytotoxic T cells to attack the tumor cells, thereby enhancing the antitumor effect in synergy with RT, chemotherapy, and CRT. However, no statistical significance of sCTLA-4 was found among these 4 groups, including RT, chemotherapy, CRT, and the whole patient groups. This suggested that the upregulated effect on sCTLA-4 of these 3 therapies has no significant difference.
The consistency analyses of sCTLA-4 and clinicopathological factors verified that sCTLA-4 level is associated with better survival from another point of view. Results showed that sCTLA-4 level after therapy was negatively correlated with TNM stage, which demonstrated that patients with early cancers in lower TNM stage usually expressed higher sCTLA-4 level. In addition, the fact that sCTLA-4 level was negatively correlated with lymph node metastasis also proved that sCTLA-4 could be considered as a favorable predictor in malignant tumors during therapy. Many other studies also demonstrated the immunomodulatory and prognostic roles of sCTLA-4 in cancers. Roncella et al discovered that sCTLA-4 in pleural effusion was a statistically significant positive prognostic factor in malignant pleural mesothelioma patients.22 Grohmann et al found that sCTLA-4 expression can be enhanced by IFN-β1a in human mononuclear cells from healthy subjects.23 This result shows a selective induction of sCTLA-4 by IFN-β1a in human cells, which might exert immunomodulatory effects. Thus, we would exert further exploitation to discover the consistency of sCTLA-4 and IFN-β1 in cancer patients.
Taken together, our data suggested the favorable prognostic value of sCTLA-4 level for tumor patients. The upregulated effect of cancer therapies on sCTLA-4 could consequently enhance its blockage effect of CTLA-4 on immunoregulatory function and exhibit the antitumor effect with RT, chemotherapy, and CRT synergistically. As a retrospective design, our study has limitations. The number of patients involved in is small, and the follow-up period was relatively short to draw definite conclusions. Thus, we need prospective controlled large sampled clinical trials to validate our findings. In addition, considering that the level of sCTLA-4 is correlated with the immunosuppressive function of CTLA-4-targeting T cells, it might be used as a biomarker to predict the effectiveness of immunotherapy, such as ipilimumab, in cancer patients. Further investigation is needed to evaluate the correlation between sCTLA-4 level and the efficiency of immunotherapies, as well as the underlying possible basic mechanisms. It is hoped that further investigation of sCTLA-4 in tumor prognosis and immunotherapy will lead to the development of new immunotherapy options for cancer patients.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (grant nos 81672974 and 81602719).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet (+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37(3):501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu HM, Yang JL, Jiao SC, Wang JD, Li Y. TGF-beta1 precursor and CD8 are potential prognostic and predictive markers in operated breast cancer. J Huazhong Univ Sci Technolog Med Sci. 2014;34(1):51–58. doi: 10.1007/s11596-014-1231-2. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunturi A, McDermott DF. Potential of new therapies like anti-PD1 in kidney cancer. Curr Treat Options Oncol. 2014;15(1):137–146. doi: 10.1007/s11864-013-0268-y. [DOI] [PubMed] [Google Scholar]
- 7.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oaks MK, Hallett KM. Cutting edge: a soluble form of CTLA-4 in patients with autoimmune thyroid disease. J Immunol. 2000;164(10):5015–5018. doi: 10.4049/jimmunol.164.10.5015. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S, Carrega P, Saverino D, et al. CTLA-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum Immunol. 2010;71(10):934–941. doi: 10.1016/j.humimm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Aberrant production of soluble costimulatory molecules CTLA-4, CD28, CD80 and CD86 in patients with systemic lupus erythematosus. Rheumatology. 2005;44(8):989–994. doi: 10.1093/rheumatology/keh663. [DOI] [PubMed] [Google Scholar]
- 11.Purohit S, Podolsky R, Collins C, et al. Lack of correlation between the levels of soluble cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) and the CT-60 genotypes. J Autoimmune Dis. 2005;2:8. doi: 10.1186/1740-2557-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XB, Kakoulidou M, Giscombe R, et al. Abnormal expression of CTLA-4 by T cells from patients with myasthenia gravis: effect of an AT-rich gene sequence. J Neuroimmunol. 2002;130(1–2):224–232. doi: 10.1016/s0165-5728(02)00228-x. [DOI] [PubMed] [Google Scholar]
- 13.Saverino D, Brizzolara R, Simone R, et al. Soluble CTLA-4 in autoimmune thyroid diseases: relationship with clinical status and possible role in the immune response dysregulation. Clin Immunol. 2007;123(2):190–198. doi: 10.1016/j.clim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(Pt 2):728–734. [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Fujimoto M, Hasegawa M, et al. Serum soluble CTLA-4 levels are increased in diffuse cutaneous systemic sclerosis. Rheumatology (Oxford) 2004;43(10):1261–1266. doi: 10.1093/rheumatology/keh303. [DOI] [PubMed] [Google Scholar]
- 18.Oaks MK, Hallett KM, Penwell RT, Stauber EC, Warren SJ, Tector AJ. A native soluble form of CTLA-4. Cell Immunol. 2000;201(2):144–153. doi: 10.1006/cimm.2000.1649. [DOI] [PubMed] [Google Scholar]
- 19.Liu MF, Wang CR, Chen PC, Fung LL. Increased expression of soluble cytotoxic T-lymphocyte-associated antigen-4 molecule in patients with systemic lupus erythematosus. Scand J Immunol. 2003;57(6):568–572. doi: 10.1046/j.1365-3083.2003.01232.x. [DOI] [PubMed] [Google Scholar]
- 20.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7–1 (CD80) and B7–2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 21.Saverino D, Simone R, Bagnasco M, Pesce G. The soluble CTLA-4 receptor and its role in autoimmune diseases: an update. Auto Immun Highlights. 2010;1(2):73–81. doi: 10.1007/s13317-010-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roncella S, Laurent S, Fontana V, et al. CTLA-4 in mesothelioma patients: tissue expression, body fluid levels and possible relevance as a prognostic factor. Cancer Immunol Immunother. 2016;65(8):909–917. doi: 10.1007/s00262-016-1844-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3(11):1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]