Abstract
Background
An increasing number of studies is now devoted to immunotherapy of cancer. We evaluated the clinical benefit of hepcortespenlisimut-L (Hepko-V5 [formerly known as V5])—an oral therapeutic vaccine designated by the United States Food and Drug Administration (FDA) as an orphan drug for treatment of hepatocellular carcinoma (HCC). V5 was initially developed by us in 2002 to treat hepatitis B or C viral infections and liver cirrhosis.
Methods
The outcome of open-label Phase II trial of daily dose of V5 pill was analyzed retrospectively. Over a period of 5 years, 75 patients with advanced HCC were enrolled, consisting of 29 (38.7%) females and 46 (61.3%) males with a median age of 60 years (mean 61.6±8.1 years). Out of these, 23 (30.7%) had hepatitis B and 34 (45.3%) had hepatitis C infections, including 9 (12%) with dual infection, 4 (5.3%) negative for both viruses, and 5 (6.7%) without established viral diagnosis. Most patients (94.7%) had underlying liver cirrhosis of varying severity.
Results
After a median of 2 months of treatment, 50 out of 75 patients had experienced a decline in serum levels of the tumor marker, alpha-fetoprotein (AFP) (66.7%; P=0.006 by Wilcoxon signed rank test). Baseline median AFP levels were 245.2 IU/mL (mean 4,233; range 7.2–92,407; 95% confidence interval [CI] 1,186–7,280) and post-treatment values were 102.3 IU/mL (mean 2,539; range 0.9–54,478; 95% CI 503–4,575). The decrease in AFP was correlated either with tumor clearance or regression on computed tomography scans. The median overall survival time could not be established since 68 out of 75 (90.7%) patients were still alive after median follow-up of 12 months (mean 15±9.7; range 7–59; 95% CI 12.8–17.2). The first patient in this study received immunotherapy 5 years ago and still remains in complete remission. None of the patients experienced any serious adverse effects or toxicity.
Conclusion
The results indicate that hepcortespenlismut-L is a safe, effective, and fast-acting immunomodulatory intervention for HCC. The Phase III, randomized, double-blind, placebo-controlled trial is now initiated at the Mongolian National Cancer Center to confirm these promising findings.
Keywords: allogeneic, inflammation, immune tolerance
Background
Hepatocellular carcinoma (HCC) is the second most common cause of death from cancer, estimated to be responsible for 818,000 deaths or 9.9% of total cancer fatalities in 2013.1 Even though the incidence of HCC is low in North America and most of Europe, it has almost doubled in these places since the 1990s. Liver cancer is largely a problem of countries in East Asia and sub-Saharan Africa where 83% (50% in China alone) of the estimated 792,000 new cases occurred in 2013. Unfortunately, Mongolia occupies the leading position in the world; it has 6 times higher than average global incidence of HCC, which is attributed to the excessive prevalence of chronic viral B and C hepatitis, alcohol consumption, and liver cirrhosis.2–9
Close to 90% of HCC cases in Mongolia are diagnosed in an advanced stage when common therapeutic interventions, such as resection, chemoembolization, radiofrequency ablation, or liver transplantation, are not feasible anymore.2 Although sorafenib (Nexavar) is available as a chemotherapy option, it is seldom used due to high cost and low efficacy combined with adverse effects. Thus, the need for a safer, more effective, and affordable treatments remain unfulfilled. The concept of liver cancer immunotherapy has gained popularity in recent years.10 Several clinical trials of HCC immunotherapy were reported; some of these suggested that this approach might be useful for patients with liver tumors.10–21 Nevertheless, despite intensive research effort during last 20 years, no proven immunotherapy has emerged for HCC indication.
Orally administered immune modulator previously known as V5 has been used in Mongolia since 2007. The accumulated clinical experience in over 10,000 patients in about 30 additional countries indicates that V5 is safe and effective for a range of clinical indications. Published clinical trials have shown the outstanding safety and beneficial effect of V5 in patients with chronic viral hepatitis and liver cirrhosis.22–25 V5 is a tableted, off-shelf formulation derived from heat-inactivated, hydrolyzed, pooled blood of hepatitis B and C carriers, prepared according to proprietary technology developed by us back in 2002.22
In 2010, we had our first patient who presented with viral hepatitis C and decompensated liver cirrhosis along with diagnosis of inoperable HCC. The patient was discharged from the hospital as incurable with less than 3 months to live. As there were no other treatment options available, he decided to take 1 pill of V5 a day so that his failing liver can be rescued. Surprisingly, after about 4 months on V5, the patient experienced complete remission. The tumor clearance coincided with the normalization of baseline plasma level of HCC tumor marker, alpha-fetoprotein (AFP), dropping from 428.7 to 2.5 IU/mL. This favorable outcome led to signing up additional HCC patients, and changes in AFP were employed as surrogate marker of response to immunotherapy since most patients could not afford the cost of computed tomography (CT) scan. Results were encouraging to the point that V5 has received an orphan drug status designation from the United States Food and Drug Administration (FDA) with assigned generic name hepcortespenlisimut-L. Here, we present detailed account of earlier reported conference abstract on hepcortespenlisimut-L (Hepko-V5) intervention in a 75-patient cohort in advanced stage of HCC.26
Methods
Patients
A total of 75 HCC patients were evaluated in this retrospective study conducted from December 2010 till November 2015 (Table 1). The patients consisted of 29 (38.7%) females and 46 (61.3%) males with a median age of 60 years (mean 61.6±8.1 years). Out of these, 23 (30.7%) had hepatitis B and 34 (45.3%) had hepatitis C infections, including 9 (12%) with dual infection, 4 (5.3%) negative for both viruses, and 5 (6.7%) without established viral diagnosis. Most patients (94.7%), except 4, had hepatic cirrhosis due to hepatitis B or C virus chronic infection, often with history of alcohol abuse. One case of cirrhosis due to hereditary hemochromatosis and 3 cryptogenic cases without known etiology were part of the cohort. All patients were in advanced stage of HCC and unfit for standard therapy, ie, surgical intervention, either due to tumor size or their location or multiplicity. Many patients had single or multiple events of recurrent lesions after surgical intervention, such as resection, transarterial embolization, radiofrequency ablation, percutaneous ethanol injection, or their combinations. Only 3 patients received FDA-approved first-line drug, sorafenib, which in all 3 cases has been discontinued shortly after treatment initiation due to intolerable adverse side effects, perceived lack of efficacy, and/or excessive cost. At the presentation, all the patients were either under palliative care or pronounced incurable without available treatment options—underlining the disease gravity. As the majority of our HCC patients were in terminal stage of the disease, they often presented with a variety of symptoms related to decompensated cirrhosis including ascites, edema, variceal bleeding, portal thrombi, and hepatic encephalopathy. Besides these symptoms, abdominal discomfort and less frequently pain were common (80%). Other encountered complaints were fatigue or weakness, cachexia, anorexia, skin itch, bleeding from gums and nose, vomiting, and jaundice. As no other intervention options except palliative care were available, patients consented to receive once-per-day pill of V5 in an open-label setting. In such a way, we had a cohort representative of real-life situation in Mongolia, where almost every patient is diagnosed only after symptoms of the disease become apparent or when tumors recur. The primary endpoints were changes in serum AFP levels, tumor burden, and overall survival (OS). This study was registered on September 24, 2014 at ClinicalTrials.gov registry site with ID: NCT02256514.
Table 1.
Baseline characteristics of 75 HCC patients in this study
| Variables | Values | Percent |
|---|---|---|
| Age (years) | ||
| Mean ± SD (median) | 61.6±8.1 (60) | |
| Range (95% CI) | 43–81 (59.7–63.5) | |
| Sex | ||
| Females | 29 | 38.7 |
| Males | 46 | 61.3 |
| Ethnicity | ||
| Asian | 72 | 96 |
| Caucasian | 3 | 4 |
| Clinical symptoms | ||
| Weight loss | 66 | 88 |
| Abdominal discomfort | 60 | 80 |
| Abdominal pain | 12 | 16 |
| Fatigue | 68 | 90.7 |
| Liver cirrhosis | 71 | 94.7 |
| Child-Pugh A | 12 | 16 |
| Child-Pugh B | 38 | 50.6 |
| Child-Pugh C | 25 | 33.4 |
| Mean ALT (U/L) | 74.3 | 16.7 |
| Mean AST (U/L) | 135.2 | 7.2 |
| Viral hepatitis | ||
| HBV+ | 23 | 30.7 |
| HCV+ | 34 | 45.3 |
| Dual HBV+/HCV+ | 9 | 12 |
| Negative | 4 | 5.3 |
| Missing data | 5 | 6.7 |
| AFP | 75 | 100 |
| ≤40 IU/mL | 19 | 25.3 |
| ≤200 IU/mL | 36 | 48 |
| ≤1,000 IU/mL | 53 | 70.6 |
| Mean ± SD (median) | 4,333±13,243 (245.2) | |
| Range (95% CI) | 7.2–92,407 (1,186–7,280) | |
| Tumor | ||
| Single (<3 cm) | 0 | 0 |
| Single (>3 cm) | 7 | 9.3 |
| Multifocal | 51 | 68.1 |
| Missing data | 17 | 22.6 |
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SD, standard deviation.
Inclusion and exclusion criteria
The following criteria were set up for participation in this study.
Inclusion criteria
1) HCC diagnosis documented prior to study either by disease history, histology, CT scan, or positive AFP serum test prior to entry and 2) both men and non-pregnant women who were at least 18 years old and willing to provide informed consent. Those who met inclusion criteria were eligible for retrospective evaluation as long as they had baseline AFP values available.
Exclusion criteria
1) Pregnant or breast-feeding women; 2) subjects who have already taken V5 in prior trials or had no baseline lab data; 3) subjects who have taken other immunomodulating drugs within 2 months prior to entry, including systemic corticosteroids, intravenous immune globulin, interferons, interleukins, pentoxifylline, thalidomide, filgrastim (granulocyte colony-stimulating factor), sargramostim (granulocyte macrophage colony-stimulating factor), dinitrochlorobenzene, thymosin alpha, thymopentin, inosiplex, polyribonucleoside (Ampligen), ditiocarb sodium (Imuthiol), or any other locally available immune modulators, or any other cancer vaccine; 4) those with evidence of active or acute cardiac disease, epilepsy, or life-threatening acute diseases unrelated to HCC; and 5) medical conditions, such as active alcohol or substance abuse, or psychological issues that in the opinion of the local investigator would interfere with adherence to treatment regimen of this study.
Hepcortespenlisimut-L (Hepko-V5)
V5 is derived from hydrolyzed pooled blood of hepatitis B and C carriers, whereas Hepko-V5 is derived from pooled blood of HBV+ and HCV+ donors diagnosed with HCC. The founding principle for making V5 is not much different from established practice of manufacturing old-fashioned killed vaccines, eg, hepatitis B vaccine made from pooled plasma. The process of manufacturing is described in detail – it involves heat and chemical inactivation with subsequent formulation into a tablet capable to withstand digestive degradation in the stomach.22 The manufacturing process eliminates potentially infectious hepatitis B and C viruses as evidenced by serology and PCR analysis of the tablets. V5 has been approved as an immunomodulating biologically active product in Mongolia since 2007. The recommended dose is 1–2 pills per day. The preparation is stable at ambient room temperature for 3 years. In December 2014, hepcortespenlisimut-L received orphan drug status designation (#457714) for treatment of HCC from the FDA. Along with this generic name, the brand name Hepko-V5 has been chosen for use in Phase III, placebo-controlled, randomized, double-blinded trial currently underway in Mongolia at the National Cancer Center (ClinicalTrials.gov ID: NCT02232490).
Administration schedule and monitoring
Treatment was administered in an outpatient setting. The patients were instructed to take orally once-daily pill of V5. They were free to stop treatment at any time. This report comprises only those patients who had baseline and post-treatment AFP values measured by routine lab methods. No other exclusion criteria were applied. In such a manner, the patient sample was maximally close to a real-life demographic distribution of HCC in the Mongolian population.
Statistical analysis
The primary endpoint for this study was effect of V5 on serum AFP levels along with OS and tumor burden changes. Secondary objectives were safety, adverse effects, and changes in clinical symptoms. The difference between pre- and post-treatment parametric values was assessed by Student’s t-test and linear regression analysis. Wilcoxon matched pairs ranking test and c2 contingency table analysis were employed for categorical data. Comparison of survival curves was analyzed by log-rank test (StatMost, Datamost Inc., South Sandy, UT, USA). The significance level was set at P≤0.05.
Ethics approval and consent to participate
All patients provided informed consent (oral or written) and were explained benefits and risks of the intervention. The study was approved by the Institutional Review Board of Immunitor LLC (#IRB00010671) and conducted in accordance with declaration of Helsinki with good clinical practice as defined by the International Conference on Harmonization.
Results
Subjects
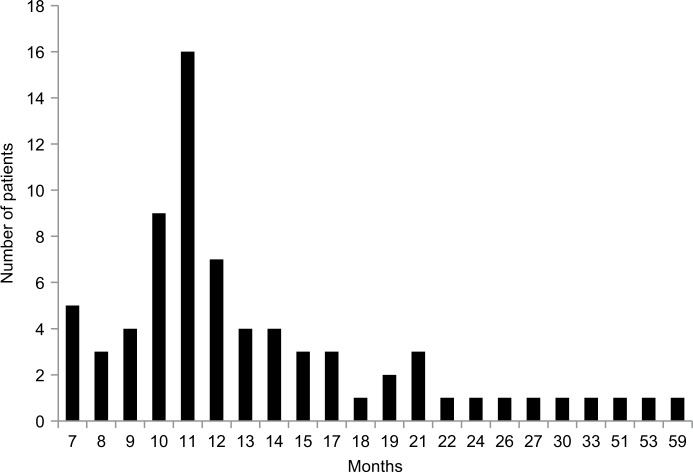
The patients entered the study in a staggered fashion over the period of 59 months between December 2010 and November 2015—the censoring time point. They have been receiving immunotherapy for various periods of time. The median duration of the therapy between time of AFP measurement at baseline and next AFP test was 2 months (mean ± standard deviation [SD] 2.75±2.45; 95% confidence interval [CI] 2.2–3.3) (Figure 1). Treatment was extremely well tolerated; no adverse side effects due to V5 were observed in any of treated patients. As per previous experience with hepatitis and cirrhosis patients, V5 produced almost immediate relief from symptoms of the disease. Fatigue and abdominal pain or discomfort were the most common symptoms that were reported as improved within 1 week from treatment initiation. The period over which patients have been followed up was considerably longer. The average follow-up time was 15±9.7 months with median 12 months (range 7–59 months; 95% CI 12.8–17.2). Figure 2 shows distribution of patients in terms of the length of follow-up in relation to number of patients in each time category, eg, 5 patients were followed up for 7 months and 1 patient for 59 months, bars on the left and right side of the histogram, respectively. Unpaired t-test has not revealed any disparity between the length of follow-up and type of viral hepatitis infection. The mean ± SD (median) of those with hepatitis B virus (HBV) vs. hepatitis C virus (HCV) were practically identical, ie, 13.7±5.4 (11.5) vs. 15.8±11.4 (12) months, respectively (P=0.34). Among 7 deceased patients, 1 had dual infection, 1 was negative for both viruses, with 2 and 3 individuals diagnosed with HBV and HCV, respectively. As numbers were small, no statistical power has been reached to determine which type of virus was more lethal (P=0.56 by c2 analysis). Due to low number of double negatives (N=4), the statistical power was not sufficient to conclude anything of significance. Nevertheless, in 3 out of 4 double negative patients, AFP levels declined (75%), which is in line with the response rate of remaining patients, ie, 38 out of 54 (70.4%) patients. The difference between outcomes was negligible (P=0.94). Similar results were seen when double positives were compared to the remainder of patients with the response rate being 6 out of 9 (66.7%) and 43 out of 65 (66.2%), respectively (P=0.99). Thus, regardless of distinct viral diagnoses, the outcome was essentially the same, at least statistically, meaning that V5 acts independently from the underlying viral status.
Figure 1.

The distribution of patients according to duration of treatment between baseline and post-treatment AFP measurements. Vertical axis shows the absolute number of patients in each time category (X-axis). Median duration between first and consecutive AFP tests was 2 months (mean ± SD 2.75±2.45; 95% CI 2.2–3.3)
Abbreviations: CI, confidence interval; SD, standard deviation.
Figure 2.
Absolute number of HCC patients, as shown on Y-axis, followed up for 7–59 months (X-axis). The median follow-up time is 12 months (mean ± SD 15±9.7; range 7–59; 95% CI 12.8–17.2).
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; SD, standard deviation.
Safety and adverse effects
We have not seen any toxicity at any point in this study. V5 has been clinically investigated since 2002—the time point when we initiated series of studies to assess its safety, first in animals and then in human subjects. No toxicity or adverse effects were observed in the past in animals or in vitro, nor in any 21 clinical trials of the same drug, nor in any of 10,000 individuals who used V5 since 2007—year when it has been first approved for commercial use for a wide variety of unrelated indications.
Overall survival
At censoring cutoff time point, ie, November 2015, 7 out of 75 patients died, which corresponds to OS rate of 90.7% as shown in Figure 3. The median OS has not been reached. To Figure 3, we have added the survival curve from Phase III Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) study for comparison, which evaluated FDA-approved first-line chemotherapy, sorafenib, in HCC patients with relatively normal liver function.27 Visually and statistically, 2 survival curves were significantly different in favor of V5 (P<0.001). This difference was not biased by patients’ baseline health status. Judging from baseline Child-Pugh (CP) scores reflecting liver performance, patients in our trial were in worse shape than participants in SHARP study, ie, in CP-A 16% vs. 95% and CP-B 50.6% vs. 5%, respectively. No patients in CP-C stage were enrolled in SHARP study, while one-third (33.4%) of V5 recipients were in the last stage of liver disease. The c2 2×2 contingency table analysis comparing baseline CP-A and CP-B classes among patients on V5 and sorafenib confirms this impression (P<0.0001). When we analyzed our data, we did employ various subgroup analyses to see if any correlates or predictors of V5 efficacy might have been revealed. Obviously, liver performance was one of the parameters that we were interested to evaluate in relation to the clinical outcome. Although one may assume that patients with poor liver function would fare worse, in reality this was not the case. No correlation was found between liver cirrhosis stages and rate of AFP response. We interpret this as if the immune response was not dependent from status of the liver. The response rate of HCC patients with CP-B and CP-C classes was essentially the same, with intergroup difference that was not statistically significant.
Figure 3.
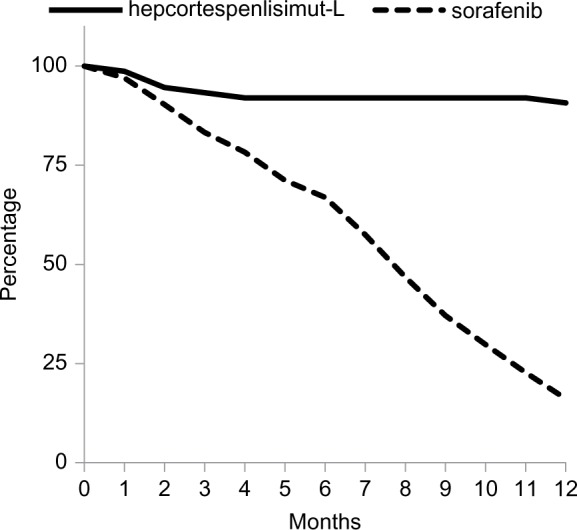
Overall survival curve (solid line) of 75 HCC patients treated with hepcortespenlisimut-L as compared to survival of sorafenib-treated population (dotted line), based on tabulated data shown at the bottom of Figure 2 of published SHARP study over the same 12-month time frame.27 Y-axis shows percent of survival vs. horizontal axis corresponding to number of months on follow-up. The difference between 2 curves is statistically significant (P<0.001).
Abbreviations: HCC, hepatocellular carcinoma; SHARP, Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol.
AFP
Median baseline AFP level was 245.2 IU/mL (mean ± SD 4,332.6±13,243.6; range 7.2–92,407; 95% CI 1,186–7,280) and corresponding post-treatment value was 102.3 IU/mL (mean ± SD: 2,538.4±8,848.6; range 0.9–54,478; 95% CI 503–4,575). Judging from AFP response, 50 out of 75 patients had experienced a decrease in AFP levels (66.7%). Wilcoxon signed rank test used as non-parametric counterpart to the parametric paired t-test has revealed highly significant difference (P=0.006). The response rate of patients with low baseline levels of AFP compared to those with high levels of AFP was similar. According to stratified analysis, 11 out of 19 patients with AFP ≤40 IU had their tumor marker levels declined (57.9%), while 38 out of 55 with higher AFP appeared to respond to treatment (69.1%). One patient whose AFP level has not changed has been excluded from this analysis. The c2 contingency 2×2 table analysis reveals that 2-tailed P-value is equal to 0.68, meaning that there is no statistical difference in outcome between groups with low and high baseline AFP levels. Figure 4 shows the dynamics of changes in AFP levels over median of 2 months. At study initiation, patients were distributed in a classic Gaussian manner across pre-specified ranges of AFP values with peak number of patients belonging to 40–400 IU/mL range with smaller populations having AFP either below 10 IU/mL (N=4) or above 50,000 IU/mL (N=2) at the tails of the bell curve. Post-treatment distribution curve of patients had shifted to the left with number of patients increasing 3-fold in <10 IU/mL range (N=12), which is considered as normal level of AFP. Only 1 patient remained in high >50,000 IU/mL AFP group; however, this was patient #9 who had a baseline value of 13,298 IU/mL, which then increased to 54,478 IU/mL. Coincidentally, this patient deceased after 3 months of treatment. Original 2 patients in this group, #12 and #67, had their AFP values going down from 92,407 and 60,844 IU/mL to 2.3 and 48,619 IU/mL, respectively. Linear regression analysis suggests that the difference between 2 curves is significant (R2=0.76; P=0.02), confirming this visual impression.
Figure 4.
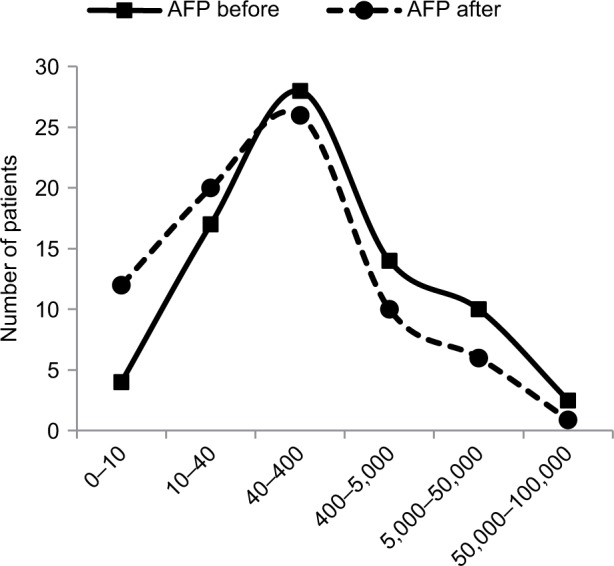
Increase in proportion of patients having lower levels of AFP after a median of 2 months, as shown in their absolute number plotted against Y-axis in relation to pre-set range of AFP plasma concentrations (shown on horizontal axis) into which they fall before (solid line) and after (dotted line) immunotherapy. Baseline median AFP level was 245.2 IU/mL (mean 4,233; range 7.2–92,407; 95% CI 1,186–7,280) and post-treatment value was 102.3 IU/mL (mean 2,539; range 0.9–54,478; 95% CI 503–4,575). There is a clear shift to the left toward lower concentrations of post-treatment AFP values compared to baseline distribution curve, which was statistically significant (R2=0.76; P=0.02).
Abbreviations: AFP, alpha-fetoprotein; CI, confidence interval.
Tumor burden
Both of the aforementioned clinical endpoints, ie, OS and decrease in AFP levels, were in favor of V5 intervention. Abdominal CT scans, when available, indicated that AFP-lowering effect of V5 coincided with reduced tumor burden. Unfortunately, only about 1 out of 10 patients could afford the cost of CT scans. Nevertheless, we provide representative images to illustrate our results. The decrease in AFP correlated with tumor shrinkage or clearance. This is clearly visible, for example, in patient #24 who could afford serial monthly measurements of AFP and bimonthly CT scans (Figures 5 and 6). The tumor-shrinking effect of hepcortespenlisimut was obvious within a very short period of time, clearly visible within 3 weeks from treatment initiation (Figure 7). In cases where AFP levels were down below upper limit of normal levels, lesions were cleared (Figures 8 and 9). These examples indicate that reduction in tumor burden is positively associated with decline in AFP levels.
Figure 5.
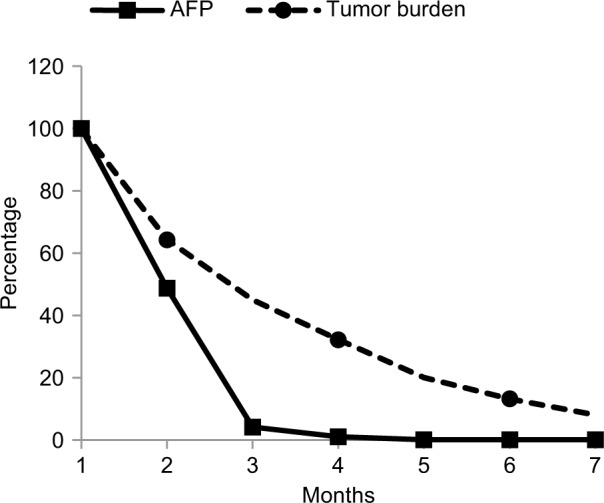
Plots expressed as percent (Y-axis) of baseline monthly values of AFP and bimonthly tumor size measurements by CT scan in patient #24 who had them evaluated for 7 months (X-axis). Changes in tumor burden were estimated by summing up perpendicular diameters of each lesion. AFP level in this patient was measured in ng/mL instead of usual units expressed in IU/mL. These values were converted into percentage figures relative to baseline values shown as 100% on Y-axis against X-axis showing number of months on follow-up. Linear regression analysis based on observed values shows strong dependency between these variables (R2=0.96; P=0.02) indicating that this relationship is unlikely to be random meaning that decrease in AFP was positively correlated with tumor burden reduction.
Abbreviations: AFP, alpha-fetoprotein; CT, computed tomography.
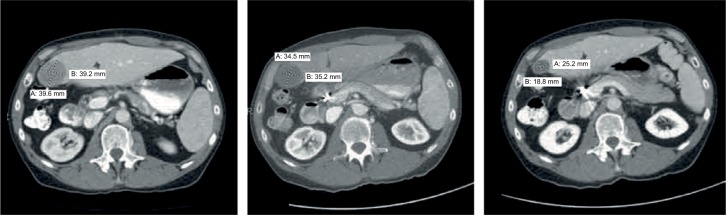
Figure 6.
Three consecutive abdominal CT scans of patient #24 carried out in June, August, and December 2014 as shown in three panels from left to right. When treatment initiated in July 2014, the patient had an AFP level of 47,937 ng/mL and 7 malignant lesions including thrombus in the portal vein, which recurred after ablation of 2 original primary tumors (12×11 and 11.1×10.4 cm in right lobe) in February 2014; in August 2014, after 3 weeks on V5, tumor sizes decreased by one third at which point AFP declined to 11,557 ng/mL. After 5 months on daily pill of hepcortespenlisimut-L, 4 out 7 lesions including thrombus cleared with remaining shrinking to under 1 cm at which time point AFP dropped down to 10.5 ng/mL—slightly above of what is considered the normal range between 0 and 8 ng/mL. Scans show planes with location of the largest lesion, which was later recognized as a cyst. By September 2016 all malignant lesions were cleared and AFP declined to 6.8 ng/mL.
Abbreviations: AFP, alpha-fetoprotein; CT, computed tomography; Hepko-V5, hepcortespenlisimut-L.
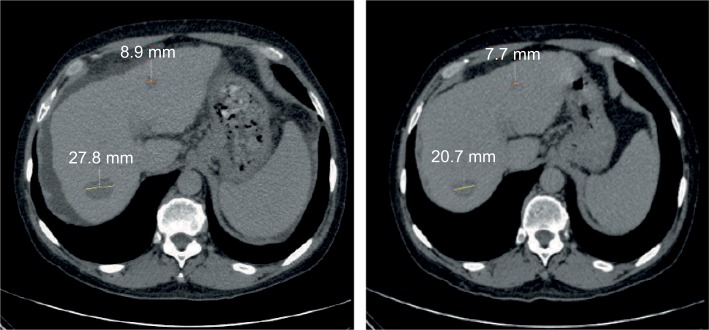
Figure 7.
Patient #63 started treatment in January 2015 (left panel); 3 weeks later, in February 2015 (right panel), both initially presented lesions shrank from 27.8 and 8.9 mm to 20.7 and 7.7 mm, respectively. Ascites clearly visible as dark gray band surrounding the liver at treatment initiation resolved after less than 1 month. After 5 months, AFP levels dropped from 5,034 to 48 IU/mL.
Abbreviation: AFP, alpha-fetoprotein.
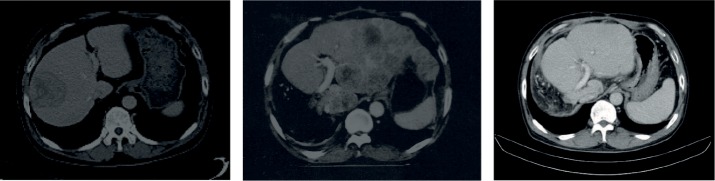
Figure 8.
Transversal and coronal CT planes of patient #1 who presented with large (>5 cm) and small (<3 cm) lesions and an AFP level of 428.7 IU/mL in December 2010 (A and B on the left). As patient was not eligible for resection due to proximity to portal vein, he consented to receive daily pill of V5. On the next visit, 4 months later, his AFP level dropped to 2.5 IU/mL at which time point lesions were cleared (C and D). Patient’s health improved remarkably; he gained weight, went back to work, and has remained in complete remission since then.
Abbreviations: AFP, alpha-fetoprotein; CT, computed tomography; Hepko-V5, hepcortespenlisimut-L.
Figure 9.
Patient #12 presented with single large tumor in September 2013 (shown in the left panel), which was removed surgically. In January 2014, multiple new lesions appeared in left lobe, which were not amenable to surgical intervention (panel in the center). At this time, his AFP concentration was extremely high (92,407 IU/mL)—no one in our entire cohort of 75 patients had more than that. In September 2014, the patient’s AFP levels reduced to 2.3 IU/mL and there were no visible lesions on CT scan (right panel). Between January and September, the patient received V5 intermittently for a total of 7 months. The patient is still alive and doing well.
Abbreviations: AFP, alpha-fetoprotein; CT, computed tomography; Hepko-V5, hepcortespenlisimut-L.
Discussion
The annual incidence of HCC and liver cirrhosis in Mongolia is the highest in the world standing at 61.9 and 350 per 100,000.2–9 Such a high rate has been attributed to the excessive prevalence of chronic viral hepatitis estimated to occur in about one-third of the population. Most of patients with HCC are diagnosed at an advanced stage of the cancer and commonly present with impaired liver function. As a result, over 90% of patients are not eligible for locoregional intervention, and systemic chemotherapy with sorafenib is seldom used due to high cost and limited benefit. Better therapies to treat liver cancer and underlying hepatic dysfunction are badly needed.
This retrospective study summarizes our experience of administering Hepko-V5 to patients with advanced HCC, all of whom had exhausted other treatment options. Similarly to our prior experience with V5 in patients with chronic hepatitis B and C or liver cirrhosis, the treatment was safe and well tolerated; not a single patient had experienced any serious adverse reaction or toxicity. In only one instance, a patient had complained of deleterious side effects, but these were found to be caused by concomitant sorafenib use. The patients who received one daily pill of V5 were, as a rule, extremely satisfied; the resolution of their clinical symptoms such as fatigue or abdominal pain often occurred after the first administered dose. Due to the tangible effect on well-being and improvement in liver function the drug adherence was outstanding. For example, Figure 7 shows the resolution of ascites resulting from dysfunctional liver within 3 weeks. Nevertheless, it is clear that V5 is not a panacea; 7 patients out of 75 had died by study termination in November 2015 (Figure 3).
The major objective of any oncology trial is OS, which determines the value of an experimental drug. The median follow-up of patients was 12 months, by that time point 90.7% of patients were still alive, which is significantly better (P<0.0001 by χ2 contingency table) than historical, 1-year survival rate among Mongolian patients with HCC.3 Due to low mortality rate, the median OS of patients on V5 was not established. As our drug was administered in an open-label setting, we have used published survival curve from Phase III sorafenib trial to compare our outcome (Figure 3). The difference in survival curves is visually distinct and statistically significant in favor of V5 as supported by log-rank test (P<0.001). While such results appear outstanding, it is clear that one needs controlled trial to confirm our initial findings. The placebo-controlled, double-blind, randomized Phase III trial has been initiated last year at the National Cancer Center in Mongolia.
Higher serum levels of AFP, the most common HCC biomarker, are associated with severity of the disease. As the SHARP study has not revealed the evidence of correlation between changes in AFP and clinical response or improved survival, most experts in HCC field assumed that this marker is not reliable as a surrogate for clinical outcome.28 However, there is a significant body of evidence arguing against this consensus. There are, for example, multiple case reports of spontaneous regression of HCC; in cases where AFP was evaluated at baseline, there is a consistent correlation with decrease or normalization of tumor marker.29–32 There are also various immunotherapy trial reports pointing out that decrease in AFP was associated with favorable clinical response.15–21 Similar findings were reported in retrospective studies that have analyzed changes in AFP as a prognostic marker in systemic and locoregional interventions for HCC.33–34 Finally, post-SHARP clinical trials have indicated that the decline in AFP was a strong predictive factor of response to sorafenib.35–40 These studies lend support to our rationale of relying on AFP as a surrogate marker. Thus, HCC is not different from other cancer types where tumor-associated markers decline or disappear if drugs are truly effective.
HCC is a classic example of cancer caused by chronic inflammation.41 This is not a secret and has been known since Rudolf Virchow’s times in the 19th century that cancer is an inflammatory disease.42 According to Virchow, immune reactive cells that attack the tumor actually stimulate proliferation of cancerous cells. The only way to overcome this unhealthy relationship is to induce the immune tolerance. There are 2 practical ways to induce immune tolerance: parenterally, by injecting very small doses of offending antigen or by feeding antigen orally.43 Judging from our preliminary in vitro investigations, physiologically relevant doses of V5 produce within 2 days a potent anti-inflammatory response reflected in a 10-fold decrease of key inflammatory cytokine tumor necrosis factor-α and at the same time manifold increase in frequency of interferon-γ secreting CD4 and CD8 T cells expressing proliferation markers Ki-67 and CD69 (unpublished, 2010). V5 has been extensively used by us to treat hepatitis and tuberculosis—2 diseases that have in common with HCC only one underlying characteristic—the inflammatory immune reaction directed against self.22–25,44
The precise mechanism of V5 action remains to be elucidated. Our working hypothesis is based on a counterintuitive premise that is opposite to the prevailing dogma in the field, which postulates that the immune response in cancer patients is weakened or rendered immune tolerant and thus the immune reaction needs to be unleashed in order to destroy malignant cells.45 We strongly believe that this notion is wrong in most cases. In fact, all of the current cancer immunotherapy approaches, eg, checkpoint inhibitors, based on such a principle have resulted in modest clinical success (<10%) and triggered autoimmune reactions of inflammatory nature in most patients (~85%), which are directed against a wide range of bystander cells unrelated to tumor cells.46,47 The outcome of our immunotherapy, which is far more effective and safer, suggests that we are on a path that the mainstream approach has not taken into account. Nevertheless, a growing body of evidence indicates that neutralizing tumor-promoting inflammation is, perhaps, the most optimal course we should be heading to.48
Conclusion
The anti-cancer activity of V5 was discovered by us accidentally 7 years ago when we treated our first patient with incurable HCC who also happened to have hepatitis C and liver cirrhosis—indications for which V5 has been developed originally. Encouraged by complete clearance of lesions in that individual, other HCC patients came forward asking for V5.26 The present report details the outcome of this immunotherapy in 75 non-selected patients with advanced HCC. Our results indicate that V5 is a safe, effective, and fast-acting immune intervention for HCC. The AFP appears to be a valid predictive biomarker for assessing clinical response. We do acknowledge that there are several deficiencies in our study that are evident, but due to retrospective nature it is too late to fix them. In order to substantiate these preliminary findings a prospective placebo-controlled, randomized, double-blind study has been initiated and we expect to have results in near future.
Acknowledgments
We are grateful to all our patients and their families who entrusted their lives to us. We regret profoundly for not being able to help them all. We are particularly indebted to Dr. Steve and Mrs. Jane Kramer and many other patients and well-wishers in Mongolia and around the globe who provided their generous donations, which we humbly accepted to advance our clinical work. Without them, this project could have been halted or delayed indefinitely. We thank Prof. Peter Nyasulu, biostatistician at the Monash University, for his valiant effort to unravel the survival data from SHARP study. The abstract of this paper was presented at the Cutting-Edge Clinical Trials session (poster #212) of the 30th annual Conference of Society for Immunotherapy of Cancer, National Harbor, MD, November 4–8, 2015. The abstract of this poster presentation was published online in the Journal for Immunotherapy of Cancer 2015;3(Suppl 2): P200 and can be found in the following link https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4649454/.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
AAR, VB, AIB, VJ, and ASB are officers for Immunitor. The other authors report no conflicts of interest in this work.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcorn T. Mongolia’s struggle with liver cancer. Lancet. 2011;377(9772):1139–1140. doi: 10.1016/s0140-6736(11)60448-0. [DOI] [PubMed] [Google Scholar]
- 3.Jazag A, Puntsagdulam N, Chinburen J. Status quo of chronic liver diseases, including hepatocellular carcinoma, in Mongolia. Korean J Intern Med. 2012;27(2):121–127. doi: 10.3904/kjim.2012.27.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baatarkhuu O, Kim DY, Nymadawa P, et al. Clinical features and prognosis of hepatocellular carcinoma in Mongolia: a multicenter study. Hepatol Int. 2012;6(4):763–769. doi: 10.1007/s12072-011-9325-4. [DOI] [PubMed] [Google Scholar]
- 5.Baatarkhuu O, Kim DY, Bat-Ireedui P, Han KH. Current situation of hepatocellular carcinoma in Mongolia. Oncology. 2011;81(Suppl 1):148–151. doi: 10.1159/000333278. [DOI] [PubMed] [Google Scholar]
- 6.Dondog B, Lise M, Dondov O, Baldandorj B, Franceschi S. Hepatitis B and C virus infections in hepatocellular carcinoma and cirrhosis in Mongolia. Eur J Cancer Prev. 2011;20(1):33–39. doi: 10.1097/cej.0b013e32833f0c8e. [DOI] [PubMed] [Google Scholar]
- 7.Sandagdorj T, Sanjaajamts E, Tudev U, Oyunchimeg D, Ochir C, Roder D. Cancer incidence and mortality in Mongolia - National Registry Data. Asian Pac J Cancer Prev. 2010;11(6):1509–1514. [PubMed] [Google Scholar]
- 8.Oyunsuren T, Kurbanov F, Tanaka Y, et al. High frequency of hepatocellular carcinoma in Mongolia; association with mono-, or co-infection with hepatitis C, B, and delta viruses. J Med Virol. 2006;78(12):1688–1695. doi: 10.1002/jmv.20755. [DOI] [PubMed] [Google Scholar]
- 9.Oyunsuren T, Sanduijav R, Davaadorj D, Nansalmaa D. Hepatocellular carcinoma and its early detection by AFP testing in Mongolia. Asian Pac J Cancer Prev. 2006;7(3):460–462. [PubMed] [Google Scholar]
- 10.Sun Z, Zhu Y, Xia J, et al. Status of and prospects for cancer vaccines against hepatocellular carcinoma in clinical trials. Biosci Trends. 2016;10(2):85–91. doi: 10.5582/bst.2015.01128. [DOI] [PubMed] [Google Scholar]
- 11.Sawada Y, Yoshikawa T, Ofuji K, et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5(5):e1129483. doi: 10.1080/2162402X.2015.1129483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterfield LH, Economou JS, Gamblin TC, Geller DA. Alpha fetoprotein DNA prime and adenovirus boost immunization of two hepatocellular cancer patients. J Transl Med. 2014;12:86. doi: 10.1186/1479-5876-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang M, Peng BG, Lu MD, et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10(5):1574–1579. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 14.Takayama T, Sekine T, Makuuchi M, et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Alfa GK, Capanu M, O’Reilly EM, et al. A phase II study of cixutumumab (IMC-A12, NSC742460) in advanced hepatocellular carcinoma. J Hepatol. 2014;60(2):319–324. doi: 10.1016/j.jhep.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breitbach CJ, Moon A, Burke J, et al. A Phase 2, open-label, randomized study of Pexa-Vec (JX-594) administered by intratumoral injection in patients with unresectable primary hepatocellular carcinoma. Methods Mol Biol. 2015;1317:343–357. doi: 10.1007/978-1-4939-2727-2_19. [DOI] [PubMed] [Google Scholar]
- 17.Palmer DH, Midgley RS, Mirza N, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Staveley-O’Carroll KF, Li G. Immune-based therapy clinical trials in hepatocellular carcinoma. J Clin Cell Immunol. 2015;6(6) doi: 10.4172/2155-9899.1000376. pii:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S, Li B, Xie H, et al. Phase I clinical trial of targeted therapy using 131I-Hepama-1 mAb in patients with hepatocellular carcinoma. Cancer Biother Radiopharm. 2004;19(5):589–600. doi: 10.1089/cbr.2004.19.589. [DOI] [PubMed] [Google Scholar]
- 20.Knox JJ, Qin R, Strosberg JR, et al. A phase II trial of bevacizumab plus temsirolimus in patients with advanced hepatocellular carcinoma. Invest New Drugs. 2015;33(1):241–246. doi: 10.1007/s10637-014-0169-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee JH, Lee Y, Lee M, et al. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br J Cancer. 2015;113(12):1666–1676. doi: 10.1038/bjc.2015.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batdelger D, Dandii D, Dahgwahdorj Y, et al. Clinical experience with therapeutic vaccines designed for patients with hepatitis. Curr Pharm Des. 2009;15(11):1159–1171. doi: 10.2174/138161209787846793. [DOI] [PubMed] [Google Scholar]
- 23.Batdelger D, Dandii D, Jirathitikal V, Bourinbaiar AS. Open label trial of therapeutic hepatitis B vaccine V-5 Immunitor (V5) delivered by oral route. Lett Drug Des Discov. 2007;4(8):540–544. [Google Scholar]
- 24.Batdelger D, Dandii D, Jirathitikal V, Bourinbaiar AS. Open label trial of therapeutic immunization with oral V-5 Immunitor (V5) vaccine in patients with chronic hepatitis C. Vaccine. 2008;26:2733–2737. doi: 10.1016/j.vaccine.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Arjanova OV, Prihoda ND, Yurchenko LV, et al. Phase 2 trial of V-5 Immunitor (V5) in patients with chronic hepatitis C co-infected with HIV and Mycobacterium tuberculosis. J Vaccines Vaccin. 2010;1:103. [Google Scholar]
- 26.Tarakanovskaya MG, Chinburen J, Purevsuren G, et al. Immunotherapy of liver cancer with hepcortespenlisimut-L: open-label Phase II clinical study in patients with advanced HCC. J Immunother Cancer. 2015;3(Suppl 2):P200. [Google Scholar]
- 27.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 28.Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Mizuno H, Fushimi H. Spontaneous necrosis of hepatocellular carcinoma: a case report. Dig Surg. 2002;19(5):413–418. doi: 10.1159/000065822. [DOI] [PubMed] [Google Scholar]
- 30.Stoelben E, Koch M, Hanke S, et al. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg. 1998;383(6):447–452. doi: 10.1007/s004230050158. [DOI] [PubMed] [Google Scholar]
- 31.Okano A, Ohana M. Spontaneous regression of hepatocellular carcinoma: its imaging course leading to complete disappearance. Case Rep Oncol. 2015;8(1):94–100. doi: 10.1159/000375486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alqutub A, Peck D, Marotta P. Spontaneous regression of a large hepatocellular carcinoma: case report. Ger Med Sci. 2011;9:Doc07. doi: 10.3205/000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14(7):717–725. doi: 10.1634/theoncologist.2009-0038. [DOI] [PubMed] [Google Scholar]
- 34.Riaz A, Ryu RK, Kulik LM, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009;27(34):5734–5742. doi: 10.1200/JCO.2009.23.1282. [DOI] [PubMed] [Google Scholar]
- 35.Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116(19):4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- 36.Kuzuya T, Asahina Y, Tsuchiya K, et al. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81(3–4):251–258. doi: 10.1159/000334454. [DOI] [PubMed] [Google Scholar]
- 37.Yau T, Yao TJ, Chan P, et al. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011;16(9):1270–1279. doi: 10.1634/theoncologist.2011-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakazawa T, Hidaka H, Takada J, et al. Early increase in α-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2013;25(6):683–689. doi: 10.1097/MEG.0b013e32835d913b. [DOI] [PubMed] [Google Scholar]
- 39.Kawaoka T, Aikata H, Kan H, et al. Clinical outcome and prognostic factors of patients with hepatocellular carcinoma and extrahepatic metastasis treated with sorafenib. Hepatol Res. 2014;44(13):1320–1328. doi: 10.1111/hepr.12307. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Kim BK, Kim SU, et al. Early α-fetoprotein response predicts survival in patients with advanced hepatocellular carcinoma treated with sorafenib. J Hepatocell Carcinoma. 2015;2:39–47. doi: 10.2147/JHC.S79353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 43.Silin DS, Lyubomska OV, Jirathitikal V, Bourinbaiar AS. Oral vaccination: where we are? Expert Opin Drug Deliv. 2007;4(4):323–340. doi: 10.1517/17425247.4.4.323. [DOI] [PubMed] [Google Scholar]
- 44.Bourinbaiar AS, Mezentseva MV, Butov DA, et al. Immune approaches in tuberculosis therapy: a brief overview. Expert Rev Anti Infect Ther. 2012;10(3):381–389. doi: 10.1586/eri.12.1. [DOI] [PubMed] [Google Scholar]
- 45.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22(6):1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 46.Gay N, Prasad V. Few people actually benefit from ‘breakthrough’ cancer immunotherapy. STAT. 2017. [Accessed March 8, 2017]. Available from https://www.statnews.com/2017/03/08/immunotherapy-cancer-breakthrough/
- 47.Menis J, Litière S, Tryfonidis K, Golfinopoulos V. The European Organization for Research and Treatment of Cancer perspective on designing clinical trials with immune therapeutics. Ann Transl Med. 2016;4(14):267. doi: 10.21037/atm.2016.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164(6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]