Abstract
Recent advances in biosensors, medical instrumentation, and information processing and communication technologies (ICT) have enabled significant improvements in healthcare. However, these technologies have been mainly applied in clinical environments, such as hospitals and healthcare facilities, under managed care by well-trained and specialized individuals. The global challenge of providing quality healthcare at affordable cost leads to the proposed paradigm of P reventive, Personalized, and Precision Medicine that requires a seamless use of technology and infrastructure support for patients and healthcare providers at point-of-care (POC) locations including homes, semi or pre-clinical facilities, and hospitals. The complexity of the global healthcare challenge necessitates strong collaborative interdisciplinary synergies involving all stakeholder groups including academia, federal research institutions, industry, regulatory agencies, and clinical communities. It is critical to evolve with collaborative efforts on the translation of research to technology development toward clinical validation and potential healthcare applications. This special issue is focused on technology innovation and translational research for POC applications with potential impact in improving global healthcare in the respective areas. Some of these papers were presented at the NIH-IEEE Strategic Conference on Healthcare Innovations and POC Technologies for Precision Medicine (HI-POCT) held at the NIH on November 9–10, 2015. The papers included in the Special Issue provide a spectrum of critical issues and collaborative resources on translational research of advanced POC devices and ICT into global healthcare environment.
Keywords: Point-of-care technologies, healthcare innovation, preventive medicine, personalized medicine, precision medicine, patient monitoring, medical informatics, medical devices, wearable devices, home based monitoring
This paper introduces the concepts and articles included in the IEEE JTEHM special issue on Healthcare Innovations and Point-of-Care Technologies - 2015.
I. Introduction
Collaborative synergies in science, engineering and pharmaceutical research have led to the development of medical technologies, drugs and rehabilitation protocols to diagnose, treat and manage critical illnesses such as cancer, heart diseases and neurological disorders improving healthcare and life expectancy. Though successful, these advances are delivered mainly in managed clinical environments such as physician offices and hospitals for diagnosis, treatment and rehabilitation. The traditional research and technology development synergies have been somewhat limited by a lack of cross-disciplinary interaction among the academic and federal research, industry, regulatory, and clinical communities. Furthermore, these traditional approaches not only have prolonged the time from research and innovation to clinical translation and acceptance, they have also significantly contributed to overall higher healthcare costs. The global challenge of providing quality healthcare with preventive, personalized and precision medicine at affordable cost calls for a collaborative paradigm with active partnership among all stakeholder groups including researchers and technology developers, healthcare providers, patients, regulatory sectors, and payors in treating patients effectively and helping them stay healthy. It is critical to further develop this paradigm with advanced technological and communication protocols that connect patients with clinicians and provide seamless healthcare from point-of-care to clinics, hospitals and intensive care units.
Most biomedical research is constrained within academic, federal or industrial sectors where clinical needs and challenges are identified by researchers without prominent participation from healthcare providers, and data from patient responses on specific clinical protocols and procedures. Even though major advances have recently been made in the development of devices for environment and lifestyle sensing, cancer diagnosis and monitoring, less progress has been made in the detection of infectious and non-communicable diseases, and other applications. A collaborative global platform involving researchers with active participation from clinicians and industry in a data-rich environment with patient-centered information databases could develop the much-needed synergy and foundation for development and implementation of future POC technologies in diverse clinical or semi-clinical settings for quality global healthcare. POC devices have the potential to transform medicine by making laboratory tests simpler, faster, more affordable, and portable, even in regions with little laboratory infrastructure. Such devices can deliver large amounts of continuous or real-time data to researchers, clinics, and public health agencies. With these advances come enormous challenges associated with standardization and data integration.
An initiative of bringing stakeholder groups to discuss global healthcare challenges and future trends in biomedical POC technologies for monitoring, diagnosis and therapeutic interventions was launched in partnership of Engineering in Medicine and Biology Society (EMBS) of the Institute of Electrical and Electronics Engineers (IEEE) and National Institutes of Health (NIH) with the First Special Topic International Conference on Point of-Care Healthcare Technologies in 2013 In Bangalore, India. This initiative was followed by Second Special Topic Conference on Healthcare Innovations and Point-Of-Care Technologies held in 2014 The NIH-IEEE Strategic Conference on Healthcare Innovations and Point-of-Care Technologies for Precision Medicine (HI-POCT) held at the NIAID Conference Center on November 9-10, 2015 brought representatives from all stakeholder groups to discuss critical issues in developing and implementing point-of-care technologies.
II. Background: POC for Global Quality Helathcare Challenge
To address the grand challenge of providing preventive, personalized and precision medicine based quality global healthcare at affordable cost, specifically with POC technologies, we started an initiative of bringing stakeholder groups in partnership of Engineering in Medicine and Biology Society (EMBS) of the Institute of Electrical and Electronics Engineers (IEEE) and National Institutes of Health (NIH). The First IEEE International Conference on Point-of-Care Healthcare Technologies was held in Bangalore, India on January 16-18, 2013. The conference provided an international forum with clinicians, healthcare providers, industry experts, innovators, researchers, and students to discuss clinical needs and technology solutions toward commercialization and translation to clinical applications across different environments and infrastructures. The conference, with sponsorships and participation from NIH, IBM, Phillips, Kyoto Hospital, Apollo Hospital, and other healthcare agencies also focused on challenges in developing and using point-of-care technologies for quality global healthcare. This meeting was then followed by the IEEE Conference on Healthcare Innovations and POC Technologies held in Seattle on October 8-10, 2014 that further emphasized collaborative opportunities and resources for applications of POC technologies in developing and developed economies to address regional priorities for clinical needs with technology innovations in medical devices, translational engineering, information and communication technologies, infrastructure support, and patient and clinician acceptance of POC healthcare technologies [1]–[4].
It was emphasized that POC developers focus on regional needs towards the overall goal of global healthcare since the environment and social behavior vary according to a region’s economic growth, gross domestic product as well as geographical location. These regional need priorities include pre-natal monitoring, hypertension, infectious and chronic disease management and monitoring, therapeutic intervention in treatment of critical diseases such as cancer, rehabilitation, and medical information system to allow remote and evidence based medicine.
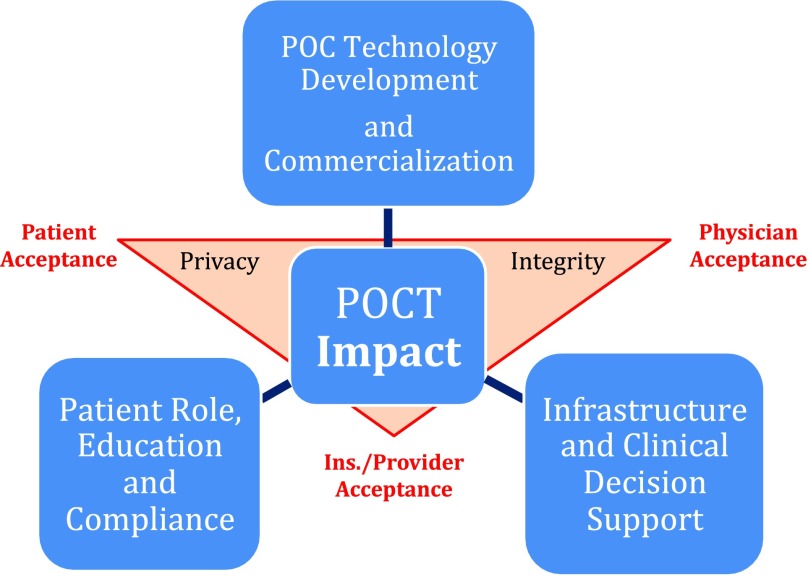
Discussion in previous conferences led to the development of a patient-centered data rich environment with technology innovation, information processing and communication protocols towards acceptances from patients, clinicians and payors. Figure 1 shows that data privacy, security and integrity must be integrated with POC technologies to be effective and accepted in the clinical environment.
Fig. 1.
POCT impact and paradigm with major infrastructure support and issues (taken from [1]).
The Precision Medicine Initiative (PMI), announced during the January 2015 State of the Union address, is designed “to enable a new era of medicine through research, technology, and policies that empower patients, researchers, and providers to work together toward development of individualized treatments.” [2]–[5] In keeping with these goals, the NIH-IEEE 2015 Strategic Conference on Healthcare Innovations and Point-of-Care Technologies for Precision Medicine held on November 9-10, 2015 at the National Institute of Allergy and Infectious Disease (NIAID) Conference Center, Bethesda, brought together patients, clinicians, engineers, industry leaders, and representatives of government to discuss clinical needs for expeditious, continuous, and/or real-time physiological and lifestyle data and emerging technologies to enable the delivery of accurate and affordable personalized medicine.
III. Procedures and Methods: Conference Program
The NIH-IEEE 2015 Strategic Conference on Healthcare Innovations and POC Technologies for Precision Medicine was planned and organized by representatives from the IEEE Engineering in Medicine & Biology Society (EMBS), National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Bill & Melinda Gates Foundation (BMGF), the National Cancer Institute (NCI), the NIAID, the National Heart, Lung, and Blood Institute (NHLBI), the Point of Care Technologies Research Network (POCTRN), CVS Caremark, PATH, and Arizona State University. The conference was attended by more than 218 representatives from academic, federal, industry, clinical, FDA and entrepreneurial communities. The distribution of stakeholders is shown in Table 1.
TABLE 1. Percentage of conference attendees representing major stakeholder groups.
| Stakeholder Group | Attendance Percentage |
|---|---|
| Academic Researchers and Students | 29% |
| Federal Agencies and Research Labs | 18% |
| Industry Representatives | 27% |
| Clinical Researchers and Practitioners | 26% |
The conference program included invited keynote and panel presentations on clinical needs, enabling POC technologies for monitoring, regulatory issues for POC devices, business models for sustainable POC businesses and funding opportunities on the first day. Four breakout sessions on the second day addressed strategic needs and challenges with the following topics:
-
1.
POC Technologies in Resource-Limited Settings
-
2.
Clinical Use and Acceptance of POC Technologies
-
3.
Patient Education and Acceptance of POC Technologies
-
4.
Comprehensive Diagnostic Evaluation and Validation of POC Devices
The conference featured 81 oral and poster presentations on current POC technologies for a spectrum of clinical applications including diagnosis and monitoring of cancer, infectious diseases, neurological disorders, hypertension and cardiac arrhythmia. The conference concluded with a panel discussion that included reports from breakout sessions, future plans and specific recommendations. A detailed report of the conference discussion and presentations is available on online on the website URL [3].
IV. Overall Goal: Clinical Impact
The overall goal of this strategic conference was to encourage and support the next generation of scientists to pursue creative new advances for detecting, measuring, and analyzing a wide range of biological information including molecular, genomic, cellular, clinical, behavioral, physiological, and environmental parameters and to provide opportunities for stakeholders to explore collaborations and synergies to accelerate healthcare system development, validation, deployment, and adoption of point-of-care technologies (POCT) for precision medicine to improve global healthcare at affordable cost.
It is important to improve accessibility and quality of healthcare while reducing costs and, optimally, to develop technologies that are networked among patient informational and clinical management systems to enable precision medicine in various environments including low-resource settings. Challenges that the POC field is beginning to address include early detection and prevention of diseases, anywhere access to healthcare, equity in healthcare outcomes, therapeutic interventions, and increased care for chronic diseases in an aging population.
The concept of precision medicine is not new, but POCTs will be very important for its future advancement. In 2004 a commentary laid out the potential use of genomics for a prospective cohort study of genes and environment in the United States [2] however, the cost of sequencing at that time was prohibitive. Since then, the cost of sequencing has decreased, and therefore its use has increased, both substantially. In 2009, the Health Information Technology for Economic and Clinical Health (HiTECH) Act directed the Office of the National Coordinator for Health Information Technology (ONC) to promote the adoption of electronic health records (EHRs). In parallel, the POC field has seen a large increase in the uptake of wearable sensors and mobile technologies. For example, a short-term goal of the Precision Medicine Initiative (PMI) may be to apply personalized strategies to cancer treatments, but the longer-term goal is to apply the same principles to all areas of future medicine. The PMI will support research to create new approaches to detect, measure, and analyze a wide variety of molecular, genomic, cellular, physiological, behavioral, and environmental variables, test these approaches in small pilot studies, and utilize the most promising approaches in larger numbers of people over longer periods of time [4].
V. Enabling POC Technologies
The plenary sessions focused on the importance of collecting more data and making it available for biomedical research to allow longitudinal studies for healthcare and preventive medicine. It is important to include social and health risk behavior parameters such physical activity, smoking, sun exposure, environmental exposures, and dietary intake in computer models for risk assessment to determine the target screening-group for POC devices to collect healthcare related data. Innovative POC devices to collect data may include the affinity dual hormone sensor, transdermal alcohol sensor, non-contact electrocardiogram sensor, edible adherence sensor, real-time food intake sensor, and continuous pulse oximetry—but in each case the data must be intelligently integrated to provide feedback and to incentivize people to lead healthier lives.
Through special panel sessions, the conference focused on innovative POC technologies addressing clinical needs in treatment, monitoring and therapeutic intervention. Researchers and clinicians from cancer centers presented clinical case studies of using POC technologies in detection and treatment critical diseases such as cancers. A physician and a cancer surviving patient discussed their experience as a living proof of recent progress in the treatment of Chronic Myelogenous Leukemia (CML). Because of treatment advances over the past two decades, CML has become a survivable, chronic condition. POC technologies have started to make an impact in cancer treatment in developing countries. GeneExpert qPCR analyzer, one of the successful systems from Cepheid (http://www.cepheid.com/us/), is a low cost device that can be used to diagnose CML and monitor patients receiving tyrosine kinase inhibitors.
The GeneXpert devices are portable, user-friendly, cartridge-based devices for automated sample preparation and multiplex quantitative reverse transcription-PCR (qRT-PCR). They can accommodate a large number of clinical sample types (e.g., blood, plasma, serum, urine, stool, formalin-fixed paraffin-embedded tissue, sputum, and vaginal and skin swabs) and deliver test results within a few hours. The devices can perform 18 FDA-approved tests and 23 tests that are available outside the United States. GeneXpert POCT is in widespread use in developing countries: more than 9,000 systems have been deployed worldwide; at least one device is present in all but 10 countries. The GeneXpert Omni is an especially small and portable unit that can connect with cell phone towers for automated remote transmission to a data cloud and can accelerate clinical decision-making in the field.
In another presentation from the researchers and technology developers at PATH medical center (http://www.path.org/), performance and effectiveness of several POC testing devices were discussed. Laboratories in rural China reported 84 percent sensitivity and 87.5 percent specificity using care HPV, and field tests in India, Uganda, and Nicaragua reported similar results.
Several enabling technologies were presented that currently are in clinical/field evaluation of low-cost POC diagnosis for critical global challenges including optical devices for cervical cancer diagnosis, and acoustofluidics devices using acoustic waves to manipulate microfluidic flow for sputum analysis for the diagnosis and treatment of asthma, tuberculosis, lung cancer, cystic fibrosis, and chronic obstructive pulmonary disease.
NIH funded Center for Point-of-Care Tests for Sexually Transmitted Diseases has been focusing on developing home-based urine and self-collected swab tests that are as sensitive as laboratory tests. Currently, POC tests are being developed for chlamydia, gonorrhea, trichomonas, syphilis, herpes simplex virus (HSV), and HIV. Potential advantages include immediate treatment before patients leave the clinic (with no loss to follow-up), decreased chance for the disease to spread, and potential for counseling. Tests for Trichomonas include the OSOMⓇRapid TV Antigen Test (immuno-chromatographic detection) and the AmpliVueⓇAssay (colorimetric detection of amplified DNA on a lateral-flow strip). The Syphilis HealthCheckTM uses rapid immune-chromatography to detect human anti-treponemal antibodies. Chembio Diagnostic Systems offers a Dual Path Platform test that simultaneously tests for syphilis and HIV. Rapid POC tests for HIV include the OraQuick AdvanceⓇ, Uni-Gold RecombigenⓇ, Alere DetermineTM, INSTITM, ClearviewⓇComplete, and ClearviewⓇStat Pak. Tests for HSV include the Biohelix IsoAmpⓇAssay. The NIH and the Viral Hemorrhagic Fever Consortium (VHFC) have been interested in the development of new rapid POC diagnostic tests for Lassa fever and Ebola. Such POC tests and devices can provide a cost-effective means to achieving a large public health benefit in resource-limited settings and can upload data to healthcare providers through smartphones, and Information and Communication Technology (ICT). Wearable sensors along with smartphones and ICT can provide effective screening tests on high-risk patients at homes or nursing facilities.
Wearable sensors as a part of the POC monitoring devices produce large data sets with specific challenges on the accuracy, reliability, integrity and security of the data for meaningful analysis for clinical/healthcare use. In addition, for better effectiveness it is critical that there are interoperability standards to connect devices with EHR/EMR following common data formats. As professional societies and industry stakeholders get involved with the development of standards for POC devices, they also need to work with regulatory agencies towards bringing validated devices in the market for reliable clinical/healthcare use. Open ICE [8], [9] (based on a published standard, American Society for Testing and Materials (ASTM) F2761) provides a standards-based system architecture to allow for safety and performance and the required interoperability.
Booz Allen Hamilton is involved in the development of advanced data analytics and interface systems to convert data from wearable sensors and telemedicine data sources to perform clinical studies. However, integrity and authentication of data provided by the patients is a continued concern of mobile devices based data management. The data analysis at the POC information processing platforms such as smartphones is essential for managing large data sets to allow only meaningful data uploads to clinical/healthcare providers when needed. It is still an undefined challenge to determine the reliability and associated liabilities of POC diagnostics and transmission of the required amount of data to clinical/healthcare facilities or EHR/EMR systems.
VI. Future Trends and Challenges
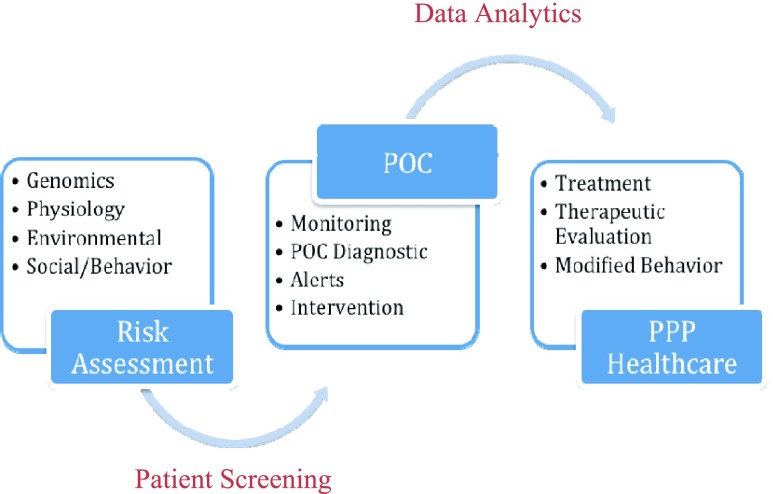
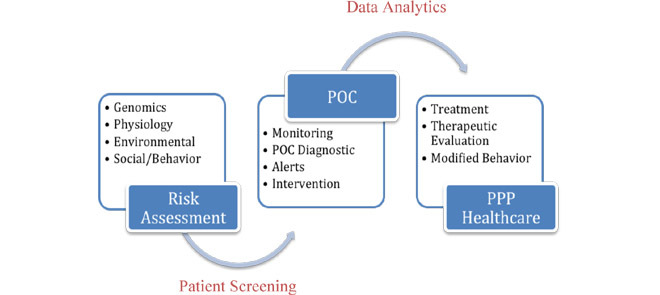
Though the top healthcare challenges and causes of death vary from low-income to middle and high income communities across developing and developed nations, there are common global healthcare challenges involving infection and communicable disease, and non-communicable diseases including ischemia and heart diseases, cancer, diabetes, strokes and neurological disorders. The trends in POC technology development are critically focused on molecular, cellular, microfluidic, acoustics based tests, and non-communicable disease screening technologies as well as biosensors based monitoring. The data collection and informatics for real-time data analysis are critical for timely POC diagnostics, alerts and preventive, personalized and précised healthcare. Thus, POC technologies may be used for screening and monitoring of high-risk patients with reliable diagnostics and data analytics for meaningful clinical use. The meaningful use of the accurate, reliable and secured data can improve the clinical management, treatment and therapeutic intervention, and help providing quality healthcare (Figure 2). The POC technologies combined with ICT can outreach patients for adherence and behavior change towards preventive medicine and staying healthy.. Changing workflows to test and treat patients in a single visit was suggested to increase efficiency [13].
Fig. 2.
Application and impact of POC Technologies in Personalized, Preventive and Precision (PPP) Medicine.
Translational of POC technologies from research and innovation stage to clinical validation and commercialization should be adopted by various stakeholders including investors, industry, clinicians, patients and regulation agencies. All stakeholders should be brought on board early in the process. Healthcare systems should be scalable and standardized in terms of setup, process, and systems, creating challenges so that they can be connected to medical records and healthcare databases. Policy drives reimbursement and regulation, which can be critical. System-level considerations to maximize adoption and sustainability in the clinical environment are data integration, financial modeling, workflow design, and clinical outcomes.
Data quality assurance, data integration, and data integrity solutions are hampered by the fact that the field is evolving at a rapid pace. Glucose monitors, for example, are being provided by many vendors using a plethora of proprietary formats. Fundamentally, for researchers to get more value out of available data, policies are needed to direct standardization. Otherwise, data will remain locked in independent silos.
The future strategic directions may be focused on developing opportunities and platforms to facilitate co-invention of future POC technologies through active collaborations among all stakeholders. The intent is to help deliver quality global healthcare with emphasis on personalized and preventive medicine. Specific tactics to build strategic alliances and partnerships may be focused on the following.
-
1.
Development a global healthcare collaborative network (GHCN) to develop databases of specific clinical needs to prioritize needs and define required specifications for potential technology and solutions and healthcare protocols.
-
2.
Development an open forum for researchers and all stakeholders to address clinical needs with potential technological solutions and to discuss challenges of the required clinical response.
-
3.
Development of protocols on data standards and interoperability requirements, including policies for data quality, integrity, consistency, connectivity, and data sharing for translation of enabling technologies and potential solutions in different environments including under-resourced, rural, and remote clinical facilities.
-
4.
Development of protocols on integration of interoperable devices, device to device communication, and data exchange and integration with EHR/EMR systems.
-
5.
Development of training protocols and means at multiple levels to educate end users to adapt technological solutions for clinical applications.
VII. Special Issue
The JTEHM Special Issue on Healthcare Innovations and POC Technologies presents selected papers from the presentations held at the NIH-IEEE 2015 Strategic Conference on Healthcare Innovations and POC Technologies for Precision Medicine and those submitted in response of the open call for papers. These papers are available through open access JTEHM website http://health.embs.org/.
National Institute of Biomedical Imaging and Bioengineering initiated a POCTRN program to emphasize multidisciplinary partnerships and close facilitation translation of POC technologies from early stage of development into clinical testing and patient use. The paper, National Institute of Biomedical Imaging and Bioengineering Point-of-Care Technology Research Network: Advancing Precision Medicine by Ford Carleton, Steven Schachter, John A. Parrish, John M. Collins, Ben Crocker, Ronald Dixon, Susan Edgman-Levitan, Kent Lewandrowski, James Stahl, Catherine Klapperich, Mario Cabodi, Charlotte A. Gaydos, Anne M. Rompalo, Yukari Manabe, Tza-Huei Wang, R Rothman, Chris D. Geddes, Lea Widdice, Joany Jackman, Rishi A. Mathura, and Tiffani Bailey Lash present a detailed introduction of the POCTRN program and the three currently funded Centers as examples of academic-based organizations that support collaborations across disciplines, institutions, and geographic regions to successfully drive innovative solutions from concept to patient care [14]. These three centers have promoted the development and translation of various POC technologies to clinical validation and pre-commercialization.
Another NIH initiative on global healthcare is described in the paper, The National Institutes of Health Affordable Cancer Technologies Program: Improving Access to Resource-Appropriate Technologies for Cancer Detection, Diagnosis, Monitoring and Treatment in Low- and Middle-Income Countries by Paul C. Pearlman, Rao Divi, Michael Gwede, Pushpa Tandon, Brian S. Sorg, Miguel R. Ossandon, Lokesh Agrawal, Vinay Pai, Houston Baker, and Tiffani Bailey Lash. This paper specifically focuses on issues related to Point-of-care (POC) technologies in cancer detection, diagnosis, monitoring, and treatment in the developed world for potential impact in the low-and-middle-income countries (LMIC) [15]. Among several POC innovations, the paper explores infectious etiologies in many preventable and treatable cancers in LMICs, such as cervical cancer, one of the most common causes of cancer death for women in many LMICs.
As mentioned above, it is critical for POC devices to be interoperable and easy to connect to data communication and analytics systems. They must be validated and celebrated with specific standards to provide accurate and reliable information for meaningful use in healthcare. Sandy Weininger, Michael B. Jaffe, Michael Robkin, Tracy Rausch, David Arney, and Julian M. Goldman argue on this important challenge in their paper, The Importance of State and Context in Safe Interoperable Medical Systems describing the roles of devices with respect to interactions such as human user workflows and device to device communication in clinical environment [16].
Point-of-Care technologies may also provide an effective solution on monitoring and management of patients with high risks of heart, lung, blood, and sleep (HLBS) disorders. HLBS disorders can be better diagnosed and treated with the monitoring of transient variables including physiological monitors and activity level. Mary Emma Gorham Bigelow, Brian G. Jamieson, Chi On Chui, Yufei Mao, Kyeong-Sik Shin, Tony Jun Huang, Po-Hsun Huang, Liqiang Ren, Bishow Adhikari, Jue Chen, and Erin Iturriaga, describe three POC technologies in their paper, Point-of-Care Technologies for the Advancement of Precision Medicine in Heart, Lung, Blood, and Sleep Disorders [17]. The paper rightly points out the critical importance of HLBS in healthcare as Cardiovascular disease (CVD) and strokes accounted for $195.6 billion in healthcare costs in United States in 2011. Cardiovascular disease (CVD) remains the leading cause of death in the United States, accounting for 1 in 3 deaths. Semiconductor Electronic Label-Free Assay (SELFA) technology is being developed and commercialized as a sample-to-answer POC diagnostic device by Selfa, Inc. Diagnostic Biochips has demonstrated the continuous monitoring of the chemotherapeutic agent doxorubicin in whole blood. The electrochemical sensors are embedded in a standard IV catheter, or the “SmartIVTM” blood monitoring platform. Such technologies can provide personalized delivery of therapies based on individual pharmacokinetics and pharmacodynamics. The acoustofluidic technology platform described in this paper is the beginning of use of POC devices in developing personalized treatment strategies to asthmatic patients in both research and community settings. These three technologies are now in translational pre-commercialization phase with a strong potential clinical impact [17].
Akilan Palaniswami, Shazia Khan, Sultan Siebel Erdem, and Tayyaba Hasan present a POC system to perform fluorescent microfluidic assay designed to rapidly determine antibiotic susceptibility for a range of bacterial pathogens that commonly occur in patients seen in clinical practice [18]. In their paper, Guiding Empiric Treatment for Serious Bacterial Infections via Point of Care B-Lactamase Characterization, authors evaluates the potential application of their assay technology in the selection of the appropriate antibiotic for personalized and precision medicine reducing the societal threat of antibiotic resistance.
The paper, Elucidating the Hemodynamic Origin of Ballistocardiographic Forces: Towards Improved Monitoring of Cardiovascular Health at Home by Abdul Qadir Javaid, Hazar Ashouri, Srini Tridandapani, and Omer T. Inan focuses on the mathematical modeling between the BCG signal and impedance cardiography (ICG), and arterial blood pressure (ABP) waveforms, to monitor cardiac output and blood pressure asynchronously. This study can lead to the development of POC tools for monitoring heart failure (HF) patients at home following the discharge from the hospital, with the goal of potentially predicting exacerbations and thus reducing unnecessary re-hospitalizations [19].
Considering the continuously increasing trends in longevity and more older people living alone, the monitoring of their wellness at home is an important global issue. As authors, Johanna Austin, Hiroko H. Dodge, Thomas Riley, Peter G. Jacobs, Stephen Thielke, and Jeffrey Kaye explain in their paper, A Smart-home System to Unobtrusively and Continuously Assess Loneliness in Older Adults, older adults often suffer from loneliness associated with increased morbidity and mortality, decreased sleep quality, and increased risk of cognitive decline [20]. They present a system to measure loneliness by assessing in-home behavior using wireless motion and contact sensors, phone monitors, and computer software as well as algorithms developed to assess key behaviors of interest. This approach, if successful, can help in improving quality of life of older adults living alone at home.
While the POC devices have to provide meaningful information for clinical or patient management, they have to be inexpensive, easy-to-use and interoperable with some form of calibration capabilities. The POC devices are often compared with more expensive and sophisticated devices used in the hospitals under trained healthcare providers. It is not clear what could be an acceptable accuracy, sensitivity and specificity for meaningful use in healthcare. Matthew Thompson, Bernhard Weigl, Annette Fitzpatrick, and Nicole Ide argue on the issues beyond accuracy in their paper, More Than Just Accuracy: A Novel Method to Incorporate Multiple Test Attributes in Evaluating Diagnostic Tests Including Point of Care Tests [21]. They suggest a novel way to weigh different attributes to devise standards so that multiple stakeholders can use to visualize test attributes, their interactions, and impacts on individual and population outcomes as needed for their respective specific application. Such adaptive protocols may facilitate better informed decisions on POC diagnostics in healthcare.
VIII. Conclusion
The POC technology development and translation to patient-centric environment for healthcare applications may lead to critical global solutions for better personalized, preventive and precision medicine. However, there is a wide spectrum of challenges including clinical acceptance, payor acceptance and patient adherence. These issues are closely correlated with the way POC technology may be used in patient or clinical management for diagnosis, treatment, therapeutic intervention, and/or rehabilitation. They also raise a challenging need of involving multiple stakeholders such as clinicians, industry, patients, and others in the early phases of research and development towards a successful technology translation in healthcare. It is not clear how clinicians, investors and other stakeholders can collaboratively participate in early phases of development of specifications of clinical needs and potential technology solutions across the globe as the regional needs and technology acceptances are often not the same in developing and developed economies among diverse income levels and regulatory infrastructures.
A summary of panel discussions from the IEEE-NIH 2015 Strategic Conference on Healthcare Innovations and POC Technologies for Precision Medicine has been presented here with comments on future trends and challenges. This special issue of selected papers demonstrates needs, significance, and translational research of some of the potential POC technologies. It is clear that we need to continue co-inventing the future of POC technology with multiple stakeholder groups towards the development of a global paradigm to address critical needs, and provide quality healthcare at affordable cost.
Acknowledgement
This paper is based on the discussions held at the IEEE-NIH 2015 Strategic Conference on Healthcare Innovations and POC Technologies for Precision Medicine. A complete meeting summary was prepared by Susan Spence, PhD, Silvia Paddock, PhD, Payal Shah Martin, MPH, and Rose Maria Li, MBA, PhD, Rose Li and Associates, Inc., under contract to the National Institute of Biomedical Imaging and Bioengineering (NIBIB Contract Number HHSN268201600011A). The views expressed in this document reflect both individual and collective opinions of the meeting participants and not necessarily those of NIBIB, the National Institutes of Health, or meeting cosponsors. Contributions in planning and during the meeting from the following individuals is gratefully acknowledged: Roderic Pettigrew, MD, PhD, Tiffani Lash, PhD, Mary Rodgers, PhD, James Gallarda, PhD, Laura Povlich, PhD, Erin Iturriaga, MSN, Bishow Adhikari, PhD, Rishi Mathura, PhD, Paul Pearlman, PhD, J. Benjamin Crocker, MD, Kent Lewandrowski, MD, Anne Rompala, MD, Bernhard Weigl, PhD, and Ed Livingston, MD, FACS, AGAF.
Biography

Atam P. Dhawan (F’04) is a Distinguished Professor of Electrical and Computer Engineering and a Vice Provost of R&D, NJIT. He has authored over 215 research papers and book chapters. He has also authored and co-authored several books in medical imaging and image analysis. His research interests include medical imaging, medical image analysis, point-of-care technologies, pattern recognition, and computer-aided-diagnosis.
Dr. Dhawan is an Elected Fellow of the National Academy of Inventors and a fellow of the American Institute of Medical and Biological Engineering for his contributions in medical imaging and image analysis, and healthcare innovations. He has been a fellow of the International Academy of Medical and Biological Engineering. He is a recipient of numerous awards, including the Martin Epstein Award (1984), the NIH FIRST Award (1988), the Sigma-Xi Young Investigator Award (1992), the IEEE EMBS Early Career Achievement Award (1995), the Doermann Distinguished Lecture Award (1999), and the EMBS Distinguished Lecturer Award (2012–2013). He has served as the Conference Chair of the IEEE 28th International Conference of Engineering in Medicine and Biology Society, NY, USA (2006). He has also served as the Senior Editor of the IEEE Transactions on Biomedical Engineering, the Editorial Board Member of the International Journal of Pattern Recognition, and the Steering Committee Member of the IEEE Transactions on Medical Imaging. He is currently serves as the Editor-In-Chief of the IEEE Journal of Translational Engineering in Health and Medicine. He is the Founding Chair of the IEEE EMBS Technical Committee on Translational Engineering and Healthcare Innovations. He has organized and chaired the IEEE-EMBS International Conferences on Point-of-Care Technologies and Healthcare Innovation, Bangalore, India (2013), and in Seattle (2014), and co-chaired the NIH-IEEE Strategic Conference on Point-of-Care Technologies for Precision Medicine held at the NIH NIAID Conference Center, Bethesda, in 2015. He served as the Conference Chair of the IEEE-NIH Conference on Healthcare Innovation and Point-of-Care Technologies held in Cancun on 2016. He has chaired numerous NIH special emphasis and review panels, including the NIH Chartered Study Section on Biomedical Computing and Health Informatics (2008–11).
References
- [1].Dhawan A. P., et al. , “Current and future challenges in point-of-care technologies: A paradigm-shift in affordable global healthcare with personalized and preventive medicine,” IEEE J. Transl. Eng. Health Med., vol. 3, 2015, Art. no. 2800110, doi: 10.1109/JTEHM.2015.2400919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].President Initiative on Precision Medicine, accessed on Jan. 25, 2016. [Online]. Available: https://www.whitehouse.gov/precision-medicine
- [3].Collins F. S., “The case for a US prospective cohort study of genes and environment,” Nature, vol. 429, pp. 475–477, May 2004. [DOI] [PubMed] [Google Scholar]
- [4].The Precision Medicine Initiative Cohort Program-Building a Research Foundation for 21st Century Medicine, accessed on Sep. 17, 2015. [Online]. Available: http://www.nih.gov/precisionmedicine/09172015-pmi-working-group-report.pdf
- [5].Dillon K. and Prokesch S., “Global challenges in health care: Is rationing in our future?” Harvard Bus. Rev., Apr. 2010. [Online]. Available: http://blogs.hbr.org/2010/04/global-challenges-in-health-ca/
- [6].Evans D. (Apr. 2011). The Internet of Things: How the Next Evolution of the Internet is Changing Everything. [Online]. Available: http://www.cisco.com/web/about/ac79/docs/innov/IoT_IBSG_0411FINAL.pdf [Google Scholar]
- [7].World Health Organization. Noncommunicable Diseases Fact Sheet, accessed on Jan. 25, 2016. [Online]. Available: http://www.who.int/mediacentre/factsheets/fs355/en/
- [8].Integrating Biology and the Bedside (i2b2), accessed on Jan. 25, 2016. [Online]. Available: https://www.i2b2.org/
- [9].Open-Source Integrated Clinical Environment (OpenICE), accessed on Jan. 25, 2016. [Online]. Available: https://www.openice.info/
- [10].Center for Medicare and Medicaid Services (CMS), accessed on Jan. 25, 2016. [Online]. Available: https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html
- [11].Federal Drug Administration (FDA), accessed on Jan. 25, 2016. [Online]. Available: http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm079632.htm
- [12].FDA Website for Medical Devices, accessed on Jan. 25, 2016. [Online]. Available: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm311176.pdf
- [13].Crocker J. B., Lee-Lewandrowski E., Lewandrowski N., Baron J., Gregory K., and Lewandrowski K., “Implementation of point-of-care testing in an ambulatory practice of an academic medical center,” Amer. J. Clin. Pathol., vol. 142, no. 5, pp. 640–646, 2014. [DOI] [PubMed] [Google Scholar]
- [14].Carleton F., et al. , “National Institute of Biomedical Imaging and Bioengineering point-of-care technology research network: Advancing precision medicine,” IEEE J. Transl. Eng. Health Med., vol. 4, Aug. 2016, Art. no. 2800614 [Online]. Available: http://ieeexplore.ieee.org/document/7544609/?arnumber=7544609, doi: 10.1109/JTEHM.2016.2598837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pearlman P. C., et al. , “The National Institutes of Health Affordable Cancer Technologies program: Improving access to resource-appropriate technologies for cancer detection, diagnosis, monitoring, and treatment in low- and middle-income countries,” IEEE J. Transl. Eng. Health Med., vol. 4, Sep. 2016, Art. no. 2800708 [Online]. Available: http://ieeexplore.ieee.org/document/7563384/, doi: 10.1109/JTEHM.2016.2604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Weininger S., Jaffe M. B., Robkin M., Rausch T., Arney D., and Goldman J. M., “The importance of state and context in safe interoperable medical systems,” IEEE J. Transl. Eng. Health Med., vol. 4, Aug. 2016, Art. no. 2800110 [Online]. Available: http://ieeexplore.ieee.org/document/7536138/?arnumber=7536138, doi: 10.1109/JTEHM.2016.2596283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bigelow M. E. G., et al. , “Point-of-care technologies for the advancement of precision medicine in heart, lung, blood, and sleep disorders,” IEEE J. Transl. Eng. Health Med., vol. 4, Jul. 2016, Art. no. 2800510 [Online]. Available: http://ieeexplore.ieee.org/xpls/icp.jsp?arnumber=7518992, doi: 10.1109/JTEHM.2016.2593920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Palanisami A., Khan S., Erdem S. S., and Hasan T., “Guiding empiric treatment for serious bacterial infections via point of care SS -lactamase characterization,” IEEE J. Transl. Eng. Health Med., vol. 4, Jun. 2016, Art. no. 2800410 [Online]. Available: http://ieeexplore.ieee.org/document/7501817/?arnumber=7501817, doi: 10.1109/JTEHM.2016.2573305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Javaid A. Q., Ashouri H., Tridandapani S., and Inan O. T., “Elucidating the hemodynamic origin of ballistocardiographic forces: Toward improved monitoring of cardiovascular health at home,” IEEE J. Transl. Eng. Health Med., vol. 4, Mar. 2016, Art. no. 1900208 [Online]. Available: http://ieeexplore.ieee.org/document/7440833/?arnumber=7440833, doi: 10.1109/JTEHM.2016.2544752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Austin J., Dodge H. H., Riley T., Jacobs P. G., Thielke S., and Kaye J., “A smart-home system to unobtrusively and continuously assess loneliness in older adults,” IEEE J. Transl. Eng. Health Med., vol. 4, Jun. 2016, Art. no. 2800311 [Online]. Available: http://ieeexplore.ieee.org/document/7488979/?arnumber=7488979, doi: 10.1109/JTEHM.2016.2579638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Thompson M., Weigl B., Fitzpatrick A., and Ide N., “More than just accuracy: A novel method to incorporate multiple test attributes in evaluating diagnostic tests including point of care tests,” IEEE J. Transl. Eng. Health Med., vol. 4, Jun. 2016, Art. no. 2800208. [DOI] [PMC free article] [PubMed] [Google Scholar]