Abstract
Objective
To evaluate the clinical and histopathologic characteristics of patients who develop proliferative vitreoretinopathy after retinoblastoma treatment.
Design
Retrospective review of three cases of proliferative vitreoretinopathy (PVR) that developed after successful treatment of retinoblastoma from 2003 to 2015.
Subjects
Three patients with treated retinoblastoma who developed severe PVR and required enucleation.
Methods
Review of clinical charts, fundus drawings, Ret-Cam 3 images, and histopathology specimens.
Main Outcome Measures
Clinical and histopathologic characterization of PVR in treated retinoblastoma.
Results
Three patients developed severe PVR after sequential thermal laser combined with systemic chemotherapy for retinoblastoma. At presentation patients were 6, 7, and 9 months of age, and all had bilateral retinoblastoma. Time to development of proliferative tissue was 9, 12, and 20 months after initial treatment. Proliferation was characterized by progressive growth of white vascularized tissue with associated traction on the retina and sometimes hemorrhage. All patients underwent enucleation. Histopathologic evaluation revealed treated retinoblastoma tumor with a Type 3 regression pattern, pre- and subretinal fibrovascular tissue consistent with PVR, and reactive changes in the retinal pigment epithelium. None of the patients developed recurrence of retinoblastoma or systemic metastasis.
Conclusion
PVR uncommonly develops after successful treatment of retinoblastoma and may result in traction or rhegmatogenous retinal detachment along with vitreous hemorrhage. Early stages of proliferation may be difficult to distinguish from recurrent tumor. Enucleation may be required due to poor vision and inability to adequately monitor for tumor recurrence.
Précis
Successfully treated retinoblastoma rarely develops fibrovascular proliferation, which can cause traction or rhegmatogenous retinal detachments. Early changes may be difficult to differentiate from recurrent tumor.
Retinoblastoma is the most common pediatric intraocular malignancy and treatment advances have allowed for excellent survival outcomes. Globe salvage has also been possible for many eyes since the advent of combined systemic chemotherapy and sequential thermal laser, or chemoreduction (CRD), in the mid 1990s1–2.With preservation of retinoblastoma eyes, more complications are being recognized, including vitreous, retinal, or choroidal hemorrhage, retinal vascular occlusion, epiretinal membrane formation, glial proliferation, retinal fold, retinal detachment, glaucoma, neovascularization, and cataracts3–4. In this series, we review the clinical and histological findings of 3 patients who developed severe proliferative vitreoretinopathy (PVR) after CRD for retinoblastoma.
Patients and Methods
We reviewed clinical charts, fundus drawings, Ret-Cam 3 images (Clarity Laboratories Inc., Pleasanton, CA), and histopathology specimens of 3 children who developed PVR after successful CRD for retinoblastoma between 2003– 2015. During this time period, all cases of advanced retinoblastoma (group C or worse) were treated with CRD unless they were treated with primary enucleation. This study was exempt from IRB approval.
The patients’ charts were reviewed to determine the age of diagnosis of retinoblastoma, the inheritance, grouping according to the International Classification, and laterality. Treatment details, time to development of PVR, and family history were also recorded.
CRD for these patients consisted of six cycles of carboplatin, etoposide, and vincristine along with sequential laser using an 810 nm diode infrared laser (Iridex, Mountain View, California). In two of the three patients, subtenon carboplatin 20 mg/2ml was given in 3 doses at 1 month intervals according to ARET0231 protocol, and one patient also had intensity-modulated radiation therapy (IMRT).
Enucleated eyes were fixed in 10% neutral buffered formalin. Pupil-optic nerve (P-O) sections as well as the calottes were submitted for routine processing. Tissue sections were cut 5 microns thick and stained with hematoxylin & eosin and Periodic acid Schiff.
Descriptive Findings
Clinical Findings
In each of these cases, there was robust proliferation of smooth, white tissue over an area of treated, regressed retinoblastoma. This proliferation gradually acquired more vascularization and progressed into a thick membrane. Initially, the growing white tissue looked similar to recurrent tumor. There were well-demarcated borders to the membranous plaque, but on careful inspection, fine fibrillar strands were noted to emanate from the edge of the membrane. These fine fibrillar strands helped distinguish these lesions from recurrent retinoblastoma. Transvitreal strands became more prominent as the proliferation progressed. The growth and contraction of this tissue lead to traction retinal detachment. In one patient the traction caused retinal stretch holes and a rhegmatogenous retinal detachment. In all cases, the retinoblastoma was bilateral, and the involved eye had a large tumor burden at the time of diagnosis (group D by the International Classification). In all cases the tumor was inactive at the onset of PVR. In all 3 patients, fellow eyes also developed fibrosis around the treated tumors, even when the tumor burden was small. Table 1 summarizes the three patients.
Table 1.
Patient Characteristics
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age of Diagnosis | 6 month old | 7 month old | 9 month old |
| Gender | Male | Female | Female |
| Laterality | Bilateral | Bilateral | Bilateral |
| Classification | Group D right eye Group D left eye |
Group D right eye Group A left eye |
Group D right eye Group D left eye |
| Number of laser treatments in affected eye |
14 | 18 | 11 |
| Other Treatment | Subconjunctival Carboplatin × 3 with CEV #2–41 |
Subconjunctival Carboplatin × 3 With CEV #3–5 |
IMRT2 Both Eyes |
| Status of fellow eye | Rb3 inactive with fibrosis |
Rb inactive with subtle fibrosis |
Rb inactive with fibrosis |
| Laterality of affected Eye |
Right | Right | Right |
| Enucleation of eye with PVR4 |
Yes | Yes | Yes |
| Time from 1st treatment to PVR4 |
9 months (2/11/10–11/29/10) |
20 months (1/4/10–8/29/11) |
12 months (5/23/03–7/12/04) |
| Family History | No | Yes | No |
| Main Histolopathologic Features |
Type 3 regressed Rb, pre-retinal anterior and posterior PVR, RPE hyperplasia and osseous metaplasia |
Type 3 regressed Rb, pre-retinal posterior PVR, RPE hypertrophy and hyperplasia |
Type 3 regressed Rb, pre- and subretinal anterior and posterior PVR, RPE osseous metaplasia |
CEV = treatment with carboplatin, etoposide, and vincristine. The cycle number is denoted.
IMRT = intensity-modulated radiation therapy
Rb = Retinoblastoma
PVR= Proliferative Vitreoretinopathy
Pathologic Findings
Examination of all the enucleated eyes showed regressed retinoblastoma tumor consisting of gliotic tissue, residual treated tumor cells, and interspersed calcification consistent with a Type 3 regression pattern. The retina was diffusely atrophic with focal traction. All eyes contained fibrovascular tissue overlying tumor and retina, consistent with PVR. In 2 cases, there was anterior PVR with the fibrovascular tissue extending to the area of the vitreous base (Case 1), ciliary body and lens (Case 3). There were reactive changes in the retinal pigment epithelium in the form of hyperplasia, hypertrophy, and osseus metaplasia.
Case Reports
Case 1
At the age of 6 months, a boy was diagnosed with bilateral retinoblastoma. The right eye presented with Group D tumor and a total retinal detachment and the left eye presented with a Group D tumor with subtotal retinal detachment. He was treated with chemotherapy, thermal laser, and subtenon carboplatin (3 doses) in both eyes. Treatment resulted in complete regression of tumors and resolution of the retinal detachments. After completion of the systemic chemotherapy, numerous recurrences were well controlled with laser. He received a total of 14 laser treatments in the right eye and 13 in the left eye. Progressive fibrosis after treatment resulted in retinal stretch holes and total retinal detachment in the right eye (Figure 1). Given the poor visual function and inability to monitor the tumors, the patient underwent enucleation of this eye. As of his last visit, four years after his diagnosis, his best-corrected visual acuity was 20/150 in the remaining eye, and there was no evidence of recurrence of retinoblastoma. There was fibrosis noted in the remaining eye.
Figure 1.
Color fundus photo depicting progression of proliferative vitreoretinopathy and retinal detachment in patient 1
Microscopic examination of the enucleated eye showed an intact cornea, iris and ciliary body. The lens was cataractous. The retina was totally detached and had diffuse atrophy of the nerve fiber and ganglion cell layers. There was an 8 × 8 mm mass arising from the retina composed of gliotic tissue, small round blue cells with bland nuclei consistent with treated tumor, and interposed areas of calcification. There was fibrovascular tissue overlying the tumor and retina extending to the vitreous base. There was pseudoadenomatous hyperplasia and osseous metaplasia of the RPE. There was no evidence of choroid or optic nerve invasion by tumor (Figure 2).
Figure 2.
A. Gross examination of the enucleated eye shows a calcified mass (asterisk) with overlying white fibrous membrane (arrow); B. Histologic section demonstrating regressed retinoblastoma (asterisk), detached retina (arrow), and PVR (arrowhead); C. The PAS stain highlights the blood vessels within the fibrovascular PVR membrane; D. Higher magnification of the area of regressed tumor shows calcification and non-viable tumor cells consistent with Type 3 regression pattern. (Hematoxylin & eosin: B. 10×; Periodic acid Schiff: C. 25×; Hematoxylin & eosin: D. 25×)
Case 2
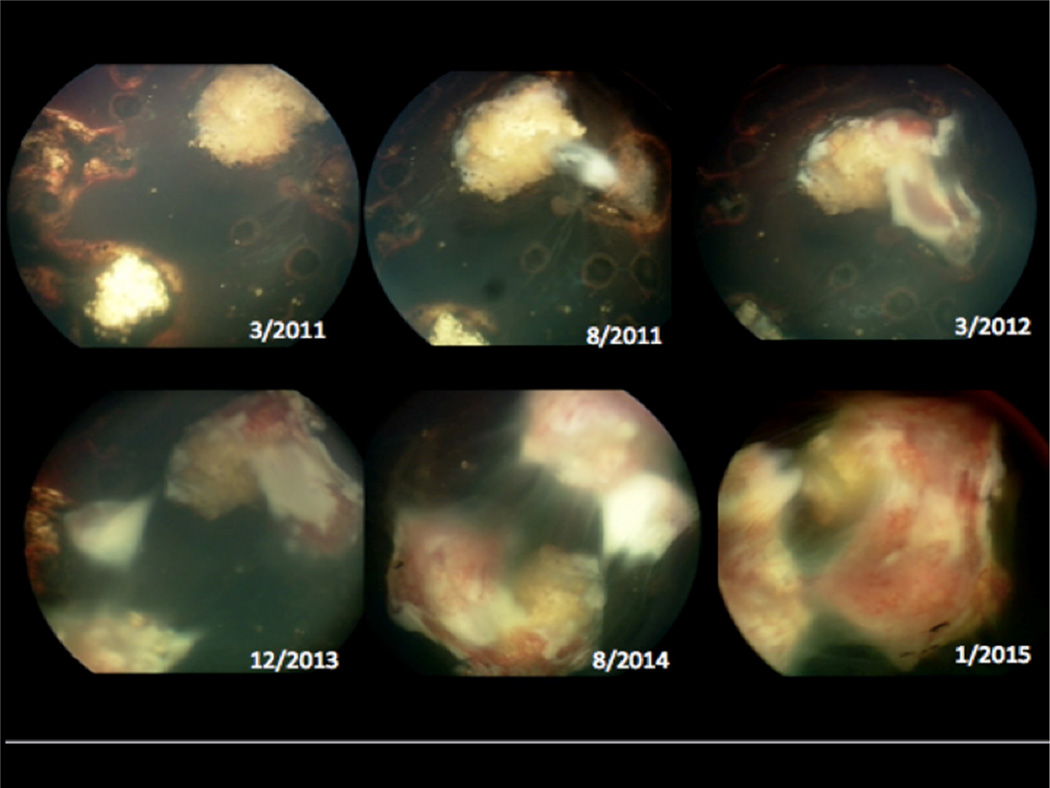
A girl with a family history of bilateral retinoblastoma in two maternal uncles was diagnosed with retinoblastoma at the age of 7 months. The right eye was classified as group D retinoblastoma with extensive subretinal seeds and the left as group A retinoblastoma. The patient was treated with CRD for both eyes, and periocular carboplatin (3 doses) in the right eye. Multiple recurrent tumors were successfully treated with thermal laser. She had a total of 18 laser treatments. She eventually developed progressive PVR in her right eye 20 months after starting treatment with resulting traction retinal detachment (Figure 3). This eye was enucleated. Five years after being diagnosed with retinoblastoma, she remains 20/25 in her left eye with no evidence of local or systemic recurrence. Mild fibrosis is present around the treated tumor in the remaining eye.
Figure 3.
Color fundus photos depicting progression of proliferative vitreoretinopathy over a four-year period in patient 2. This patient also developed a combined tractional-rhegmatogenous retinal detachment as a result of the fibrovascular proliferation.
Microscopic examination of the enucleated eye showed an intact cornea, iris and ciliary body. The lens was cataractous. There was a 10 × 5 mm mass arising from the retina composed of gliotic tissue with interposed areas of calcification, consistent with regressed tumor. There was fibrovascular tissue overlying the tumor and retina, resulting in focal areas of traction retinal detachment. There was hypertrophy and hyperplasia of the RPE (Figure 4). There was no evidence of choroid or optic nerve invasion by tumor.
Figure 4.
A. Gross examination of the enucleated eye shows white fibrous membranes (arrow) overlying a mass with a combination of “cottage cheese-like” (asterisk) and translucent “fish flesh-like” (arrowhead) areas; B. Histologic section demonstrating regressed retinoblastoma (asterisk), detached retina (arrow), and PVR (arrowhead); (Hematoxylin & eosin: B. 10×)
Case 3
A 9 month-old female was diagnosed with bilateral retinoblastoma with subretinal fluid in both eyes. The patient underwent CRD for both eyes. There was initial tumor control, but she then developed multiple subretinal recurrences in her right eye and vitreous seeds in the left eye. She underwent further thermal laser (for a total of 11 laser treatments in the right eye) in both eyes and then IMRT in the left eye with resolution of the vitreous seeds. The right eye developed extensive recurrence with blood and exudative retinal detachment and was treated with IMRT. The eye developed progressive fibrosis, traction retinal detachment, hypotony, and was enucleated (Figure 5). Ten years after her diagnosis of retinoblastoma, her best-corrected visual acuity was 20/40 on the left and there was no evidence of retinoblastoma recurrence. Of note, the left eye also had growth of white proliferative tissue but it was mild and did not cause significant traction. Microscopic examination of the enucleated eye showed an intact cornea and iris. The lens was cataractous. The retina displayed focal tractional detachment and gliosis. There was a 7 × 6 mm mass arising from the retina composed of gliotic tissue, small round blue cells with bland nuclei consistent with treated tumor, and interposed areas of calcification. There was pre- and subretinal fibrovascular and glial tissue extending from the tumor and retina up to the lens anteriorly and incorporating the ciliary body non-pigmented epithelium. There was osseous metaplasia of the RPE (Figure 6). There was no evidence of choroid or optic nerve invasion by tumor.
Figure 5.
Color fundus photos depicting progression of proliferative vitreoretinopathy over a five-month period in patient 3
Figure 6.
A. Histologic section demonstrating regressed retinoblastoma (asterisk), detached retina (arrow), and PVR (arrowhead) extending to lens (L); B. Fibroglial tissue (arrowhead) adjacent to lens (L) and incorporating peripheral retina (arrow) (Hematoxylin & eosin: A. 5×; Periodic acid Schiff: B. 25×)
Discussion
In this series, we describe features of three patients that developed severe PVR after treatment for bilateral retinoblastoma. Each of these patients was treated with CRD consisting of combined systemic chemotherapy and sequential thermal laser. In addition, two patients also received 3 doses of subconjunctival carboplatin in the affected eye per study protocol, and one patient was treated with IMRT. Each patient achieved complete control of retinoblastoma by the time of onset of PVR.
The PVR in these cases initially appeared as thick, discrete white plaques. Fine fibrils of tissue emanated from the borders of the membranes. Subsequently, a robust vascularization of the tissue developed. Contraction of the fibrous tissue lead to traction retinal detachment in all cases. Poor vision and inability to adequately monitor for recurrence prompted enucleation in each case. No patient developed local or systemic recurrence of the retinoblastoma at the last follow-up (4–10 years after diagnosis).
There have been a limited number of case reports and series of patients who develop gliosis and/or PVR after successful retinoblastoma treatment (Table 2)3–8. While this complication is uncommon, it is also likely under-reported in the literature. When reported, all cases have been in patients with advanced bilateral disease. The mean age at time of diagnosis was 14 months (range 5 days to 50 months) in the published cases. Different combinations of therapy were utilized, and included systemic chemotherapy, laser photocoagulation, local carboplatin, intravitreal chemotherapy with melphalan, cryotherapy, external beam radiation, and plaque brachytherapy. In the review of all these studies, there was no common treatment modality for all patients and thus the PVR was not the result of any one individual treatment. The PVR in our cases was likely a reactive process to the treatment. Our patients had high tumor burden that required numerous laser treatment sessions. In addition, there may have been patient specific factors that predisposed to proliferation. Mild membrane growth was visible in the remaining eye in all 3 cases (Figure 5).
Table 2.
Summary of literature reporting Proliferative Vitreoretinopathy and/or gliosis after treatment of retinoblastoma
| Author | Yr | No. Pts. |
Age at Dx |
Stage | Laterality Of Rb |
Rb Treatment | Finding | FVP Treatment |
|---|---|---|---|---|---|---|---|---|
| Hwang | 2016 | 3 | 6 m 7 m 9 m |
D D D |
Bilateral Bilateral Bilateral |
SC, PC, LC SC, PC, LC SC, PC, EBRT |
TRD TRD TRD |
All: Enuc |
| Ghassemi* | 2014 | 4 | 24 m 50 m 33 m 9 m |
D D D D |
Left Left Left Bilateral |
All: SC IVx M |
Gliosis TRD Gliosis Gliosis |
All: Enuc |
| Yokoi | 2008 | 1 | 6 m | IVa | Bilateral | SC OD:EBRT OS: enuc |
Gliosis RRD OD |
SB/PPV Biopsy OD |
| Tawansy | 2006 | 4 | 14 m 3 m 10 m 17 m |
Vb IIIa IVa Vb |
Bilateral Bilateral Bilateral Bilateral |
SC, EBRT, cryo, PC, LC SC, cryo, PC SC, XRT, cryo, PC, LC SC, EBRT, cryo, PC |
Gliosis Traction band TRD TRD |
Enuc Observation: stable PPV/MP PPV/MP |
| Warden | 2006 | 1 | 2 y | Not known |
Bilateral | OD: XRT, PC, cryo OS: EBRT, XRT, enuc |
TRD, ERM OD |
PPV/MP OD |
| Albert | 1986 | 1 | 5 d | Not known |
Bilateral | OU: EBRT | TRD OU | Enuc OU |
| Margo | 1983 | 1 | 3 m | Not known |
Bilateral | OU: cryo OD: PC |
Fibrocell ular membrane with active tumor |
Enuc OD |
Data by Ghassemi et al is based on pathology findings of enucleated retinoblastoma treated eyes.
Pt = patient, OD = right eye, OS = left eye, OU = both eyes, Rb = retinoblastoma y = year old, m = month old, d = day old
TRD = tractional retinal detachment, ERM = epiretinal membrane
RRD = rhegmatogenous retinal detachment
SC = systemic chemotherapy, IVxM = intravitreal melphalan, XRT = plaque radiotherapy, PC = photocoagulation, cryo = cryotherapy. EBRT = external beam radiation, enuc= enucleation, LC = local carboplatin, SB = scleral buckle, PPV = pars plana vitrectomy, Obs = observation, MP = membrane peel
A literature review of 7 studies, including this current study, revealed fibrovascular proliferation or PVR in 15 patients. Eleven eyes of 10 patients were enucleated, 4 patients underwent retinal surgery, and one patient was observed. The one patient who was observed remained stable. There has been significant trepidation in performing vitrectomy surgery on patients previously treated for retinoblastoma because there is risk of retinoblastoma recurrence and local and systemic spread of the disease 6–7,9. A review of cumulative data indicated a 44% risk of developing recurrence of retinoblastoma in successfully treated retinoblastoma patients who underwent vitrectomy. Some cases demonstrated tumor reactivation despite negative cytology at the time of surgery3. Given the substantial risks, vitrectomy should only be performed in patients with extraordinary circumstances. In all of the cases reviewed, vitrectomy was performed in the patients’ only remaining eye – the fellow eyes were all enucleated for inadequate tumor control.
Proliferative vitreoretinopathy is growth of membranes on the surfaces of the retina and in the vitreous and is thought to represent a reparative tissue response to retinal injury. Histologically, PVR membranes may appear gliotic, fibrovascular, or fibroglial. PVR is believed to form from inward migration and proliferation of glial cells normally present in the nerve fiber and ganglion cell layers. Aside from glial cells, PVR membranes may also contain fibroblasts and RPE cells10. In reactive retinal conditions, RPE cells typically undergo changes including hypertrophy, hyperplasia, and metaplasia as seen in these 3 cases.
Retinal gliosis as a component of PVR is a proliferation of retinal glial cells (specifically astrocytes) that is histologically characterized by uniform spindle and oval shaped cells with small nuclei and fibrillar eosinophilic cytoplasm. Gliosis of the retina can become very florid and grow to tumorous proportions with associated secondary vasculature. Once this happens, it is then called a reactive retinal astrocytic tumor (RRAT). This acquired benign tumor is thought to be within the same disease spectrum as massive retinal gliosis and vasoproliferative tumor of the retina11. It can be seen in several retinal conditions, including retinopathy of prematurity, retinal detachment, and diabetic retinopathy5, 12–13. The changes seen in the eyes reported here, however, would not be classified as reactive retinal astrocytic tumors because the gliotic areas were not very prominent, there were no Rosenthal fibers or eosinophilic granular bodies seen, and abnormal hyalinized or sclerotic vessels were absent. Although reactive retinal astrocytic tumor (RRAT)/massive retinal gliosis (MRG) has been postulated to be part of the spectrum of PVR, RRAT/MRG is essentially an intraretinal process14. For the purposes of nomenclature, the authors consider the changes described in the present 3 cases of retinoblastoma to be PVR, which is separate and distinct from RRAT/MRG15.
Clinically and microscopically, the PVR we describe here is similar to changes seen in retinopathy of prematurity. In retinopathy of prematurity, a grey-white ridge of fibrovascular tissue proliferates into the vitreous cavity and can develop extraretinal vascularization. As the tissue continues to proliferate, the retina can become tractionally detached.
In summary, severe PVR is an uncommon sequela in successfully treated retinoblastoma. This change is likely a reactive process resulting from heavy laser for eyes with high tumor burden. Distinguishing between recurrent retinoblastoma and PVR as a response to treatment can be challenging, especially in the early stages of PVR growth. Membrane growth is frequently visible in the contralateral eye and may reflect an underlying predisposition for proliferation in some patients (Figure 7).
Figure 7.
Fellow eyes in patient 1 (A), patient 2 (B), and patient 3 (C). Arrows indicate fibrosis in these eyes, even in cases of small tumor burden.
Acknowledgments
This study was funded in part by the Research to Prevent Blindness, the Foundation Fighting for Blindness, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures related to this study.
References
- 1.Shields CL, De Potter P, Himelstein BP, et al. Chemoreduction in the initial management of intraocular retinoblastoma. Arch Ophthalmol. 1996;114(11):1330–1338. doi: 10.1001/archopht.1996.01100140530002. [DOI] [PubMed] [Google Scholar]
- 2.Shields CL, Mashayekhi A, Au AK, et al. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmol. 2006;113:2276. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Warden SM, Mukai S. Pars plana vitrectomy in eyes treated for retinoblastoma. Retina. 2006;26(7):S53–S56. doi: 10.1097/01.iae.0000244289.01875.78. [DOI] [PubMed] [Google Scholar]
- 4.Tawansy KA, Samuel MA, Shammas M, et al. Vitreoretinal complications of retinoblastoma treatment. Retina. 2006;26(7):S47–S52. doi: 10.1097/01.iae.0000225350.83931.f6. [DOI] [PubMed] [Google Scholar]
- 5.Yokoi T, Hiraoka M, Suzuki Y, et al. Glial extrusion from regressed retinoblastoma after conservative treatment. Acta Ophthalmol. 2008:462–464. doi: 10.1111/j.1600-0420.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- 6.Albert DM, Walton DS, Weichselbaum RR, et al. Fibroblast radiosensitivity and intraocular fibrovascular proliferation following radiotherapy for bilateral retinoblastoma. Br J Ophthalmol. 1986;70:336–342. doi: 10.1136/bjo.70.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghassemi F, Amoli FA. Pathological findings in enucleated eyes after invtravitreal melphalan injection. Int Ophthalmol. 2014;34:533–540. doi: 10.1007/s10792-013-9851-2. [DOI] [PubMed] [Google Scholar]
- 8.Margo C, Hidayat AA, Marshall CF, et al. Cryotherapy and photocoagulation in the management of retinoblastoma: treatment failure and unusual complication. Ophthalmic Surgery. 1983;14:336–342. [PubMed] [Google Scholar]
- 9.Baumal CR, Shields CL, Shields JA, et al. Surgical repair of rhegmatogenous retinal detachment after treatment for retinoblastoma. Ophthalmology. 1998;105:2134–2139. doi: 10.1016/S0161-6420(98)91139-3. [DOI] [PubMed] [Google Scholar]
- 10.Yanoff M, Sassani JW. Ocular Pathology. 6th. Edinburgh: Mosby Elsevier; 2009. p. 494. [Google Scholar]
- 11.Poole-Perry LJ, Jakobiec FA, Zakka FR, et al. Reactive retinal astrocytic tumors (so355 called vasoproliferative tumors): histopathologic, immunohistochemical, and genetic studies of four cases. Am J Ophthalmol. 2013;155(3):593–608. doi: 10.1016/j.ajo.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields CL, Shields CL, Kaliki S, et al. Retinal vasoproliferative tumors: comparative clinical features of primary vs secondary tumors in 334 cases. JAMA Ophthalmol. 2013;131(3):328–334. doi: 10.1001/2013.jamaophthalmol.524. [DOI] [PubMed] [Google Scholar]
- 13.Houston SK, Bourne TD, Lopes MD, et al. Bilateral massive retinal gliosis associated with retinopathy of prematurity. Arch Pathol Lab Med. 2009;133(8):1242–1245. doi: 10.5858/133.8.1242. [DOI] [PubMed] [Google Scholar]
- 14.Hiscott P, Mudhar H. Is vasoproliferative tumour (reactive retinal glioangiosis) part of the spectrum of proliferative vitreoretinopathy? Eye. 2009;23(9):1851–1858. doi: 10.1038/eye.2008.351. [DOI] [PubMed] [Google Scholar]
- 15.Margo CE, Harman LE. Pathology of the vitreous. In: Heegaard S, Grossniklaus HE, editors. Eye Pathology. Heidelburg: Springer; 2015. pp. 284–285. [Google Scholar]