Abstract
Background: Direct oral anticoagulants (DOACs) have become popular alternatives to vitamin K antagonists for the treatment and prevention of thromboembolic diseases; however, there are limited data regarding the appropriate use of DOACs in clinical practice. To ensure safety and efficacy of these medications, it is important that decisions regarding their use in patients rely on the available evidence.
Objective: The purpose of this study was to evaluate the appropriateness of DOAC prescribing in adult patients before and after the implementation of a pharmacist-driven DOAC protocol.
Methods: Data were collected on adult patients admitted to a community teaching hospital who received DOAC therapy for at least 2 days between January and March 2015 (pre-intervention group) and between January and March 2016 (post-intervention group). These data were analyzed to measure inappropriately prescribed DOACs, defined based on DOAC indication, renal function, drug interactions, and other pertinent patient-specific factors. Prior to the start of data collection for the post-intervention group, a pharmacist-driven protocol was developed and implemented. DOAC education was provided to pharmacists, including an evidence-based prescribing table to guide appropriate DOAC therapy. Comparisons were made between the pre-intervention and post-intervention groups to determine the impact of the pharmacist-driven service on appropriate DOAC prescribing.
Results: Fifty patients were analyzed in the pre-intervention group compared with 85 patients in the post-intervention group, with a total of 333 and 816 doses administered, respectively. Of the total doses administered, 32.4% were considered inappropriate in the pre-intervention group compared with 13.8% in the post-intervention group (adjusted odds ratio [OR], 0.42, 95% CI, 0.19–0.96; p = 0.039).
Conclusions: Implementing a pharmacist-driven DOAC service significantly improved appropriate prescribing of these agents. Provider education regarding DOAC use is essential to further increase appropriate prescribing of DOACs, optimize patients' therapy, and prevent adverse drug events.
Keywords: anticoagulation, apixaban, dabigatran etexilate, DOAC, drug interactions, edoxaban
Anticoagulants are frequently recommended for a wide variety of indications, including the prevention of stroke in patients with atrial fibrillation and prophylaxis and/or treatment of venous thro m boembolism (VTE).1,2 These medications are an important treatment modality for patients, yet they are considered high-risk medications that require careful attention. Anticoagulants have been consistently recognized as one of the most common medication classes involved in adverse drug events (ADEs) throughout health care settings, with bleeding as the primary ADE of concern.3 This supports The Joint Commission's decision to include anticoagulants in their national patient safety goals aimed at reducing the likelihood of patient harm associated with their use.4
For many decades, vitamin K antagonists (VKAs) such as warfarin were the only oral anticoagulants available for use in patients. Over the past few years, several direct oral anticoagulants (DOACs) have been developed and now provide patients with alternative therapy options. Four DOACs are currently available in the United States, including a direct thrombin inhibitor, dabigatran etexilate, and 3 factor Xa inhibitors, rivaroxaban, apixaban, and edoxaban.5–8 These anticoagulants have become more accepted, and their use is becoming more common. Between 2009 and 2014, treatment visits involving the use of DOACs increased by more than 1 million per quarter. 9 The increased utilization of DOACs is likely due to the convenience in their dosing and monitoring, quick onset of action, and limited food and drug interactions. Additionally, large clinical trials have shown DOACs to be at least as effective as VKAs, and some trials have found them to be associated with a lower or comparable risk of bleeding complications. 10–14 Gastrointestinal bleeding risk, however, has been shown to be increased in patients treated with rivaroxaban or dabigatran compared with those treated with warfarin.15 There is insufficient evidence to determine whether this risk is also associated with the use of the other DOACs, apixaban or edoxaban.
Although DOACs provide major advantages compared with VKAs, there are many patient populations that have not been studied. All DOACs undergo renal excretion in variable amounts; therefore, patients with renal impairment may be at an increased risk of harm due to the potential for drug accumulation. The safety of DOACs in patients with severe renal impairment (creatinine clearance [CrCl] <30 mL/min), including patients on dialysis, is not well established, as this population has been underrepresented in clinical trials.15 Dose adjustments and careful selection of DOAC agents are necessary for these patients. A subgroup analysis of patients with moderate renal impairment (CrCl 30–50 mL/min) indicated that when dosed according to the manufacturers' recommendations, DOACs are at least as safe as warfarin.16,17 Dose adjustments are also warranted based on indication, age, and weight and can be further complicated by the addition of interacting drugs. Additionally, not all DOACs are approved for the same indications as VKAs. Other challenges that arise with the use of DOACs include the lack of experience managing DOAC-related bleeding, lack of approved antidotes available for factor Xa inhibitors, and lack of consensus regarding appropriate laboratory monitoring for these agents. Despite these challenges, DOAC use is continuing to rise, which makes it important to ensure these medications are being managed appropriately. Appropriate use of DOACs in clinical practice has not been well investigated. In a prospective, observational study of nonvalvular atrial fibrillation outpatients, 23% of rivaroxaban and 42% of dabigatran etexilate patients received an inappropriate dose.18 The authors assessed the appropriateness of DOAC dosage by the patient's CrCl, age, hemorrhagic risk, and concomitant drug use. Because DOAC dosing is complex and requires careful review of patients' characteristics, the implementation of a pharmacist-driven DOAC monitoring service may be valuable. Implementing a pharmacist-driven anticoagulation service has been shown to improve quality of care for patients taking warfarin in the inpatient setting.19 In hospitals without pharmacist-provided heparin and warfarin management, death rates, length of stay, bleeding complications, and transfusion rates for bleeding complications were higher.20
In this study, we evaluated the effectiveness of a pharmacist-driven DOAC service on appropriate prescribing and monitoring of DOACs in patients indicated for nonvalvular atrial fibrillation or VTE treatment/prophylaxis.
METHODS
A single-center, pre- and post-intervention study was conducted at a 504-bed community teaching hospital with an average daily admission rate of about 66.8 adult patients per day and more than 24,000 adult inpatient admissions annually. The study compared retrospectively reviewed DOAC prescribing practices (pre-intervention group) to patients prospectively managed by a pharmacist-driven DOAC protocol (post-intervention group). Institutional review board approval was obtained prior to the initiation of this study. Informed consent was waived by the institutional review board based on the study involving no more than minimal risk to patients and following best practice guidelines. All of the DOACs were on formulary at the time of implementing the DOAC protocol, however, edoxaban had not yet been approved for formulary during the retrospective data collection period.
The primary objective was to determine the proportion of inappropriately prescribed DOACs administered to patients during admission before and after the implementation of the per-pharmacy protocol. Secondary outcomes included the classification of inappropriately prescribed DOACs and the number of pharmacist interventions made. Inappropriate DOAC prescribing was defined as an incorrect medication selection, dose, and/or frequency. Appropriate DOAC prescribing was determined based on US Food and Drug Administration (FDA)–approved prescribing information (Figure 1).
Figure 1.

Direct oral anticoagulant (DOAC) prescribing table. ABW = actual body weight; BID = twice daily; CrCl = estimated creatinine clearance; DVT/PE = deep venous thrombosis/pulmonary embolism; QDay = every day; SCr = serum creatinine.
Patients were included in the study if they were admitted to the hospital, were 18 years of age or older, and were receiving a DOAC for a minimum of 2 consecutive days between January and March 2015 (pre-intervention study population) or January and March 2016 (post-intervention study population) for an indication of nonvalvular atrial fibrillation and/or VTE treatment/prophylaxis. Patients were excluded if they did not meet all of the inclusion criteria or if they had missing data that would interfere with the ability to assess for DOAC appropriateness.
The pharmacist-driven DOAC protocol was developed utilizing the FDA-approved prescribing information. The policy and protocol were approved by the institution's Clinical Practice Group and Pharmacy and Therapeutics Committee in November 2015. An electronic template for documenting DOAC interventions was developed to be incorporated in the electronic medical record (EMR) as a progress note. Education was provided to the pharmacists in December 2015; it included a presentation on all 4 DOACs (apixaban, rivaroxaban, dabigatran etexilate, and edoxaban) that highlighted key differences and important efficacy and safety points. Pharmacists were required to attend at least one educational case-based session that provided an opportunity for them to become familiar with DOAC management and to discuss appropriate protocol utilization. Pharmacists utilized the protocol to ensure appropriate DOAC prescribing. Pharmacists were also responsible for conducting daily profile reviews on all patients receiving a DOAC within the hospital. These patients were identified through daily reports that specified patients on 1 of the 4 DOACs. If an inappropriately prescribed DOAC was identified, the pharmacist was responsible for providing an appropriate alternative recommendation to the prescriber electronically or verbally and documenting any interventions in the EMR progress note. This note included pertinent information, such as current DOAC regimen, indication, labs, drug-drug interactions, and the recommended anticoagulation treatment plan. It was under the provider's discretion to accept or deny the recommendation from the pharmacist.
EMRs were reviewed to collect baseline characteristics, including age, gender, weight, height, serum creatinine (SCr), clinical indication for DOAC use, DOAC dose and frequency, and concomitant medication use that could potentially interact with the DOAC (all medications listed in Figure 2). The Cockgroft-Gault equation using actual body weight was used to calculate an estimated CrCl as recommended by the product manufacturers of rivaroxaban, dabigatran, and edoxaban. The DOACs administered were assessed for appropriateness by the primary author. Pharmacist interventions were also collected during the post-intervention study period, including the acceptance rate by prescribers.
Figure 2.
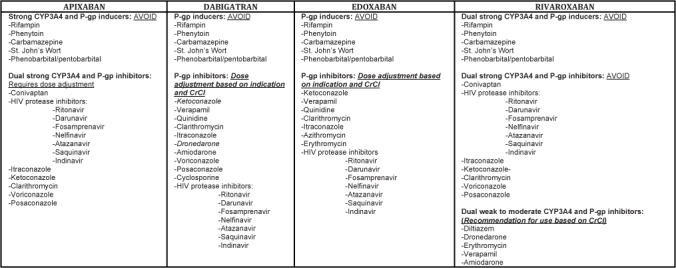
Direct oral anticoagulants (DOAC) interacting medications. CrCl = estimated creatinine clearance.
Results from descriptive analyses were reported as means or percentages. The Shapiro–Wilks test was used to test for normality. Differences between groups of patients were tested using the Wilcoxon rank-sum test or t test as appropriate for continuous variables, expressed as percentages; a chi-square test and Fisher's exact test were used for categorical variables, expressed as mean ± standard deviation. A p value < 0.05 was considered to be statistically significant. Data were initially entered into an Excel spreadsheet followed by analysis using Stata 13.0 (StataCorp LP, College Station, TX).
RESULTS
Fifty patients and 333 doses were included in the pre-intervention group, and 85 patients and 816 doses were included in the post-intervention group. There was no difference in baseline characteristics, except for age (Table 1). Patients in the pre-intervention group were approximately 7 years older on average compared with the post-intervention group (p = 0.002). Forty percent of patients in the pre-intervention group received an inappropriately prescribed DOAC during hospitalization compared to 29% of patients in the post-intervention group. There was also a decrease in the percentage of inappropriate DOAC administrations in the post-intervention group compared to the pre-intervention group (32.4% vs 13.8%; OR 0.34; 95% CI, 0.16–0.73; p = 0.005). Adjusting for age and specific DOAC resulted in a decrease in the odds of inappropriate administrations in the post-intervention group by 58% (adjusted OR, 0.42; 95% CI, 0.19–0.96; p = 0.039). The most common DOAC that was inappropriately prescribed was apixaban, followed by rivaroxaban and dabigatran. There was only one patient included in the post-intervention group who received edoxaban inappropriately. Overall, the majority of patients who received an inappropriately prescribed DOAC were classified as having been under-dosed (Table 2). This was most commonly associated with SCr, age, and weight criteria for apixaban dosing in patients with atrial fibrillation: 6 patients in the pre-intervention group and 9 patients in the post-intervention group (Table 3). One patient in the pre-intervention group received inappropriately prescribed apixaban classified as under-dosed due to prescribing once daily as opposed to twice daily. One patient in the post-intervention group received inappropriately prescribed apixaban due to dosing on indication. This patient was being treated for a new pulmonary embolism, and the dose was not reduced after 7 days of treatment. Other common causes for patient's receiving under-dosed DOACs was due to patient's renal function: 5 patients in the pre- intervention group and 4 patients in the post-intervention group. The number of patients who received an inappropriately prescribed DOAC that should have been avoided altogether was most commonly associated with renal function and/or a drug-drug interaction: 4 patients in the pre-intervention group due to renal function and a drug interaction; 2 patients in the post-intervention group due to a drug interaction; and 4 patients in the post-intervention group due to both renal function and a drug interaction. The number of inappropriate DOAC doses administered decreased in the post-intervention group compared to the pre-intervention group (14% vs 26%; p = 0.042). Patients who received an inappropriate DOAC due to being over-dosed was least common: 2 patients in the pre-intervention group and 4 patients in the post-intervention group due to SCr, age, and weight criteria; 1 patient in the post-intervention group due to renal function; and 1 patient in the post-intervention due to dosing for indication. There was no difference between groups when comparing the number of patients who received an inappropriately prescribed DOAC classified as under-dosed or over-dosed. Of the 25 patients who received inappropriately prescribed DOACs in the post-intervention group, 22 had a pharmacist intervention. Of the pharmacist interventions made, 86% were accepted from prescribers.
Table 1.
Baseline characteristics of the study population

Table 2.
Inappropriate DOAC administrations (total doses) by classification
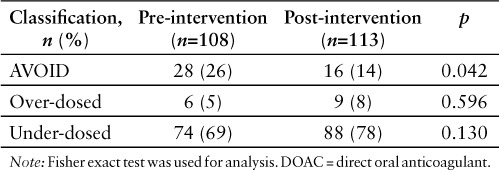
Table 3.
Number of patients with inappropriate DOAC prescribing by criterion

DISCUSSION
Although the introduction of DOACs has provided a major advantage to oral anticoagulation, these medications should still be managed carefully to prevent unwanted harm. The results of our study showed a high percentage of inappropriate DOAC doses being administered to adult patients and supported the potential usefulness of pharmacist interventions. In a previous study of patients receiving rivaroxaban in a community hospital, 36.9% of patients treated for nonvalvular atrial fibrillation and 12.4% treated for VTE were on an inappropriate regimen.21 Another study analyzing appropriate prescribing of dabigatran etexilate and rivaroxaban at a teaching hospital found that 28% of orders had an error based on indication and 26% of orders had an error in dosing.22 The implementation of a pharmacist-driven DOAC service was established at our institution to improve appropriate DOAC prescribing for all DOACs, as they were all available on formulary. This is one of several other pharmacistdriven services at our institution. The pharmacistdriven DOAC service was associated with a decrease in inappropriate DOAC prescribing in adult patients with an indication for nonvalvular atrial fibrillation and/or VTE treatment/prophylaxis.
There were several limitations to this study. First, data were not collected to measure complications associated with inappropriately prescribed DOACs, either thromboembolism or bleeding. These data may have added value to the pharmacist-driven dosing service. Additionally, the number of inappropriately prescribed DOACs found in the post-intervention group was impacted by prescribers' authority to decline pharmacist recommendations for changing a patient's DOAC regimen. The most common reason prescribers declined pharmacist recommendations was due to the prescriber not wanting to change a patient's home DOAC. Patients who received a DOAC for 1 day or less were excluded; this may not provide a complete representation of the intervention impact on appropriate DOAC dosing. Another limitation of this study was that all DOAC medications were not equally represented, with the majority of patients receiving rivaroxaban or apixaban. Also, upon protocol implementation, data collection began immediately, not allowing time for pharmacists to develop an increased level of comfort with the new protocol. Last, the time available for the completion of this study was relatively short. An extended study time could have provided the ability to draw more solid conclusions.
A pharmacist-driven DOAC dosing service was associated with decreased odds of inappropriate dosing of these agents. The utilization of this service has continued at our hospital, with changes made to improve the monitoring forms for efficiency of workflow. There is still room for improvement with overall appropriateness to optimize the care of patients receiving therapy with a DOAC. Prospective surveillance of all DOACs will be continued to further capture the effectiveness of the pharmacist DOAC dosing service. Active plans are in place to continue improving the pharmacist dosing protocol based on updated evidence and by providing physician education regarding the appropriate dosing and monitoring of DOACs to further improve DOAC use at our institution.
CONCLUSION
Pharmacist-driven DOAC monitoring significantly increased the appropriateness of DOACs administered to adult patients with an indication for use including nonvalvular atrial fibrillation and/or VTE treatment/prophylaxis. The analysis revealed an area for improvement – to provide DOAC education to providers in order to increase the acceptance rate of pharmacist interventions. Additionally, routine educational sessions are recommended in order to maintain pharmacist competence and compliance with the DOAC dosing protocol.
REFERENCES
- 1. January CT, Wann LS, Alpert JS, . et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Amer Coll Cardiol. 2014; 64( 21): 2246– 2280. [DOI] [PubMed] [Google Scholar]
- 2. Beckman MG, Hooper WC, Critchley SE, . et al. Venous thromboembolism: A public health concern. Am J Prev Med. 2010; 38( 4 suppl): S495– 501. [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services, Office of Disease Prevention and Health Promotion. . National action plan for adverse drug event prevention. http://health.gov/hcq/pdfs/ADE-Action-Plan-Anticoagulants.pdf. Accessed August 18, 2015.
- 4. The Joint Commission Hospital: 2015 National Patient Safety Goals. http://www.jointcommission.org/assets/1/6/2015_NPSG_HAP.pdf. Accessed August 1, 2015.
- 5. Eliquis® (apixaban) tablets [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2015. [Google Scholar]
- 6. Pradaxa® (etexilate mesylate) capsules [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015. [Google Scholar]
- 7. Savaysa® (edoxaban) tablets [prescribing information]. Parsippany, NJ: Daiichi Sankyo; 2015. [Google Scholar]
- 8. Xarelto® (rivaroxaban) tablets [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals; 2015. [Google Scholar]
- 9. Barnes GD, Lucas E, Alexander GC, . et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015; 128( 12): 1300– 1305.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Connolly SJ, Ezekowitz MD, Yusuf S, . et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361( 12): 1139– 1151. [DOI] [PubMed] [Google Scholar]
- 11. Patel MR, Mahaffey KW, Garg J, . et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365( 10): 883– 891. [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, Alexander JH, McMurray JJV, . et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365( 11): 981– 992. [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, Ruff CT, Braunwald E, . et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369( 22): 2093– 2104. [DOI] [PubMed] [Google Scholar]
- 14. Van der Hulle T, Koolman J, den Exter PL, . et al. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: A systematic review and meta-analysis. J Thromb Haemost. 2014; 12( 3): 320– 328. [DOI] [PubMed] [Google Scholar]
- 15. Holster IL, Valkhoff VE, Kuipers EJ, . et al. New oral anticoagulants increase risk for gastrointestinal bleeding: A systematic review and meta-analysis. Gastroenterology. 2013; 145: 105– 112. [DOI] [PubMed] [Google Scholar]
- 16. Harel Z, Scholzberg M, Shah PS, . et al. Comparisons between novel oral anticoagulants and vitamin K antagonists in patients with CKD. J Am Soc Nephrol. 2014; 25( 3): 431– 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lega JC, Bertoletti L, Gremillet C, . et al. Consistency of safety profiles of new oral anticoagulants in patients with renal failure. J Thromb Haemost. 2014; 12: 337– 343. [DOI] [PubMed] [Google Scholar]
- 18. Basaran O, Filiz Basaran N, Cekic EG, . et al. Prescription patterns of oral anticoagulants in nonvalvular atrial fibrillation (Proper Study) [pubished online ahead of print October 30, 2015]. Clin Appl Thromb Hemost. [DOI] [PubMed]
- 19. Schillig J, Kaatz S, Hudson M, . et al. Clinical and safety impact of an inpatient pharmacist-directed anticoagulation service. J Hosp Med. 2011; 6( 6): 322– 328. [DOI] [PubMed] [Google Scholar]
- 20. Bond CA, Raehl CL.. Pharmacist-provided anticoagulation management in United States hospitals: Death rates, length of stay, Medicare charges, bleeding complications, and transfusions. Pharmacotherapy. 2004; 24( 8): 953– 963. [DOI] [PubMed] [Google Scholar]
- 21. Tellor KB, Patel S, Armbruster AL, . et al. Evaluation of the appropriateness of dosing, indication and safety of rivaroxaban in a community hospital. J Clin Pharm Ther. 2015; 40( 4): 447– 451. [DOI] [PubMed] [Google Scholar]
- 22. Larock AS, Mullier F, Sennesael AL, . et al. Appropriateness of prescribing dabigatran etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: A prospective study. Ann Pharmacother. 2014; 48( 10): 1258– 1268. [DOI] [PubMed] [Google Scholar]


