In the paper “Fluorescent proteins such as eGFP lead to catalytic oxidative stress in cells” published in Volume 12 of Redox Biology, Ganini et al. report that expression of fluorescent proteins such as enhanced green fluorescent protein (eGFP) induces oxidative stress in cells [1]. The authors propose that increased formation of reactive oxygen species (ROS) such as superoxide (O2•−) and hydrogen peroxide (H2O2) can explain the cytotoxicity and tissue abnormalities reported previously in mammalian and bacterial cells and animals overexpressing fluorescent proteins [2], [3], [4], [5], [6]. Importantly, both O2•− and H2O2 induce redox signaling mechanisms, leading to altered gene expression of cell regulatory proteins involved in cell proliferation, cell differentiation, and cell death [7], [8], [9], [10]. Thus, the findings reported in this paper could have a major influence on the interpretation of results obtained from numerous studies that routinely use fluorescent protein tags [11], [12], [13].
Ganini et al. make a very strong and convincing case for nicotinamide adenine dinucleotide phosphate (NAD(P)H)-dependent O2•– formation in the presence of purified eGFP or TagRFP. Using the spin-trapping technique, they unequivocally show formation of O2•− and hydroxyl spin adducts of 5,5-dimethyl-pyrroline N-oxide (DMPO). Formation of O2•− and hydroxyl adducts of DMPO was dependent on NADH, abrogated by superoxide dismutase (SOD) and not inhibited by added catalase, indicating that both adducts derive from O2•–. NADH-dependent O2•– production from eGFP was also confirmed by SOD-inhibitable cytochrome c reduction. The results on O2•– formation were accompanied by the monitoring of H2O2 generation using a FOX assay and of the rates of NADH consumption, leading to the conclusion that the rate of H2O2 formation is equal to the rate of NADH consumption. Furthermore, the authors demonstrate that, in the presence of excess NADH, H2O2 formation is catalytic, indicating the redox cycling activity of eGFP. Importantly, this activity was attributed to one of the maturing intermediates of eGFP, rather than to the mature protein.
Because the concentrations of eGFP and NAD(P)H used in the cell-free assays were comparable to those reported in cells, the authors hypothesized that eGFP redox cycling activity occurs in GFP-expressing cells, where a steady synthesis and maturation of the protein is expected. The investigators used the Amplex Red assay to estimate extracellular H2O2 released in HeLa cells stably expressing GFP. Mason's laboratory previously investigated the effect of light on oxidation of Amplex Red to resorufin and is well aware of the light-induced oxidation of Amplex Red [14], [15], [16]. These experiments were conducted in the dark to avoid the effect of light, and all experiments involved control samples with catalase present to confirm the identity of the oxidant detected. Cells stably expressing GFP exhibited increased H2O2 production when compared with control cells.
Does GFP expression result in increased oxidative stress in cells? To address this question, Ganini et al. performed experiments with Escherichia coli expressing different levels of GFP. They monitored the expression of genes regulated by the transcription factors, SoxR and OxyR, that are activated by O2•– and H2O2. The genes activated by SoxR or OxyR were upregulated in E. coli with enhanced GFP expression. However, the investigators did not detect differences in extracellular H2O2 formation. Overexpression of GFP in HeLa mammalian cells resulted in upregulation of several genes associated with inflammation, both in eGFP stably expressing and doxycyclin-induced models.
Collectively, these findings—enhanced ROS formation and alterations in oxidative stress genes in response to GFP expression in bacterial and mammalian cells—suggest that GFP expression in cells is not innocuous and should be taken into consideration in interpreting results obtained from experiments involving expressing fluorescent proteins that probe redox-related mechanisms. Overexpression of GFP may alter cell phenotype and interfere with redox measurements with GFP-based sensors.
This study also raises important questions with regard to studies using GFP-based sensors: (1) Does the intracellular NAD(P)H level control O2•– and H2O2 formation in GFP-overexpressing cells? (2) Do other redox-sensitive fluorescent proteins enhance NAD(P)H-dependent O2•– and H2O2 in cells?
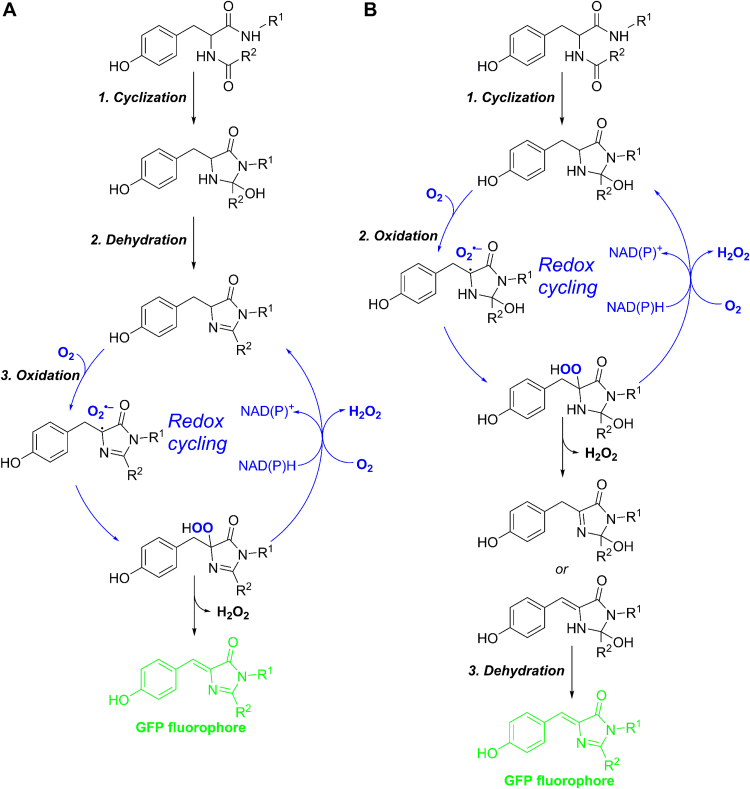
Although the authors proposed a putative mechanism of the redox cycling process, further mechanistic studies are warranted. For example, though the authors proposed a redox cycle based on the maturation sequence of cyclization>dehydration>oxidation (Scheme 1A), a possibility of the alternative sequence: cyclization>oxidation>dehydration involving redox cycle (Scheme 1B) should also be considered [17]. Several unanswered questions remain: What is the exact mechanism of oxidation during fluorophore maturation and of redox cycling in the presence of NAD(P)H? Is O2•– produced catalytically? Does NAD(P)H react directly with the putative hydroperoxide intermediate? Is the 1:3 ratio of the rates of O2•– production to NADH consumption constant over different experimental conditions, and what is the origin of this ratio? Do changes in intracellular O2•– and H2O2 in GFP-expressing cells closely relate to gene expression changes characteristic for oxidative stress? These questions should be pondered in future studies; however, they do not in any way affect the overall conclusions of this important paper.
Scheme 1.
Tentative mechanism of NAD(P)H-driven redox cycling in two possible pathways of eGFP maturation.
Acknowledgements
This work was supported by NIH NCI U01 CA178960 to Michael Dwinell and Balaraman Kalyanaraman (MPIs).
References
- 1.Ganini D., Leinisch F., Kumar A., Jiang J., Tokar E., Malone C.C., Petrovich R.M., Mason R.P. Fluorescent proteins such as eGFP lead to catalytic oxidative stress in cells. Redox Biol. 2017;12:462–468. doi: 10.1016/j.redox.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari A.M., Ahmed A.K., Matsangos A.E., Lay F., Born L.J., Marti G., Harmon J.W., Sun Z. Cellular GFP toxicity and immunogenicity: potential confounders in vivo cell tracking experiments. Stem Cell Rev. Rep. 2016;12(5):553–559. doi: 10.1007/s12015-016-9670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H.-S., Jan M.-S., Chou C.-K., Chen P.-H., Ke N.-J. Is green fluorescent protein toxic to the living cells? Biochem. Biophys. Res. Commun. 1999;260(3):712–717. doi: 10.1006/bbrc.1999.0954. [DOI] [PubMed] [Google Scholar]
- 4.Coumans J.V.F., Gau D., Poljak A., Wasinger V., Roy P., Moens P. Green fluorescent protein expression triggers proteome changes in breast cancer cells. Exp. Cell Res. 2014;320(1):33–45. doi: 10.1016/j.yexcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goto H., Yang B., Petersen D., Pepper K.A., Alfaro P.A., Kohn D.B., Reynolds C.P. Transduction of green fluorescent protein increased oxidative stress and enhanced sensitivity to cytotoxic drugs in neuroblastoma cell lines. Mol. Cancer Ther. 2003;2(9):911–917. [PubMed] [Google Scholar]
- 6.Huang W.Y., Aramburu J., Douglas P.S., Izumo S. Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat. Med. 2000;6(5):482–483. doi: 10.1038/74914. [DOI] [PubMed] [Google Scholar]
- 7.Sobotta M.C., Liou W., Stöcker S., Talwar D., Oehler M., Ruppert T., Scharf A.N.D., Dick T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015;11(1):64–70. doi: 10.1038/nchembio.1695. [DOI] [PubMed] [Google Scholar]
- 8.Murakami S., Motohashi H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 2015;88(Part B):168–178. doi: 10.1016/j.freeradbiomed.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Mikhed Y., Görlach A., Knaus U.G., Daiber A. Redox regulation of genome stability by effects on gene expression, epigenetic pathways and DNA damage/repair. Redox Biol. 2015;5:275–289. doi: 10.1016/j.redox.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez E.A., Campbell R.E., Lin J.Y., Lin M.Z., Miyawaki A., Palmer A.E., Shu X., Zhang J., Tsien R.Y. The growing and glowing toolbox of fluorescent and photoactive proteins. Trends Biochem. Sci. 2017;42(2):111–129. doi: 10.1016/j.tibs.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolbat A., Schultz C. Recent developments of genetically encoded optical sensors for cell biology. Biol. Cell. 2017;109(1):1–23. doi: 10.1111/boc.201600040. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman R.M. Use of fluorescent proteins and color-coded imaging to visualize cancer cells with different genetic properties. Cancer Metastasis. Rev. 2016;35(1):5–19. doi: 10.1007/s10555-016-9610-8. [DOI] [PubMed] [Google Scholar]
- 14.Summers F.A., Zhao B., Ganini D., Mason R.P. Photooxidation of Amplex red to resorufin: implications of exposing the Amplex red assay to light. Methods Enzymol. 2013;526:1–17. doi: 10.1016/B978-0-12-405883-5.00001-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhao B., Ranguelova K., Jiang J., Mason R.P. Studies on the photosensitized reduction of resorufin and implications for the detection of oxidative stress with Amplex red. Free Radic. Biol. Med. 2011;51(1):153–159. doi: 10.1016/j.freeradbiomed.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B., Summers F.A., Mason R.P. Photooxidation of Amplex red to resorufin: implications of exposing the Amplex red assay to light. Free Radic. Biol. Med. 2012;53(5):1080–1087. doi: 10.1016/j.freeradbiomed.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craggs T.D. Green fluorescent protein: structure, folding and chromophore maturation. Chem. Soc. Rev. 2009;38(10):2865–2875. doi: 10.1039/b903641p. [DOI] [PubMed] [Google Scholar]