Abstract
Resveratrol (RSV) is a naturally occurring polyphenolic compound endowed with interesting biological properties/functions amongst which are its activity as an antioxidant and as Sirtuin activating compound towards SIRT1 in mammals. Sirtuins comprise a family of NAD+-dependent protein deacetylases that are involved in many physiological and pathological processes including aging and age-related diseases. These enzymes are conserved across species and SIRT1 is the closest mammalian orthologue of Sir2 of Saccharomyces cerevisiae. In the field of aging researches, it is well known that Sir2 is a positive regulator of replicative lifespan and, in this context, the RSV effects have been already examined. Here, we analyzed RSV effects during chronological aging, in which Sir2 acts as a negative regulator of chronological lifespan (CLS). Chronological aging refers to quiescent cells in stationary phase; these cells display a survival-based metabolism characterized by an increase in oxidative stress. We found that RSV supplementation at the onset of chronological aging, namely at the diauxic shift, increases oxidative stress and significantly reduces CLS. CLS reduction is dependent on Sir2 presence both in expired medium and in extreme Calorie Restriction. In addition, all data point to an enhancement of Sir2 activity, in particular Sir2-mediated deacetylation of the key gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pck1). This leads to a reduction in the amount of the acetylated active form of Pck1, whose enzymatic activity is essential for gluconeogenesis and CLS extension.
Keywords: Resveratrol, Chronological aging, Sir2, Oxidative stress, Saccharomyces cerevisiae
Graphical abstract
Highlights
-
•
RSV supplementation at the diauxic shift strongly reduces chronological lifespan.
-
•
RSV supplementation increases oxidative stress during chronological aging.
-
•
RSV supplementation decreases trehalose stores during chronological aging.
-
•
RSV supplementation determines a decrease in the acetylated active form of Pck1.
-
•
Sir2 is required for the pro-aging effects elicited by RSV supplementation.
1. Introduction
Resveratrol (3,5,4′-trihydroxystilbene) (RSV) is a natural non-flavonoid, polyphenolic product belonging to the stilbenoid family, which is synthesized by a rather restricted and heterogeneous group of plant species. Grapevine (Vitaceae) is among these, and the products derived therefrom such as grapes and red wine are sources of RSV in human diet [1], [2]. In particular RSV intake with red wine has been proposed to explain the “French Paradox”, a term coined on the basis of epidemiological studies that in France revealed low rates of coronary heart diseases despite a diet rich in saturated fats [3]. This apparent paradox has been ascribed to protective/beneficial effects of RSV linked to French dietary habits of moderate consumption of red wine [3], [4]. Very numerous in vitro and in vivo studies dealing with the different aspects of the various health-promoting effects of RSV have been reported, including its antioxidative, anti-inflammatory, neuroprotective, anticancer and cardioprotective properties [5], [6], [7], [8]. Nevertheless, the exact molecular mechanisms underlying the RSV action and its direct cellular targets are still being explored [9], [10]. In this context, after a high-throughput screen in which RSV was identified as a natural SIRT1 activating compound (STAC) [11], a series of reports provided evidence that SIRT1 is required for most of RSV metabolic actions either via a direct activation of SIRT1 by RSV or an indirect one [9], [12], [13], [14]. The mammalian SIRT1 is a member of a family of evolutionarily conserved NAD+-dependent deacetylases, namely Sirtuins, In mammals, there are seven Sirtuin isoforms (SIRT1-SIRT7) endowed with multifaceted functionality comprising transcriptional regulation and chromatin structure maintenance in the nucleus as well as activation/inactivation of metabolic enzymes in the cytoplasm and mitochondria in response to nutritional and environmental stimuli [15], [16], [17], [18]. SIRT1 is the most well-studied member of the mammalian Sirtuins and its deacetylase activity plays a crucial role in metabolic responses to nutritional availability in different tissues and in physiological processes known to be affected during aging. Consequently, the modulation of SIRT1 activity can represent a potential therapeutic approach for treating age-related or metabolic diseases in order to improve the quality of life and extend health span [12], [17], [19], [20], [21].
SIRT1 is the closest mammalian orthologue of the Sirtuin founding member, namely Sir2 of Saccharomyces cerevisiae [22]. In yeast, Sir2 is a key modulator of both replicative and chronological aging [23], [24]. In the former, which is a useful model of cellular aging for mitotically active mammalian cells [25], Sir2 activity promotes replicative lifespan (RLS) [26], [27]. Furthermore, a quantitative trait locus (QTL) analysis investigating the role of natural genetic variation associated with RLS identified SIR2 as the QTL with the largest effect on RLS [28]. RLS measures the reproductive potential of individual yeast cells determined by counting how many daughter cells (buds) are generated by an asymmetrically dividing mother cell in the presence of nutrients prior to senescence [29]. Treatment with RSV and also with some of its synthetic derivatives has been reported to extend RLS and require SIR2 [11], [30], [31], although in this regard Sir2 activation by RSV has been questioned and is a topic of some controversy [32].
Yeast chronological aging is a complementary model to the replicative one: it allows us to simulate cellular aging of nondividing, albeit metabolic active, mammalian cells such as those of the brain and heart [23], [33]. The chronological lifespan (CLS) refers to the mean and maximum length of time a non-dividing culture survives in stationary phase. It is estimated, starting 72 h after the diauxic shift, by measuring the percentage of cells able to resume growth upon return to fresh rich medium [34]. Unlike RLS, Sir2 activity does not promote CLS [35], [36], [37]. In addition, lack of Sir2 along with CLS-extending mutations/deletions that reduce nutrient-responsive pathways such as Sch9 and Ras ones, further increases the CLS [36]. The same CLS-extending effect is observed in combination with a severe form of Calorie Restriction (CR) obtained by incubation of post-diauxic cells in water [35], [36], [37].
In chronologically aging cells, lack of Sir2 affects, on the one hand, carbon metabolism by increasing anabolic pathways such as gluconeogenesis and, on the other, respiratory activity by reducing non-phosphorylating respiration. The former leads to an increase of protective factors such as trehalose and the latter to a low content of the harmful superoxide anion () [35], [37]. These features together with an increased resistance to heat shock and oxidative stress [36] contribute to the establishment of an efficient protective quiescent state that favors a better long-term survival.
Herein, we investigated the effects of RSV supplementation at the onset of chronological aging. The results indicate that RSV displays pro-aging properties promoting CLS restriction. In particular, RSV interferes with the metabolic reprogramming that is required to trigger the metabolic adaptations to nutrient scarcity determining a decrease in trehalose stores and an increase in oxidative stress. Our findings also implicate an enhancement of Sir2 enzymatic activity in eliciting the RSV effects.
2. Materials and methods
2.1. Yeast strains, growth conditions and CLS determination
All yeast strains used in this work were generated by PCR-based methods and are listed in Table S1. For each strain generated, prototrophic derivatives of W303-1A were constructed by integration of the corresponding wt allele of the auxotrophic mutation at the original genomic locus. The accurancy of gene replacements and correct deletions/integrations was verified by PCR with flanking and internal primers. Cells were grown in batches at 30 °C in minimal medium (Difco Yeast Nitrogen Base without amino acids, 6.7 g/L) with 2% w/v glucose and supplements added in excess [38]. Cell growth was monitored by counting cell number using a Coulter Counter-Particle Count and Size Analyser [39] and, in parallel, the extracellular concentration of glucose and ethanol were measured in medium samples collected at different time points in order to define the growth profile (exponential phase, diauxic shift (Day 0), post-diauxic phase and stationary phase) of the cultures [38]. Duplication time (Td) was obtained by linear regression of the cell number increase over time on a semi-logarithmic plot. CLS was measured according to [36] by counting colony-forming units (CFU) starting with 72 h (Day 3, first age-point) after Day 0. The number of CFU on Day 3 was considered the initial survival (100%). Treatments were performed at Day 0 by adding resveratrol (RSV, dissolved in DMSO, Sigma-Aldrich) at the final concentrations of 100 µM. Survival experiments in water (pH adjusted to 3.2) were performed as described [40]. Every 48 h, 100 µM RSV, 5 mM nicotinamide (NAM, Sigma-Aldrich) or both were added to the culture after washing. Viability was assessed by CFU.
2.2. Metabolite measurements and enzymatic assays
At designated time points, aliquots of the yeast cultures were centrifuged, and both pellets (washed twice) and supernatants were collected and frozen at −80 °C until used. Glucose, ethanol and acetic acid concentrations in the growth medium were determined using enzymatic assays (K-HKGLU, K-ETOH, and K-ACET kits from Megazyme). Extraction and determination of intracellular trehalose as in [41]. The released glucose was quantified using the K-HKGLU kit.
Immediately after preparation of cell-free extracts [38], phosphoenolpyruvate carboxykinase (Pck1) and isocitrate lyase (Icl1) activities were assayed according to [42]. Catalase and superoxide dismutase activities were determined in cell-free extracts prepared as in [43]. The former was measured spectrophotometrically at 240 nm by following the disappearance of H2O2 [44] and the latter at 550 nm by following the rate of ferricytochrome c reduction [45]. Total protein concentration was estimated using the BCA™ Protein Assay Kit (Pierce).
2.3. Estimation of oxygen consumption rates, superoxide levels and lipid peroxidation
The basal oxygen consumption of intact cells was measured at 30 °C using a “Clark-type” oxygen electrode (Oxygraph System, Hansatech Instruments, Nortfolk, UK) as previously reported [40]. The addition of 37.5 mM triethyltin bromide (TET, Sigma-Aldrich) and 10 µM of the uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP, Sigma-Aldrich) to the oxygraph chamber accounted for the non-phosphorylating respiration and the maximal/uncoupled respiratory capacity, respectively [37]. The addition of 2 M antimycin A (Sigma-Aldrich) accounted for non-mitochondrial oxygen consumption. Respiratory rates for the basal oxygen consumption (JR), the maximal/uncoupled oxygen consumption (JMAX) and the non-phosphorylating oxygen consumption (JTET) were determined from the slope of a plot of O2 concentration against time, divided by the cellular concentration.
Staining with dihydroethidium (DHE, Sigma-Aldrich) was performed according to [46] to analyze superoxide anion (). Cells were counterstained with propidium iodide to discriminate between live and dead cells. A Nikon Eclipse E600 fluorescence microscope equipped with a Leica DC 350F ccd camera was used. Digital images were acquired using FW4000 software (Leica).
Lipid peroxidation was determined by quantifying malondialdehyde (MDA) using the BIOXYTECH® LPO-586™ Colorimetric Assay Kit (OxisResearch). The assay is based on the reaction of the chromogenic N-methyl-2-phenylindole with MDA forming a stable chromophore with maximum absorbance at 586 nm.
2.4. Immunoprecipitation and Western analysis
Total protein extracts preparation, immunoprecipitation and Western analysis were performed as described [35]. Acetylation levels of histones were analyzed on extracts prepared by mild alkaline treatment [47] and SDS-PAGE was performed on 12% polyacrylamide slab gels. Gels were blotted onto Hybond-P PVDF membranes (Amersham). Correct loading/transfer was confirmed by staining filters with Ponceau S Red (Sigma-Aldrich). The primary antibodies used were: anti-HA (12CA5, Roche), anti-acetylated-lysine (Ac-K-103, Cell Signaling), anti-3-phosphoglycerate kinase (Pgk1) (22C5, Invitrogen), anti-H4 (ab16483, Abcam) and anti-H4K16ac (ab1762, Abcam). Secondary antibodies were purchased from Amersham. Binding was visualized with the ECL Western Blotting Detection Reagent (Amersham). After ECL detection, films were scanned on a Bio-Rad GS-800 calibrated imaging densitometer and quantified with Scion Image software.
2.5. Statistical analysis of data
All values are presented as the mean of three independent experiments±Standard Deviation (SD). Three technical replicates were analyzed in each independent experiment. Statistical significance was assessed by one-way ANOVA test. The level of statistical significance was set at a P value of ≤0.05.
3. Results
3.1. Resveratrol supplementation at the diauxic shift restricts CLS and increases oxidative stress
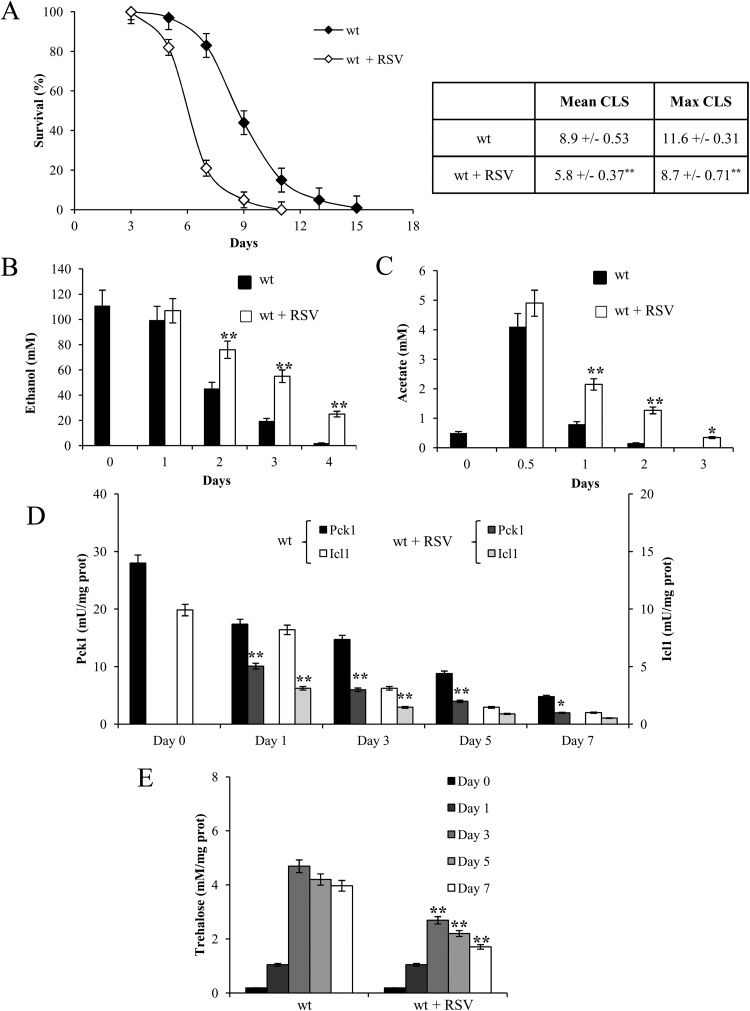
During chronological aging, an increase in oxidative stress occurs, which negatively affects CLS [48], [49], [50]. Since antioxidant properties, among others, have been reported for potential RSV health benefits, we evaluated the effects of its supplementation at the diauxic shift (Day 0) on both CLS and cellular metabolism. RSV-treated culture showed a decrease of CLS (Fig. 1A) in concert with increased extracellular levels of ethanol and acetate compared with the untreated one (Fig. 1B and C). Ethanol and acetate are two well-known by-products of yeast glucose fermentation that are transiently accumulated in the culture medium. In fact, after the diauxic shift, these C2 compounds are used as substrates of a respiration-based metabolism that, in addition to functional mitochondria, requires the involvement of the glyoxylate/gluconeogenesis pathways. In RSV-treated cells, the enzymatic activities of isocitrate lyase (Icl1), one of the signature enzymes of the glyoxylate shunt, and of the key gluconeogenic enzyme phosphoenolpyruvate carboxykinase (Pck1), were significantly lower than those in untreated cells (Fig. 1D). In line with a down-regulation of the glyoxylate-requiring gluconeogenesis, a reduction in these cells of intracellular trehalose stores took place (Fig. 1E). Indeed, the production of trehalose stores relies upon gluconeogenesis, which yields glucose-6-phosphate from the oxaloacetate provided by the glyoxylate shunt. In addition, since this shunt is fed with acetyl-CoA generated from acetate a reduction in the glyoxylate/gluconeogenesis flux also implies a slower depletion of extracellular ethanol and acetate as observed (Fig. 1B and C).
Fig. 1.
RSV supplementation at the diauxic shift determines a short-lived phenotype. Wild type (wt) cells were grown in minimal medium/2% glucose and the required supplements in excess (see Materials and methods). At the diauxic shift (Day 0), resveratrol (RSV, 100 μM) was added to the expired media and (A) survival over time of treated and untreated cultures was assessed by colony-forming capacity on YEPD plates. 72 h after the diauxic shift (Day 3) was considered the first age-point, corresponding to 100% survival. Data referring to the time points where chronological aging cultures showed 50% (Mean CLS) and 10% (Maximum CLS) of survival are reported in the Table. In parallel, for the same cultures the concentrations of extracellular ethanol (B) and acetate (C) together with Icl1 and Pck1 enzymatic activities (D) and intracellular trehalose content (E) were measured. All data refer to mean values of three independent experiments with three technical replicates each. Standard deviations (SD) are indicated. Statistical significance as assessed by one-way ANOVA test is indicated (*P≤0.05 and **P≤0.01).
Moreover, RSV supplementation at Day 0 increased cellular respiration rate (JR) as well as the non-phosphorylating respiration rate (JTET). The latter estimated in the presence of the FoF1-ATPase inhibitor triethyltin bromide (TET, [51]) (Table 1). However, in these cells the net respiration (netR), measured by subtracting JTET from JR, was extremely low especially 3 days after the diauxic shift (Table 1) indicating that in the presence of RSV the respiration is predominantly uncoupled. No significant differences were detected in the maximal oxygen consumption rate (JMAX, see Materials and Methods) between treated and untreated cultures reflecting a similar maximal respiratory capacity (Table 1).
Table 1.
RSV supplementation at the diauxic shift affects respiration.
| JR |
JTET |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 3 | Day 5 | Day 0 | Day 1 | Day 3 | Day 5 | |||||
| wt | 9.51 ± 0.24 | 14.11 ± 0.31 | 7.22 ± 0.29 | 4.31 ± 0.12 | 1.64 ± 0.24 | 3.68 ± 0.19 | 3.93 ± 0.24 | 4.18 ± 0.29 | ||||
| wt + 100 μM RSV | 17.49** ± 0.18 | 9.45** ± 0.22 | 8.35** ± 0.26 | 6.81* ± 0.24 | 7.95** ± 0.21 | 8.27** ± 0.26 | ||||||
| sir2Δ | 7.89** ± 0.26 | 10.52** ± 0.13 | 5.72** ± 0.19 | 3.34** ± 0.14 | 1.09** ± 0.24 | 1.23** ± 0.21 | 1.49** ± 0.17 | 1.68** ± 0.19 | ||||
| sir2Δ + 100 μM RSV | 10.44** ± 0.21 | 5.59** ± 0.15 | 3.27** ± 0.21 | 1.28** ± 0.25 | 1.55** ± 0.16 | 1.72** ± 0.23 | ||||||
| JMAX |
netR |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 3 | Day 5 | Day 0 | Day 1 | Day 3 | Day 5 | |||||
| wt | 19.11 ± 0.34 | 26.41 ± 0.13 | 25.36 ± 0.16 | 12.01 ± 0.20 | 7.38 ± 0.28 | 10.41 ± 0.32 | 3.21 ± 0.27 | 0.28 ± 0.29 | ||||
| wt + 100 μM RSV | 26.62 ± 0.25 | 25.22 ± 0.23 | 12.18 ± 0.18 | 9.18* ± 0.22 | 1.65** ± 0.17 | 0.08** ± 0.09 | ||||||
| sir2Δ | 18.89 ± 0.22 | 26.19 ± 0.19 | 24.98 ± 0.27 | 11.35 ± 0.18 | 6.83 ± 0.16 | 9.87** ± 0.32 | 4.40** ± 0.27 | 1.45** ± 0.24 | ||||
| sir2Δ + 100 μM RSV | 26.33 ± 0.16 | 26.11 ± 0.25 | 11.21 ± 0.22 | 9.92** ± 0.23 | 4.35** ± 0.16 | 1.38** ± 0.21 | ||||||
Oxygen uptake rates (J) are expressed as pmol/106 cells/s. Basal respiration rate (JR), non-phosphorylating respiration rate (JTET), uncoupled respiration rate (JMAX) and net respiration (netR = JR - JTET). Substrates and inhibitors used in the measurements of the respiratory parameters are detailed in the text. Day 0, diauxic shift. Data refer to mean values determined in three independent experiments with three technical replicates each. SD is indicated. Values obtained for untreated wt cells were used as reference for comparisons with the corresponding ones determined for RSV-treated and sir2Δ cells. (*P ≤ 0.05 and **P ≤ 0.01, one-way ANOVA test).
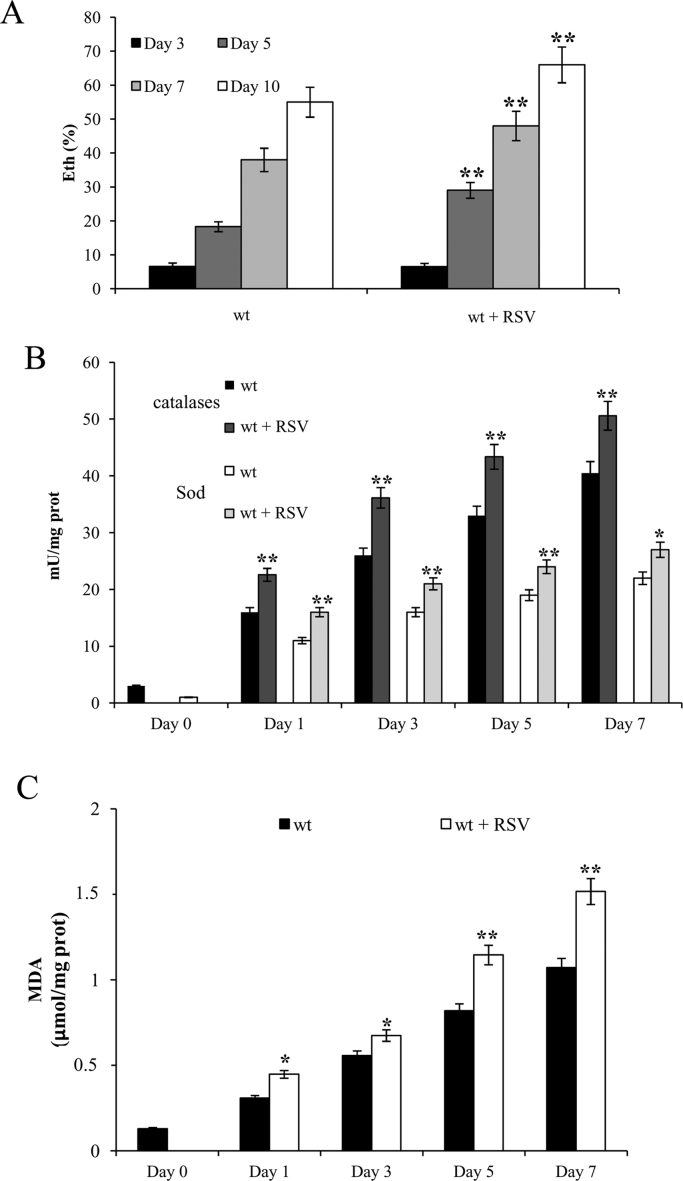
It is known that a non-phosphorylating respiration state is the source of the superoxide anion () [52], [53], which is produced by one electron reduction of oxygen due to leakage of electrons from the respiratory chain. and the resulting cascade of reactive oxygen species (ROS) accumulate as a function of age and contribute to the chronological aging phenotype [48], [50], [54]. In RSV-treated cells an increase in content compared to that of the untreated ones was observed (Fig. 2A) in line with an increased non-phosphorylating respiration (Table 1).
Fig. 2.
RSV supplementation at the diauxic shift triggers oxidative stress. Cultures of Fig. 1 were analyzed for the presence of intracellular superoxide by the conversion of non-fluorescent dihydroethidium into fluorescent ethidium (Eth). (A) Summary graphs of the percentage of fluorescent/superoxide positive cells (% Eth) are reported. About 1000 cells for each sample (three technical replicates) in three independent experiments were examined. At the indicated time points, catalases/Sod enzymatic activities (B) and the levels of intracellular malondialdehyde (MDA) (C) were measured. All the measurements were normalized to the protein content. Day 0, diauxic shift. SD is indicated. Statistical significance as in Fig. 1 (*P≤0.05 and **P≤0.01).
An increased content reflects a serious imbalance between production and the cellular antioxidant capacity. In fact, cells have developed endogenous antioxidant defenses for scavenging /ROS in order to offset their accumulation and the consequential damaging effects. In particular, starting from the diauxic shift, when cells shift to a respiratory metabolism and the cellular redox environment shifts to a more oxidized state [55], [56] these protective mechanisms become increasingly engaged as yeast cells age [49], [57]. In this context, antioxidant enzymes such as superoxide dismutases (Sod) and catalases are involved [48], [58]. The former catalyze the dismutation of into hydrogen peroxide (H2O2) and the latter reduce H2O2 to water [49]. Consistent with published data [59], in untreated cells both the enzymatic activities of Sod and catalases increased after the diauxic shift (Fig. 2B). Notably, at the same time-points, in RSV-treated cells the same enzymatic activities were always higher (Fig. 2B) indicating that RSV supplementation stimulates the antioxidant defences. Despite this, the generated in the RSV presence exceeds the cellular antioxidant capacity resulting in an increase of levels.
Oxidative stress is associated with the accumulation of macromolecular damages including lipid peroxidation (LPO). This process is initiated by attack of ROS to lipids containing carbon-carbon double bond(s), in particular polyunsaturated fatty acids. Afterwards, a cascade of reactions takes place generating different types of aldehydes, many of which are highly reactive and toxic [60], [61]. Malondialdehyde (MDA) is one of these end-products and is used as a convenient and reliable biomarker for LPO analysis [62]. After the diauxic shift in wt cells MDA levels increased (Fig. 2C), in line with the fact that LPO increases as chronological aging progresses [59], [63]. At the same time-points, in chronologically aging RSV-treated cells MDA levels were always higher (Fig. 2C) indicating that RSV supplementation exacerbated LPO. All this can contribute to the shortened CLS.
3.2. Lack of Sir2 results in cells unresponsive to RSV supplementation at the diauxic shift
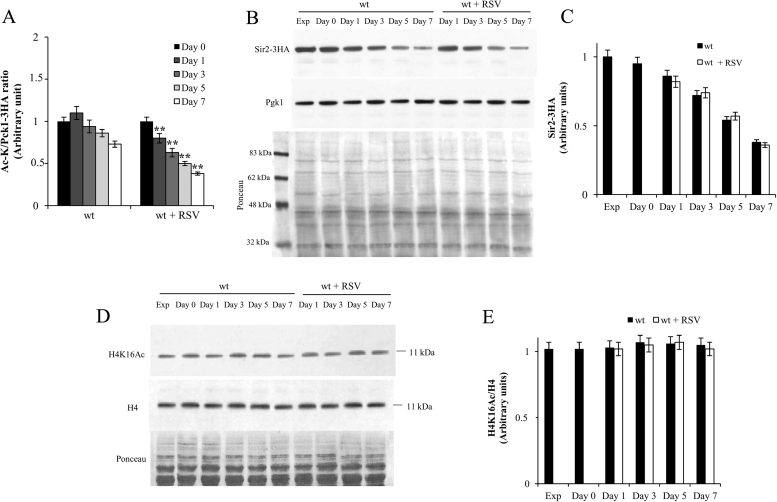
Pck1 is a metabolic enzyme that is highly regulated and, in particular, its gluconeogenic activity strictly depends on its de/acetylation state [35], [64]. In agreement with the decrease in Pck1 activity measured following RSV supplementation at the diauxic shift (Fig. 1D), a decrease in the level of the acetylated active form of the enzyme compared with that in the untreated culture was observed (Fig. 3A). No significant difference in the total Pck1 was detected between the two cultures (data not shown). Since RSV has been classified as a naturally occurring STAC [12], [14] and Sir2 is the enzyme responsible for Pck1 deacetylation [64], it is plausible to hypothesize that the deacetylase activity of Sir2 can be involved in the metabolic/physiologic changes observed in the presence of RSV. To investigate this, we initially compared total cellular levels of Sir2 in untreated and RSV-treated cells: no significant difference was found (Fig. 3B and C), ruling out any influence of RSV on Sir2 synthesis/stability. In parallel, we also compared the levels of H4 lysine 16 acetylation (H4K16ac). Indeed, the deacetylase activity of Sir2 is required for gene silencing at telomeres, mating-type loci (HML and HMR) and rDNA locus, where it maintains a hypoacetylated chromatin state by removing H4K16ac [65]. We did not observe any significant changes in H4K16ac levels between untreated and RSV-treated cells (Fig. 3D and E).
Fig. 3.
RSV supplementation at the diauxic shift increases Pck1 deacetylation without affecting Sir2 levels. Wt cells expressing Pck1-3HA were grown and supplied at the diauxic shift (Day 0) with RSV as in Fig. 1. At different time points, total protein extracts from both treated and untreated cultures were prepared and immunoprecipitated with anti-HA antibody. Afterwards, Western analyses were performed with anti-HA and anti-Ac-K antibodies followed by densitometric quantification of signal intensity of the bands relative to the total Pck1 (Pck1-3HA) and the acetylated form (Ac-K). The ratios of Ac-K to correspondent Pck1-3HA values are reported (A). (B) Wt cells expressing Sir2-3HA were grown and supplied at Day 0 with RSV as in Fig. 1. At different time points, total protein extracts from both treated and untreated cultures were prepared and subjected to Western analysis using anti-HA antibody. Protein extracts from exponentially growing cells were also analyzed (Exp). Pgk1 was used as loading control. The filter stained with Ponceau S Red is also shown. One representative filter is shown. (C) Bar chart of the relative levels of Sir2-3HA expressed in arbitrary units. For each time point, band intensities relative to Sir2-HA and Pgk1 detected by Western analysis were quantified and the values of Sir2-3HA signals were normalized against Pgk1 ones. Then, all values obtained were further normalized against the normalized Sir2-3HA level measured in exponential phase, which was arbitrary set to 1. (D) Total protein extracts prepared from cultures in (B) were subjected to Western analysis using antibodies anti-H4K16ac and anti-H4. The filter stained with Ponceau S Red is also shown. (E) Bar chart of the relative levels of H4K16ac expressed in arbitrary units. Densitometric quantification of signal intensity of the bands relative to the total H4 and the acetylated form (K16ac) was performed. The ratios of H4K16ac to correspondent H4 values are reported. All data refer to mean values determined in three independent experiments with three technical replicates each. SD is indicated. Statistical significance as in Fig. 1 (**P≤0.01).
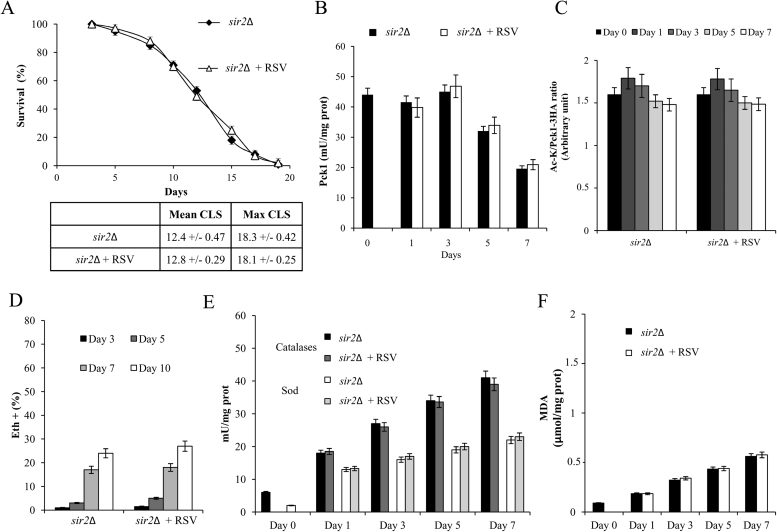
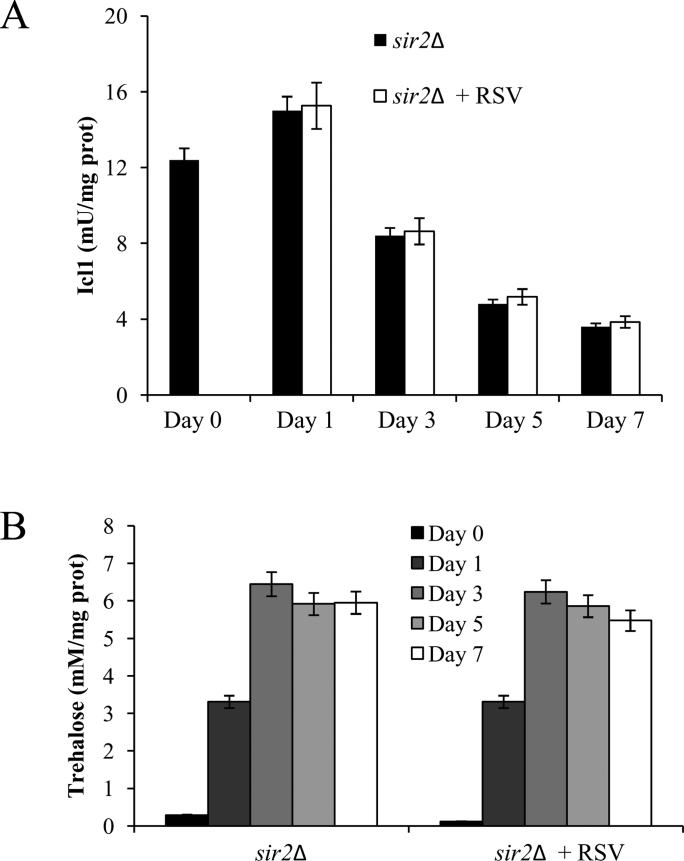
A way to assess if RSV works via Sir2 is to analyze its effects in the absence of the enzyme. Thus, RSV was supplied to sir2Δ cells at Day 0: all the RSV effects were abolished when SIR2 was deleted and unlike wt cells, sir2Δ ones were unresponsive to RSV (Figs. 4 and S1). Indeed, CLS of sir2Δ cells was unaffected (Fig. 4A), as were the metabolic features that characterize a chronologically aging sir2Δ culture [35], [37] such as fast depletion of extracellular ethanol and low levels of acetate (data not shown), high levels of Icl1 and Pck1 enzymatic activities (Figs. S1A and 4B), high levels of the acetylated Pck1 (Fig. 4C) and enhanced trehalose stores (Fig. S1B). Similarly, also the respiratory activity of chronologically aging sir2Δ cells that is particularly characterized by a low JTET [37], was unaffected by RSV presence (Table 1). This was accompanied by low levels (Fig. 4D). As far as the enzymatic activities of Sod and catalases were concerned, in sir2Δ cells their levels were similar to those measured in the wt ones (Figs. 4E and 2B) in line with published gene expression profiles [36]. RSV supplementation to sir2Δ cells had no effect on both the enzymatic activities (Fig. 4E) indicating that in the absence of Sir2 the cellular antioxidant defences are not stimulated by RSV treatment. Finally, lack of Sir2 also determined during chronological aging a strong reduction of LPO as shown by the lower MDA levels compared with those of the wt culture and, in the same way as for the other parameters so far analyzed, they were unaffected by RSV (Figs. 2C and 4F). Thus, overall, following RSV supplementation at the diauxic shift, wt cells acquire metabolic traits that are opposite to those of chronologically aging sir2Δ cells. Moreover, taken together these data indicate that Sir2 is required in the acquirement of these traits.
Fig. 4.
RSV supplementation at the diauxic shift tosir2Δ cells does not determine a short-lived phenotype. Sir2Δ cells were grown and supplied at the diauxic shift (Day 0) with RSV as in Fig. 1. (A) Survival over time of treated and untreated cultures was assessed. In the Table are shown Mean CLS and Maximum CLS values. Bar charts report Pck1 enzymatic activity (B), the ratio between Ac-K and Pck1-3HA values (C), the percentage of intracellular superoxide-accumulating cells (D), catalases/Sod enzymatic activities (E) and the levels of intracellular malondialdehyde (MDA) (F) determined for both cultures. All data refer to mean values of three independent experiments with three technical replicates each. SD is indicated.
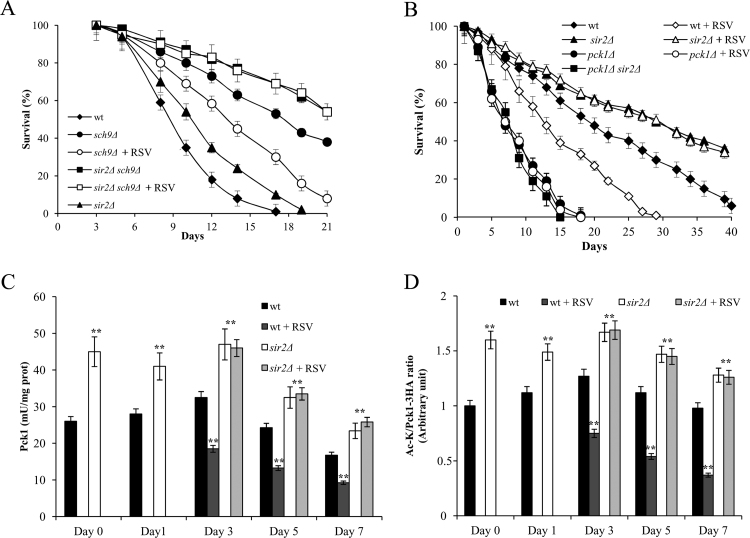
3.3. RSV supplementation at the diauxic shift promotes Sir2-mediated deacetylation of Pck1
The AGC protein kinase Sch9, which is a direct substrate for TORC1 [66], is a negative regulator of CLS and its lack significantly extends CLS ([67] and Fig. 5A). This long-lived phenotype is further exacerbated by SIR2 deletion ([36] and Fig. 5A). On the contrary, RSV supplementation to sch9Δ cells at Day 0 caused a survival reduction, whereas the synergistic effect of the SIR2 deletion with the SCH9 one on CLS was unaffected (Fig. 5A). Another condition in which SIR2 deletion further exacerbates a long-lived phenotype is that of a severe form of CR obtained by transferring post-diauxic cells from their expired medium to water ([35], [36] and Fig. 5B). Supplementing RSV to water strongly reduced the beneficial effect on the survival of wt cells produced by the severe CR, whilst had no impact on the extreme CLS extension of the sir2Δ culture (Fig. 5B). These data are consistent with the interpretation that the effects of RSV on CLS are mediated by Sir2.
Fig. 5.
RSV reduces the beneficial effects induced bySCH9deletion and severe CR on CLS. (A) CLS of sch9Δ and sch9Δsir2Δ cells supplemented with RSV at the diauxic shift (Day 0) as in Fig. 1. Survival of wt and sir2Δ strains is also shown for comparison. (B) The indicated strains grown as in Fig. 1, at Day 1 were transferred to water containing 100 μM RSV. Every 48 h, cultures were resuspended in fresh water and each time RSV was added. Survival of both treated and untreated cells was evaluated. In parallel, Pck1 enzymatic activity (C) and the ratio of Ac-K to Pck1-3HA values (D) were determined. All data presented are the mean values±SD of three biological replicates. Statistical significance as in Fig. 1 (**: P≤0.01).
Afterwards, since Pck1 acetylation/enzymatic activity is, on the one hand, necessary and sufficient for CLS extension in water and, on the other, the key substrate of Sir2 controlling this form of longevity [64], we measured both parameters after RSV supplementation to wt and sir2Δ cultures. Once wt cells were switched to water, the presence of RSV determined a decrease in the levels of Pck1 enzymatic activity compared to those detected without RSV (Fig. 5C). This decrease was associated with a reduction in the amount of the acetylated active form of the enzyme (Fig. 5D) in line with a reduced CLS (Fig. 5B). The sir2Δ culture in water, characterized by an extreme long-lived phenotype, had higher levels of both the enzymatic activity and acetylated form of Pck1 compared to those of the wt culture (Fig. 5C and D). Moreover, similarly to all the other cellular parameters analyzed, neither did the enzymatic activity and acetylated form of Pck1 change following RSV supplementation to sir2Δ cells (Fig. 5C and D). As previously reported, PCK1 deletion strongly reduced CLS in both water (Fig. 5B and [64]) and in expired medium [64]. The short-lived phenotype of pck1Δ cells was unaffected by SIR2 deletion (Fig. 5B and [35], [64]) being PCK1 epistatic to SIR2 in this form of longevity [64]. As SIR2 deletion, RSV supplementation did not affect the CLS of pck1Δ cells in both water (Fig. 5B) and in expired medium (data not shown).
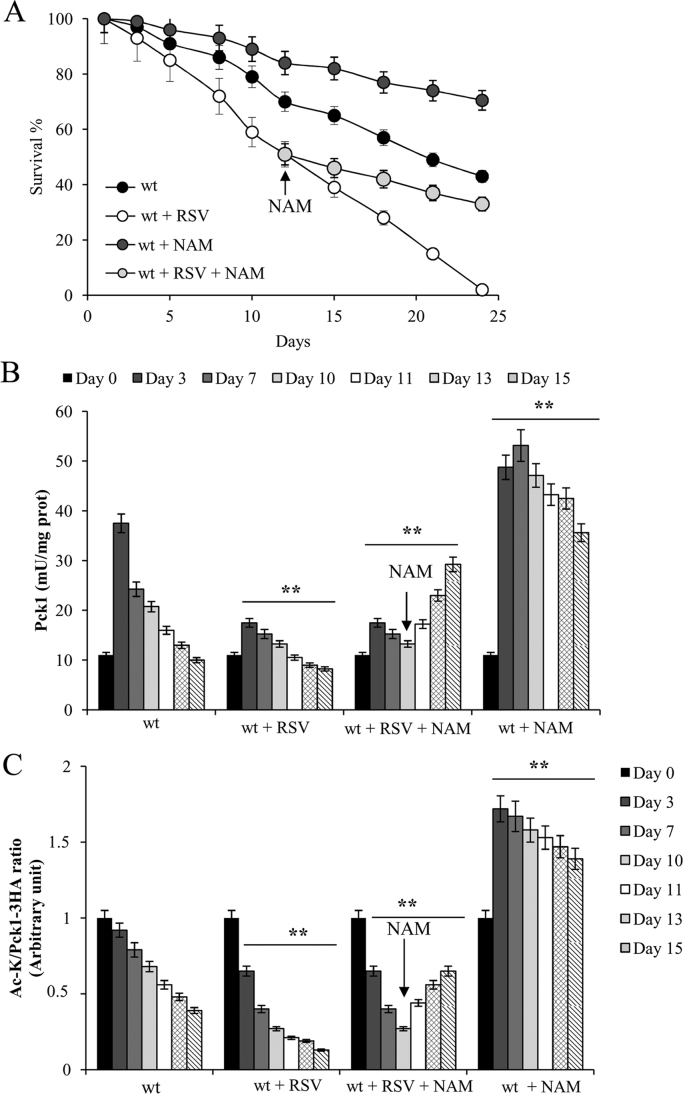
Finally, considering that we recently found that nicotinamide (NAM) supplementation at the diauxic shift inhibits Sir2 activity, in particular Sir2-mediated deacetylation of Pck1 resulting in a phenocopy of SIR2 deletion [37], to further delineate the link between RSV and Sir2, we set out to evaluate whether NAM would cause any effect on RSV-supplemented cells. To this end, post-diauxic wt cells were transferred from their expired medium to RSV-supplemented water. Then, at the time-point where the RSV stationary culture showed 50% (mean CLS) of survival, NAM (5 mM) was added. As shown in Fig. 6A, the negative effect on CLS exerted by RSV presence was almost completely abolished by the longevity-extending activity of NAM as a function of time in culture. Concomitantly, in the same time-frame, the trend of decrease of the enzymatic activity and the acetylation form of Pck1 was reversed and both increased (Fig. 6B and C). Taken together all these data reinforce the notion that the RSV effects observed herein are mediated by an enhancement of the Sir2 activity and involve its cellular target Pck1.
Fig. 6.
NAM supplementation during chronological aging rescues the negative effects of RSV on CLS and Pck1 activity. (A) Wt cells were transferred to water as in Fig. 5B supplemented with 100 μM RSV or 5 mM nicotinamide (NAM). Every 48 h, cultures were resuspended in fresh water containing RSV or NAM and survival of the cells over time was monitored. In addition, at the time point corresponding to 50% of survival RSV-treated cells were switched to water supplemented with both NAM and RSV. Survival of wt cells in water is also shown for comparison. During the treatments in (A), at the indicated time points, Pck1 enzymatic activity (B) and the ratio of Ac-K to Pck1-3HA values (C) were determined. All data refer to mean values of three independent experiments with three technical replicates each. SD is indicated. Statistical significance as in Fig. 1 (**: P≤0.01).
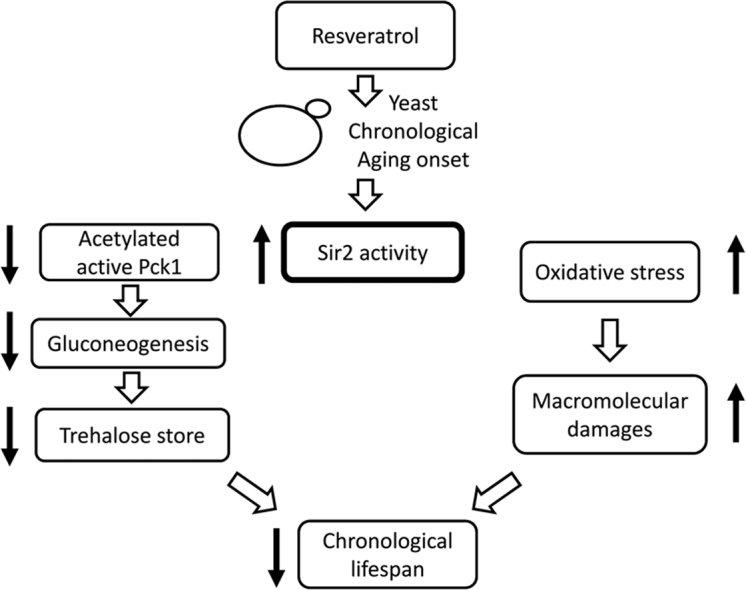
4. Discussion
In this study we investigated the effects of RSV supplementation at the onset of chronological aging, namely at the diauxic shift, on yeast metabolism and CLS. In effect, at the diauxic shift when cells shift from glucose-driven fermentation to ethanol/acetate-driven respiration, a massive metabolic reconfiguration takes place, leading cells to acquire a set of characteristics that ensure survival in stationary phase. These characteristics are specific to quiescent cells and influence the CLS. The metabolic reconfiguration mainly involves an increase in mitochondrial respiration and activation of gluconeogenesis that allows cells to utilize the glucose-derived fermentation products, ethanol and acetate, and accumulate sufficient stores for surviving starvation [68]. In particular trehalose, the accumulation of which is beneficial for CLS extension [69], apart from its unequivocal protective activity and contribution as an energy storage carbohydrate in quiescent cells, it plays a key role in fueling cell cycle reentry from quiescence [70]. We found that RSV supplementation affects this metabolic reconfiguration leading to negative outcomes on CLS, such as increased levels and a decrease in trehalose stores. Interestingly, all data indicate that the RSV effects on both metabolism and CLS are Sir2-dependent. With regard to the reduction in trehalose stores, it relies on a decrease in gluconeogenesis whose main flux-controlling enzymatic activity, namely Pck1, strongly decreases following RSV supplementation. This decrease is mirrored by the decrease in the acetylated active form of Pck1, which is targeted for deacetylation by Sir2 causing downregulation of gluconeogenesis [35], [37], [64]. Since Sir2 levels are unaffected by RSV presence, this suggests that the Sir2 enzymatic activity is enhanced. Furthermore, it is well known that the acetylation control of Pck1 gluconeogenic activity is crucial for CLS extension both in expired medium and in extreme CR condition (water) [64]. In both conditions, RSV supplementation determines CLS restriction and reduced levels of both enzymatic activity and acetylated form of Pck1. Notably, the negative effects of RSV on CLS and Pck1 activity/acetylation level are reversed by the supplementation of a non-competitive inhibitor of Sir2 activity, such as NAM, which reacts with an intermediate of the deacetylation reaction reforming NAD+ and the acetylated substrate [71]. In this context, we recently showed that NAM supplementation at the diauxic shift inhibits Sir2-mediated deacetylation of Pck1 generating a phenocopy of chronologically aging sir2Δ cells [37]. Since these cells display features that are opposite to those of RSV-supplied cells and SIR2 deletion makes cells unresponsive to RSV, taken as a whole, this supports the view that RSV effects stem from an enhancement of Sir2 activity and involve its cellular target Pck1.
In addition, chronologically aging sir2Δ cells and NAM-supplied ones display lower levels compared to wt cells (Fig. 4D and [37]). On the contrary, as far as levels are concerned, in RSV-treated cells, these levels remain higher compared with untreated ones despite the accompanying stimulation of endogenous cellular antioxidant defenses such as catalases and Sod. It is usually accepted that RSV has antioxidant properties relying in part upon the modulation of enzymes involved in the oxidative stress response [72]; in the chronological context this takes place, but strikingly it seems not to be enough to offset the increased flux of generated. It is well known that when cellular antioxidant responses are overwhelmed, oxidative stress occurs leading to the accumulation of macromolecular damages. Indeed, RSV-supplemented cells display enhanced lipid peroxidation. Furthermore, RSV supplementation also reduces trehalose stores and consequently reduces a protection against oxidative damage to proteins and lipids. All this may contribute thereby to restrict CLS.
To sum up, collectively these data are consistent with the notion that RSV supplementation at the diauxic shift requires Sir2 for eliciting its effects. In particular, it negatively influences chronological aging by enhancing Sir2 deacetylase activity. A common mechanism of allosteric activation/stimulation of SIRT1 activity has been proposed for RSV and synthetic STACs in which their binding to the N-terminal domain of SIRT1 leads to lowering the Km for the substrate [14]. Such an N-terminal regulatory domain is also present in Sir2 and provides a means for allosteric regulation of its enzymatic activity. Indeed, interactions between Sir4 and Sir2 involve this domain and enhance Sir2-mediated deacetylation of H4K16 [73], [74]. Although we cannot rule out the possibility than RSV might directly target proteins other than Sir2, it is plausible to hypothesize that RSV can interact with Sir2 by a similar mechanism and modify/increase the affinity of Sir2 towards its physiological substrate, the acetylated Pck1, the enzymatic activity of which is essential for gluconeogenesis and CLS extension.
Acknowledgement
We thank Rossella Ronzulli for technical support. The authors are grateful to Neil Campbell for English editing. This work was supported by FAR 2014 to M.V. M.S. and G.S were supported by fellowships from SYSBIONET, Italian roadmap of ESFRI.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.04.015.
Contributor Information
Ivan Orlandi, Email: ivan.orlandi@unimib.it.
Giulia Stamerra, Email: g.stamerra@campus.unimib.it.
Maurizio Strippoli, Email: maurizio.strippoli@unimib.it.
Marina Vai, Email: marina.vai@unimib.it.
Appendix A. Supplementary material
Fig. S1.
Cells lacking Sir2 are irresponsive to RSV supplementation at the diauxic shift. Bar charts of Icl1 enzymatic activity (A) and intracellular threalose content (B) determined for RSV-treated and untreated sir2Δ cultures of Fig. 4. Data refer to mean values determined in three independent experiments with three technical replicates each. SD is indicated.
.
Supplementary material
.
References
- 1.Burns J., Yokota T., Ashihara H., Lean M.E., Crozier A. Plant foods and herbal sources of resveratrol. J. Agric. Food Chem. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 2.Kursvietiene L., Staneviciene I., Mongirdiene A., Bernatoniene J. Multiplicity of effects and health benefits of resveratrol. Medicina. 2016;52(3):148–155. doi: 10.1016/j.medici.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 4.Catalgol B., Batirel S., Taga Y., Ozer N.K. Resveratrol: french paradox revisited. Front. Pharmacol. 2012;3 doi: 10.3389/fphar.2012.00141. (Article ID:141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdogan C.S., Vang O. Challenges in analyzing the biological effects of resveratrol. Nutrients. 2016;8(6) doi: 10.3390/nu8060353. (Article ID: E353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novelle M.G., Wahl D., Dieguez C., Bernier M., de Cabo R. Resveratrol supplementation: where are we now and where should we go? Ageing Res. Rev. 2015;21:1–15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park E.J., Pezzuto J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta. 2015;1852(6):1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Tang P.C., Ng Y.F., Ho S., Gyda M., Chan S.W. Resveratrol and cardiovascular health - Promising therapeutic or hopeless illusion? Pharmacol. Res. 2014;90:88–115. doi: 10.1016/j.phrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Kulkarni S.S., Canto C. The molecular targets of resveratrol. Biochim. Biophys. Acta. 2015;1852(6):1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Tennen R.I., Michishita-Kioi E., Chua K.F. Finding a target for resveratrol. Cell. 2012;148(3):387–389. doi: 10.1016/j.cell.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., Scherer B., Sinclair D.A. Small molecule activators of Sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 12.Hubbard B.P., Sinclair D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014;35(3):146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S.J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., Kim M.K., Beaven M.A., Burgin A.B., Manganiello V., Chung J.H. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sinclair D.A., Guarente L. Small-molecule allosteric activators of Sirtuins. Annu. Rev. Pharmacol. Toxicol. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gertz M., Steegborn C. Using mitochondrial sirtuins as drug targets: disease implications and available compounds. Cell. Mol. Life Sci. 2016;73(15):2871–2896. doi: 10.1007/s00018-016-2180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houtkooper R.H., Pirinen E., Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell. Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai S., Guarente L. NAD+ and Sirtuins in aging and disease. Trends Cell. Biol. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris B.J. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013;56:133–171. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- 19.Kitada M., Ogura Y., Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging (Albany, NY) 2016;8(10):2290–2307. doi: 10.18632/aging.101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A., Chauhan S. How much successful are the medicinal chemists in modulation of SIRT1: a critical review. Eur. J. Med. Chem. 2016;119:45–69. doi: 10.1016/j.ejmech.2016.04.063. [DOI] [PubMed] [Google Scholar]
- 21.Poulose N., Raju R. Sirtuin regulation in aging and injury. Biochim. Biophys. Acta. 2015;1852(11):2442–2455. doi: 10.1016/j.bbadis.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye R.A. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem. Biophys. Res. Commun. 2000;273(2):793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 23.Longo V.D., Shadel G.S., Kaeberlein M., Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16(1):18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nystrom T., Liu B. The mystery of aging and rejuvenation - a budding topic. Curr. Opin. Microbiol. 2014;18:61–67. doi: 10.1016/j.mib.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Longo V.D., Kennedy B.K. Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Imai S., Armstrong C.M., Kaeberlein M., Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 27.Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stumpferl S.W., Brand S.E., Jiang J.C., Korona B., Tiwari A., Dai J., Seo J.G., Jazwinski S.M. Natural genetic variation in yeast longevity. Genome Res. 2012;22(10):1963–1973. doi: 10.1101/gr.136549.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinkraus K.A., Kaeberlein M., Kennedy B.K. Replicative aging in yeast: the means to the end. Annu. Rev. Cell. Dev. Biol. 2008;24:29–54. doi: 10.1146/annurev.cellbio.23.090506.123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarolim S., Millen J., Heeren G., Laun P., Goldfarb D.S., Breitenbach M. A novel assay for replicative lifespan in Saccharomyces cerevisiae. FEMS Yeast Res. 2004;5(2):169–177. doi: 10.1016/j.femsyr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Yang H., Baur J.A., Chen A., Miller C., Adams J.K., Kisielewski A., Howitz K.T., Zipkin R.E., Sinclair D.A. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell. 2007;6(1):35–43. doi: 10.1111/j.1474-9726.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaeberlein M., Kennedy B.K. Does resveratrol activate yeast Sir2 in vivo? Aging Cell. 2007;6(4):415–416. doi: 10.1111/j.1474-9726.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 33.MacLean M., Harris N., Piper P.W. Chronological lifespan of stationary phase yeast cells; a model for investigating the factors that might influence the ageing of postmitotic tissues in higher organisms. Yeast. 2001;18(6):499–509. doi: 10.1002/yea.701. [DOI] [PubMed] [Google Scholar]
- 34.Fabrizio P., Longo V.D. The chronological life span of Saccharomyces cerevisiae. Methods Mol. Biol. 2007;371:89–95. doi: 10.1007/978-1-59745-361-5_8. [DOI] [PubMed] [Google Scholar]
- 35.Casatta N., Porro A., Orlandi I., Brambilla L., Vai M. Lack of Sir2 increases acetate consumption and decreases extracellular pro-aging factors. Biochim. Biophys. Acta. 2013;1833(3):593–601. doi: 10.1016/j.bbamcr.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Fabrizio P., Gattazzo C., Battistella L., Wei M., Cheng C., McGrew K., Longo V.D. Sir2 blocks extreme life-span extension. Cell. 2005;123(4):655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Orlandi I., Pellegrino Coppola D., Strippoli M., Ronzulli R., Vai M. Nicotinamide supplementation phenocopies SIR2 inactivation by modulating carbon metabolism and respiration during yeast chronological aging. Mech. Ageing Dev. 2017;161:277–287. doi: 10.1016/j.mad.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Orlandi I., Coppola D., Vai M. Rewiring yeast acetate metabolism through MPC1 loss of function leads to mitochondrial damage and decreases chronological lifespan. Microb. Cell. 2014;1(12):393–405. doi: 10.15698/mic2014.12.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanoni M., Vai M., Popolo L., Alberghina L. Structural heterogeneity in populations of the budding yeast Saccharomyces cerevisiae. J. Bacteriol. 1983;156(3):1282–1291. doi: 10.1128/jb.156.3.1282-1291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlandi I., Ronzulli R., Casatta N., Vai M. Ethanol and acetate acting as carbon/energy sources negatively affect yeast chronological aging. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/802870. (article ID: 802870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee D.H., Goldberg A.L. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol. Cell. Biol. 1998;18(1):30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jong-Gubbels P., Vanrolleghem P., Heijnen S., van Dijken J.P., Pronk J.T. Regulation of carbon metabolism in chemostat cultures of Saccharomyces cerevisiae grown on mixtures of glucose and ethanol. Yeast. 1995;11(5):407–418. doi: 10.1002/yea.320110503. [DOI] [PubMed] [Google Scholar]
- 43.Giannattasio S., Guaragnella N., Corte-Real M., Passarella S., Marra E. Acid stress adaptation protects Saccharomyces cerevisiae from acetic acid-induced programmed cell death. Gene. 2005;354:93–98. doi: 10.1016/j.gene.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Petrova V.Y., Drescher D., Kujumdzieva A.V., Schmitt M.J. Dual targeting of yeast catalase A to peroxisomes and mitochondria. Biochem. J. 2004;380(2):393–400. doi: 10.1042/BJ20040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flohe L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 46.Madeo F., Frohlich E., Ligr M., Grey M., Sigrist S.J., Wolf D.H., Frohlich K.U. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 1999;145(4):757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kushnirov V.V. Rapid and reliable protein extraction from yeast. Yeast. 2000;16(9):857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 48.Breitenbach M., Rinnerthaler M., Hartl J., Stincone A., Vowinckel J., Breitenbach-Koller H., Ralser M. Mitochondria in ageing: there is metabolism beyond the ROS. FEMS Yeast Res. 2014;14(1):198–212. doi: 10.1111/1567-1364.12134. [DOI] [PubMed] [Google Scholar]
- 49.Herrero E., Ros J., Belli G., Cabiscol E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta. 2008;1780(11):1217–1235. doi: 10.1016/j.bbagen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Pan Y. Mitochondria, reactive oxygen species and chronological aging: a message from yeast. Exp. Gerontol. 2011;46(11):847–852. doi: 10.1016/j.exger.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Cain K., Griffiths D.E. Studies of energy-linked reactions. localization of the site of action of trialkyltin in yeast mitochondria. Biochem. J. 1977;162(3):575–580. doi: 10.1042/bj1620575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrero-Castillo S., Araiza-Olivera D., Cabrera-Orefice A., Espinasa-Jaramillo J., Gutierrez-Aguilar M., Luevano-Martinez L.A., Zepeda-Bastida A., Uribe-Carvajal S. Physiological uncoupling of mitochondrial oxidative phosphorylation. studies in different yeast species. J. Bioenerg. Biomembr. 2011;43(3):323–331. doi: 10.1007/s10863-011-9356-5. [DOI] [PubMed] [Google Scholar]
- 53.Hlavata L., Aguilaniu H., Pichova A., Nystrom T. The oncogenic RAS2val19 mutation locks respiration, independently of PKA, in a mode prone to generate ROS. EMBO J. 2003;22(13):3337–3345. doi: 10.1093/emboj/cdg314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barros M.H., da Cunha F.M., Oliveira G.A., Tahara E.B., Kowaltowski A.J. Yeast as a model to study mitochondrial mechanisms in ageing. Mech. Ageing Dev. 2010;131(7–8):494–502. doi: 10.1016/j.mad.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Ayer A., Sanwald J., Pillay B.A., Meyer A.J., Perrone G.G., Dawes I.W. Distinct redox regulation in sub-cellular compartments in response to various stress conditions in Saccharomyces cerevisiae. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065240. (Article ID: e65240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drakulic T., Temple M.D., Guido R., Jarolim S., Breitenbach M., Attfield P.V., Dawes I.W. Involvement of oxidative stress response genes in redox homeostasis, the level of reactive oxygen species and ageing in Saccharomyces cerevisiae. FEMS Yeast Res. 2005;5(12):1215–1228. doi: 10.1016/j.femsyr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Longo V.D., Gralla E.B., Valentine J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996;271(21):12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 59.Reverter-Branchat G., Cabiscol E., Tamarit J., Ros J. Oxidative damage to specific proteins in replicative and chronological-aged Saccharomyces cerevisiae: common targets and prevention by calorie restriction. J. Biol. Chem. 2004;279(30):31983–31989. doi: 10.1074/jbc.M404849200. [DOI] [PubMed] [Google Scholar]
- 60.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/360438. (Article ID: 360438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauck A.K., Bernlohr D.A. Oxidative stress and lipotoxicity. J. Lipid Res. 2016;57(11):1976–1986. doi: 10.1194/jlr.R066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 63.Cabiscol E., Piulats E., Echave P., Herrero E., Ros J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J. Biol. Chem. 2000;275(35):27393–27398. doi: 10.1074/jbc.M003140200. [DOI] [PubMed] [Google Scholar]
- 64.Lin Y.Y., Lu J.Y., Zhang J., Walter W., Dang W., Wan J., Tao S.C., Qian J., Zhao Y., Boeke J.D., Berger S.L., Zhu H. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell. 2009;136(6):1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gartenberg M.R., Smith J.S. The nuts and bolts of transcriptionally silent chromatin in Saccharomyces cerevisiae. Genetics. 2016;203(4):1563–1599. doi: 10.1534/genetics.112.145243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loewith R., Hall M.N. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fabrizio P., Pozza F., Pletcher S.D., Gendron C.M., Longo V.D. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 68.De Virgilio C. The essence of yeast quiescence. FEMS Microbiol. Rev. 2012;36(2):306–339. doi: 10.1111/j.1574-6976.2011.00287.x. [DOI] [PubMed] [Google Scholar]
- 69.Ocampo A., Liu J., Schroeder E.A., Shadel G.S., Barrientos A. Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 2012;16(1):55–67. doi: 10.1016/j.cmet.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi L., Sutter B.M., Ye X., Tu B.P. Trehalose is a key determinant of the quiescent metabolic state that fuels cell cycle progression upon return to growth. Mol. Biol. Cell. 2010;21(12):1982–1990. doi: 10.1091/mbc.E10-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sauve A.A., Wolberger C., Schramm V.L., Boeke J.D. The biochemistry of Sirtuins. Annu. Rev. Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 72.Diaz-Gerevini G.T., Repossi G., Dain A., Tarres M.C., Das U.N., Eynard A.R. Beneficial action of resveratrol: how and why? Nutrition. 2016;32(2):174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 73.Hsu H.C., Wang C.L., Wang M., Yang N., Chen Z., Sternglanz R., Xu R.M. Structural basis for allosteric stimulation of Sir2 activity by Sir4 binding. Genes Dev. 2013;27(1):64–73. doi: 10.1101/gad.208140.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanny J.C., Kirkpatrick D.S., Gerber S.A., Gygi S.P., Moazed D. Budding yeast silencing complexes and regulation of Sir2 activity by protein-protein interactions. Mol. Cell. Biol. 2004;24(16):6931–6946. doi: 10.1128/MCB.24.16.6931-6946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material