Abstract
Introduction
Though a meta-analysis reported the effect of diabetes on colorectal prognosis in 2013, a series of large-scale long-term cohort studies has comprehensively reported the outcome effect estimates on the relationship between diabetes and colorectal prognosis, and their results were still consistent.
Methods
We carried out an extensive search strategy in multiple databases and conducted a meta-analysis on the effect of diabetes on colorectal prognosis, based on the included 36 cohort studies, which contained 2,299,012 subjects. In order to collect more data, besides conventional methods, we used the professional software to extract survival data from the Kaplan-Meier curves, and analyzed both the 5-year survival rate and survival risk in overall survival, cancer-specific survival, cardiovascular disease—specific survival, disease-free survival, and recurrence-free survival, to comprehensively reflect the effect of diabetes on colorectal prognosis.
Results
The results found that compared to patients without diabetes, patients with diabetes will have a 5-year shorter survival in colorectal, colon and rectal cancer, with a 18%, 19% and 16% decreased in overall survival respectively. We also found similar results in cancer-specific survival, cardiovascular disease—specific survival, disease-free survival, and recurrence-free survival, but not all these results were significant. We performed the subgroup analysis and sensitivity analysis to find the source of heterogeneity. Their results were similar to the overall results.
Conclusions
Our meta-analysis suggested that diabetes had a negative effect on colorectal cancer in overall survival. More studies are still needed to confirm the relationship between diabetes and colorectal prognosis in cancer-specific survival, cardiovascular disease—specific survival, disease-free survival, and recurrence-free survival.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in global incidence and the fourth in mortality all over the world, and the incidence and mortality are higher in men than in women in most parts of the world [1]. In recent years, diagnosis and treatment had made a certain degree of progress, but CRC is still a very important public health problem in the world. Thus, early diagnosis, effective treatment and analysis prognosis were of great significance to reducing the CRC mortality. To guide decision-making for therapeutic strategies for CRC patients and improve their prognosis, a better understanding of the relevant factors affecting CRC prognosis is urgently needed.
Diabetes mellitus (DM) is one of the most common chronic and metabolism diseases. The number of people with DM worldwide has increased by two times in the past three decades[2]. An estimated 285 million people worldwide had diabetes mellitus in 2010, and the number of DM sufferers will rise to 439 million by 2030, represents 7.7% of the total adult population of the world aged 20–79 years[3]. The concurrence of DM pandemics with the growing burden of cancer globally has generated interest in defining the epidemiological and biological relationships between these medical conditions[3, 4].
DM can seriously affect quality of life. DM can not only cause neurological and vascular complications, but is also closely related to the occurrence, development and prognosis of cancer. Currently, more and more clinicians are considering whether patients have suffered from diabetes during the treatment of cancer, and diabetologists often have to manage diabetes in patients who are being treated for cancer[4]. Insulin resistance or compensatory hyperinsulinemia leads to hormonal and metabolic alterations, and is involved in the formation of the microenvironment for tumorigenesis and tumor progression. Diabetes mellitus might influence survival of CRC patients due to insulin-stimulated growth of colorectal cancer cells or inadequate treatment of persons with concomitant disease. However, it is unclear whether colorectal cancer patients with DM are more likely to receive a worse colorectal cancer prognosis compared to patients without DM. A meta-analysis has reported the effect of DM on CRC prognosis[5], but since 2013, a series of large-scale long-term cohort studies had comprehensively reported the outcome effect estimates on the relationship between DM and CRC prognosis, and their results were still consistent[6–20]. For example, in overall survival (OS) of CRC, several studies found that DM showed a significant decreased risk in OS[6, 7, 12–14, 17], and others found no link[8–11, 15, 16, 18–20]. The data from these studies has also allowed us to evaluate the relationship between DM and CRC prognosis more accurately. Thus we want to perform a meta-analysis to determine the relationship between DM and CRC prognosis, and provide a theoretical basis for clinical research. Our meta-analysis first reported the 5-year survival estimates on the effect of DM on CRC prognosis, and respectively analyzed the effects of DM on the colorectal, colon and rectal cancer from OS, cancer-specific survival (CSS), cardiovascular disease—specific survival (CVDS), disease-free survival (DFS), or recurrence-free survival (RFS).
Methods
Literature search
A systematic literature review was independently carried out by two groups (Bo Zhu, Bo Wu as a group, and Lu Zhang, Lixuan Wei as another group) in multiple databases (Pubmed, Web of Science, Embase and Google Scholar) up to March 19, 2017. In order to collect as many relevant studies as possible, we set the following search terms: (diabetes OR hyperglycemia OR glucose intolerance) AND (colorectal cancer OR colorectal neoplasms OR colon cancer OR colonic neoplasms OR rectal cancer OR rectal neoplasms) AND (prognosis OR survival analysis OR survival OR survival rate OR mortality). The reviewed reference lists from all the relevant original research and reviews were also searched to identify additional potentially eligible studies. There were no language or other restrictions. All retrieved studies were initially selected by reading the title and abstract. S1 File showed the detailed methods used for searching all the databases.
Inclusion and exclusion criteria
The final included studies were identified by reading the full text, according to the inclusion and exclusion criteria. Three authors (Bo Zhu, Xiaomei Wu and Bo Wu) participated in this process, and any disagreements were solved by discussion.
The included studies in our meta-analysis should meet the following criteria: the study should (1) investigate the relationship between DM and CRC prognosis; (2) be cohort study; (3) provide the hazard ration (HR) or rate, which reflected overall survival (OS), cancer-specific survival (CSS), cardiovascular disease—specific survival (CVDS), disease-free survival (DFS), or recurrence-free survival (RFS); (4) provide the relevant data to calculate the corresponding outcome effect estimates.
The diagnostic criterion for DM and hyperglycemia was used by the World Health Organization (WHO) 1999 criteria or American Diabetes Association (ADA) 2010 guidelines. OS was defined as the time from the date of surgery to death from any cause. CSS was defined as the time from the date of surgery to death from colorectal cancer-specific cause of death. CVDS defined as the time from the date of surgery to death from cardiovascular disease -specific cause of death. DFS was defined as time from the date of surgery to tumor recurrence or occurrence of a new primary colorectal tumor or death from any cause. RFS was defined as the time from the surgery to tumor recurrence or occurrence of a new primary colon tumor[8, 21].
The exclusion criteria of our meta-analysis are: (1) the study did not investigate the relationship between the relationship between DM and CRC prognosis; (2) the study did not provide the relevant data to calculate outcome effect estimates (including HR and/or rate), which reflected OS, CSS, CVDS, DFS, or RFS; (3) the type of study excluded animal experiment, chemistry and cell-line research, letters to the editor, meetings abstracts, communications or review.
Data extraction and conversion
The data from the final included studies were extracted independently by two authors (Bo Zhu and Xiaomei Wu). These authors used the standard table to extract the information, which included author, year of publication, country, type of study, sample size, population source, recruitment time, age, gender, patients with DM, DM ascertainment, type of cancer, outcomes, and adjusted variables. If the study provided more than two outcome effect estimates adjusted for different numbers of potential confounders, we extracted the estimate that adjusted for the highest number of potential confounders for analysis. If more than two studies provided the outcome effect estimates from the same population, we extracted the latest or highest-quality outcome effect estimates.
Quality assessment
Two authors (Bo Zhu and Xiaomei Wu) independently conducted the quality assessment of the final studies included by using the Newcastle-Ottawa Quality Assessment Scale (NOS)[22]. The NOS is a semi quantitative method for assessing the quality of studies, and consisted of three main parts: selection (4 points), comparability (2 points) and outcome (3 points). Thus, the quality of study was determined on a scale from zero to nine points. Studies with seven or more points were regarded as “high quality”, studies with the points from four to six were regard as “moderate quality”, and otherwise, the study was regarded as “low quality”[23].
Statistical analysis
The Stata v.12.0 software was used to conduct our meta-analysis and used the pooled outcome effect estimates and corresponding 95% confidence interval (CI) for OS, CSS, CVDS, DFS or RFS to analyze the relationship between DM and CRC prognosis. If the study did not provide the corresponding results, we used the Engauge Digitizer v.4.1 software (http://digitizer.sourceforge.net/) to extract survival rates from the Kaplan-Meier curves [24–26], the survival rates were entered in the spreadsheet by the method in Tierney’s article[24]. The process of extracting survival rates was performed by two independent authors (Dan Pei and Lixuan Wei) to make the extracted data more accurate. The heterogeneity in the included studies was evaluated by the Chi-square-based Q-test and I2 (I2 = 0% to 25%, no heterogeneity; I2 = 25% to 50%, moderate heterogeneity; I2 = 50% to 75%, high heterogeneity; I2 = 75% to 100%, extreme heterogeneity). When I2 was larger than 50%, a random effects model was used; otherwise, the fixed effects model was used.
We used subgroup analysis by region, type of study, sample size, population source and DM ascertainment to find the potential heterogeneity among the included studies. If the number of study was less than or equal to 1, we did not carry out the subgroup analysis. We used the sensitivity analysis to evaluate the robustness of the results by excluding each study in turn and obtaining the pooled estimates from the remaining studies. The purpose of sensitivity analysis was to evaluate the effect of a single study on the overall pooled estimates. If the number of study was less than or equal to 1, we did not carry out the subgroup analysis and sensitivity analysis.The possibility of publication bias was assessed using Begger's and Egger's test. Where publication bias existed, we also performed the Duval and Tweedie nonparametric “trim and fill” procedure to further assess the possible effect of publication bias in our meta-analysis. If the number of study was less than or equal to 2, we did not carry out the sensitivity analysis and publication bias test. A two-sided P value <0.05 in statistical process was considered significantly different.
Results
Search results
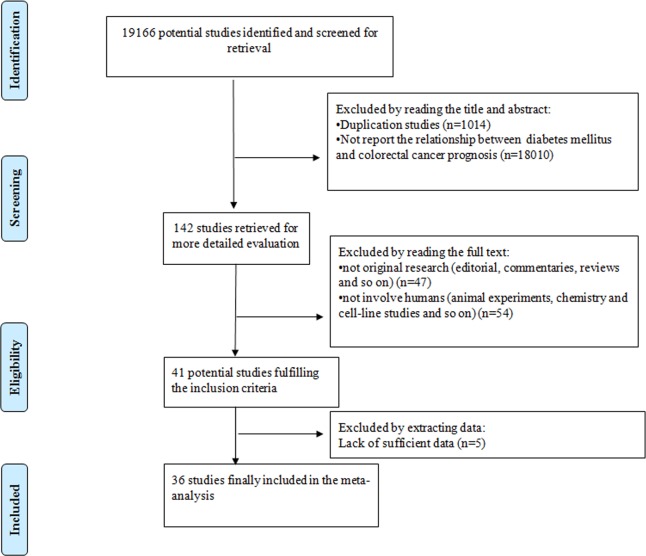
Originally, we retrieved 19166 potential studies from four electronic databases. By reading the title and abstract, we found that 1014 studies were repetitive and 18010 studies did not report the relationship between DM and CRC Prognosis. By reading the full text, 101 studies were excluded for different reasons, and 5 studies did not provide sufficient data to calculate the outcome effect estimates. Finally, 36 studies were included in our meta-analysis[6–20, 27–47]. The study selection process for inclusion in our meta-analysis was shown in Fig 1.
Fig 1. The study selection process for inclusion in our meta-analysis.
Study characteristics and quality
In our meta-analysis, year of publication ranged from 2003 to 2016, and the regions included 2 American countries[7, 13–15, 18, 19, 27, 30, 33, 37, 41, 42, 45, 46], 6 European countries[6, 11, 17, 28, 32, 39, 40, 44], 2 Asian countries[8, 9, 12, 16, 20, 29, 34–36, 38, 43, 47] and 1 Oceania country[31]; the included studies contained 15 retrospective[9, 10, 14, 16–20, 27, 33, 36, 37, 39, 41, 47] and 21 prospective[6–8, 11–13, 15, 28–32, 34, 35, 38, 40, 42–46] cohort studies; the sample size ranged from 391 to 1056243, and the mean age of study ranged from 46.4 to 72.07. In DM ascertainment, 25 studies[6, 8, 9, 11–15, 18, 19, 28, 29, 31, 33–37, 39–42, 44–46] used the method of medical records, 5 studies[16, 20, 38, 43, 47] used the method of blood sugar test, and 6 studies[7, 10, 17, 27, 30, 32] used the method of self-reported. To avoid the effects of confounders, we preferred to extract the adjusted outcome effect estimates, but we still found that the outcome effect estimates of 4 studies were not adjusted.
The quality score ranged from 5 to 9. 11 studies were evaluated as 9 scores, 7 studies were evaluated as 8 scores, 12 studies were evaluated as 7 scores, 4 studies were evaluated as 6 scores, and 2 studies were evaluated as 5 scores. All the included studies were regarded as moderate and high quality.
The characteristic and quality of the included studies is shown in Table 1.
Table 1. The characteristic and quality of the included studies.
| Author | Year | Region | Type of Study | Sample Size | Population source | Recruitment time | Age (Year) | Gender (male/female) | Patients with DM (n) | DM ascertainment | Type of cancer | Outcomes | Adjusted variable | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee, S. J. | 2016 | Korea | retrospective | 741 | Hospital-based | 1999–2010 | 65.20 | 440/301 | 634 | Blood glucose test | colon cancer | adjusted HROS; 5-year OS | age and sex, WBC, CRP, total cholesterol, high density lipoprotein, low density lipoprotein, triglycerides | 9 |
| Paulus, J. K. | 2016 | USA | retrospective | 21292 | population-based | 2001–2008 | 69.16 | 20866/426 | 4983 | Medical records | colorectal cancer | adjusted HROS; 5-year OS | age, race, AJCC stage, BMI, co-morbidity index, CRC treatment, smoking status | 8 |
| Fransgaard, T. | 2016 | Denmark | retrospective | 29353 | Hospital-based | 2003–2012 | 70.05 | 15495/13858 | 3250 | Self-reported | colorectal cancer | adjusted HROS | age, gender, ASA score, BMI, blood transfusions, smoking, alcohol consumption, elective or emergency surgery, AL, type of cancer (colon or rectal) and year of operation | 9 |
| Yang, I. P. | 2016 | Chinese Taiwan | retrospective | 520 | Hospital-based | 2005–2011 | 64.56 | 310/210 | 135 | Blood glucose test | colorectal cancer | adjusted HROS and DFS | age, gender, stage, tumor size, location, invasive depth, vascular invasion, perineural invasion and serum blood sugar of CRC patients | 9 |
| Ramjeesingh, R. | 2016 | Canada | retrospective | 1304 | Hospital-based | 2005–2011 | 71.09 | 764/540 | 277 | Medical records | colorectal cancer | adjusted HROS; 5-year OS | age, gender, co-morbidities (cardiac, diabetic, renal,and respiratory), diabetes treatments (metformin or not), BMI, smoking history, alcohol history, family history of CRC, location of cancer (rectal vs. colon), stage at diagnosis and differentiation | 9 |
| Cui, G. | 2015 | China | retrospective | 391 | Hospital-based | 2008–2013 | — | 222/169 | 58 | Blood glucose test | colorectal cancer | unadjusted HROS; 5-year OS | — | 5 |
| Chen, K. H. | 2014 | Chinese Taiwan | Prospective | 6937 | Population-based | 2004–2008 | 67.3 | 3946/2991 | 1371 | Medical records | colon cancer | adjusted HROS and HRCSS; 5-year OS and 5-year CSS | age, gender, tumor stage, treatment, cirrhosis, and all other co-morbidities | 8 |
| Luo, J. | 2014 | USA | Prospective | 46400 | Population-based | 2003–2009 | >65 | 20638/25762 | 14813 | Medical records | colorectal cancer; colon and rectal cancer | adjusted HROS, HRCSS, and HRCVDS; 5-year OS and 5-year CSS | age at diagnosis, gender, race, marital status, grade, census tract median income and co-morbidity | 8 |
| Waheed, S. | 2014 | USA | Prospective | 16977 | Population-based | 2000–2005 | >67 | 7094/9883 | 4414 | Medical records | colorectal cancer | unadjusted HROS, HRCSS and HRCVDS; 5-year OS, 5-year CSS and 5-year CVDS | — | 6 |
| Tong, L. | 2014 | USA | retrospective | 375462 | Population-based | 1975–2009 | — | 190189/185273 | — | Medical records | colorectal cancer | adjusted HROS | age, gender, race, and regions | 6 |
| Walker, J. J. | 2013 | Scotland | Prospective | 19505 | Population-based | 2000–2007 | — | 10417/9088 | 2387 | Medical records | colon and rectal cancer | adjusted HROS | age, SES, stage and treatment | 7 |
| Bella, F. | 2013 | Italy | Prospective | 1039 | Hospital-based | 2003–2005 | — | 593/446 | 373 | Medical records | colorectal cancer; colon and rectal cancer | adjusted HROS and HRCSS; 5-year OS and 5-year CSS | age, gender, stage, type of treatment, morphology and grade | 7 |
| Jeon, J. Y. | 2013 | Korea | Prospective | 4131 | Hospital-based | 1995–2007 | 59 | 2479/1652 | 517 | Medical records | colorectal cancer; colon and rectal cancer | adjusted HROS, HRRFS, HRDFS and HRCSS; 5-year DFS | age, gender, BMI, family history of CRC, TNM stage, adjuvant therapy and the year of surgery. | 9 |
| Morrison, D. S. | 2013 | Asia Pacific region | retrospective | 600427 | Population-based | 1961–1999 | 46.4 | 216154/384273 | 182569 | Self-reported | Colorectal cancer, rectal and colon cancer | adjusted HROS | age, BMI, physical activity, height, drink, smoke, cholesterol, diabetes and education | 9 |
| Liu, D. | 2013 | China | retrospective | 525 | Hospital-based | 2004–2011 | 63.2 | 310/215 | 86 | Medical records | colorectal cancer | unadjusted HROS and HRDFS; 5-year OS and 5-year DFS | — | 6 |
| Cossor, F. I. | 2013 | USA | Prospective | 2066 | Population-based | 1993–1998 | 71.92 | 0/2066 | 212 | Self-reported | colorectal cancer | adjusted HROS and HRCSS; 5-year OS and 5-year CSS | age and stage at diagnosis | 7 |
| Huang, C. W. | 2012 | Chinese Taiwan | Prospective | 1197 | Hospital-based | 2002–2008 | 64.18 | 673/524 | 283 | Medical records | colorectal cancer | adjusted HROS and HRCSS; 5-year OS and 5-year CSS | age, gender, location, tumor size, BMI, albumin, histology, AJCC stage, Pre-op CEA, Post-op CEA, vascular invasion and perineurial invasion | 8 |
| Dehal, A. N. | 2012 | USA | Prospective | 2278 | Population-based | 1992–1993 | — | — | 393 | Self-reported | colorectal cancer | adjusted HROS, HRCVDS and HRCSS; 5-year OS, 5-year CSS and 5-year CVDS | gender, age at CRC diagnosis, BMI, smoking status, physical activity, red meat intake, and surveillance, epidemiology, and end results summary stage | 6 |
| van de Poll-Franse, L. V. | 2012 | Netherlands | Prospective | 10862 | Hospital-based | 1997–2007 | 68.34 | 5806/5056 | 1224 | Medical records | colon cancer | adjusted HROS, HRCSS; 5-year OS and 5-year CSS | age at diagnosis, gender, stage, number of examined lymph nodes, adjuvant therapy, SES, year of diagnosis, hypertension, CVD, cerebrovascular disease, previous cancer and lung disease | 9 |
| Yeh, H. C. | 2012 | USA | retrospective | 18240 | Population-based | 1989 | 51.8 | 7795/10445 | 599 | Self-reported | colorectal cancer | adjusted HROS | age, the square of age, gender, BMI, smoking, education level, hypertension treatment, and high cholesterol treatment | 9 |
| Morrison, D. S. | 2011 | UK | Prospective | 17949 | Population-based | 1967–1970 | — | 17949/0 | 236 | Self-reported | colon and rectal cancer | adjusted HROS | age at risk, height, BMI, plasma cholesterol, diastolic blood pressure, systolic blood pressure, physical activity, socioeconomic position and smoking | 7 |
| Huang, Y. C. | 2011 | Chinese Taiwan | Prospective | 2762 | Hospital-based | 1998.1–2008.1 | — | 1756/1006 | 469 | Medical records | colon cancer | adjusted HROS and HRCSS; 5-year OS and 5-year CSS | age, gender, stage, bowel perforation at diagnosis, bowel obstruction at diagnosis, poorly differentiated or undifferentiated histology | 7 |
| Lai, C. C. | 2011 | Korea | Prospective | 2529 | Hospital-based | 1995–2008 | — | 1315/1214 | 307 | Medical records | colon cancer | adjusted HROS; 5-year OS | age, gender, hypertension, cardiac disease, old CVA, liver cirrhosis, other disease, CEA level, albumin level, morbidity, tumorphology, histologic type, histologic grade and TNM stage | 7 |
| Sarfati, D. | 2011 | New Zealand | Prospective | 11524 | Hospital-based | 1996–2003 | — | 5477/6047 | 1107 | Medical records | colon cancer | adjusted HROS | age, gender, ethnicity, NZ deprivation quintiles and extent of disease; | 7 |
| Lieffers, J. R. | 2011 | Canada | retrospective | 574 | Population-based | 2004–2006 | 64 | 335/239 | 72 | Medical records | colorectal cancer | adjusted HROS | age, gender, stage, and all co-morbidities | 8 |
| Chiao, E. Y. | 2010 | USA | retrospective | 470 | Hospital-based | 1999–2006 | 67.7 | 464/6 | 122 | Medical records | colorectal cancer | adjusted HROS; 5-year OS | age, race, BMI, stage, treatment received and Deyo co-morbidity score | 9 |
| Chen, C. Q. | 2010 | China | Prospective | 945 | Hospital-based | 1994–2002 | 62.3 | 556/389 | 26 | Blood glucose test | colorectal cancer | adjusted HROS and HRDFS; 5-year OS and 5-year DFS | gender, surgery type, chemotherapy, TNM, gross type, differentiation, intestinal obstruction and location | 8 |
| Noh, G. Y. | 2010 | Korea | retrospective | 657 | Hospital-based | 1997–2004 | 57.97 | 374/283 | 67 | Medical records | colorectal cancer | adjusted HROS and HRRFS; 5-year OS and 5-year RFS | age, gender, BMI, stage, grade | 7 |
| Jullumstro, E. | 2009 | Norway | retrospective | 1194 | Hospital-based | 1980–2004 | 72.07 | 628/566 | 97 | Medical records | colorectal cancer | adjusted HROS and unadjusted HRCSS; 5-year OS and 5-year CSS | age, gender, cardiac disease, pulmonary disease, ASA, bowel obstruction, bowel perforation, location, stage, poor differentiation, mean percentage positive nodes after resection and adjuvant chemotherapy | 9 |
| van de Poll-Franse, L. V. | 2007 | Netherlands | Prospective | 8328 | Hospital-based | 1995–2002 | 68.15 | 4465/3863 | 913 | Medical records | colon and rectal cancer | adjusted HROS; 5-year OS | age, gender, stage, treatment and CVD | 7 |
| Shonka, N. A. | 2006 | USA | retrospective | 1853 | Hospital-based | 1986–2003 | — | 891/962 | 255 | Medical records | colon cancer | unadjusted HROS; 5-year OS | — | 5 |
| Polednak, A. P. | 2006 | USA | Prospective | 9395 | Population-based | 1994–1999 | — | 4487/4908 | 1014 | Medical records | colorectal cancer | adjusted HROS | age at diagnosis, gender, race, extent of disease at diagnosis, lymph-node status and poverty-rate category | 7 |
| Park, S. M. | 2006 | Korea | Prospective | 14578 | Population-based | 1996–2004 | 50.8 | 14578/0 | 1223 | Blood glucose test | colorectal cancer | adjusted HROS | age, alcohol consumption, BMI, fasting serum glucose level, cholesterol level, physical activity, food preference, blood pressure, and other co-morbidities (heart disease, liver disease, and cerebrovascular disease | 8 |
| Lemmens, V. E. | 2005 | Netherlands | Prospective | 6931 | Population-based | 1995–2001 | — | 3660/3271 | — | Medical records | colon and rectal cancer | adjusted HROS | age, gender, tumor stage, treatment and number of co-morbid conditions or single concomitant diseases | 7 |
| Coughlin, S. S. | 2004 | USA | Prospective | 1056243 | Population-based | 1982 | — | 467922/588321 | 52803 | Medical records | colon and rectal cancer | adjusted HROS | age, race, years of education, BMI, cigarette smoking history, alcohol consumption, total red meat consumption, consumption of citrus fruits and juices, consumption of vegetables, physical activity | 7 |
| Meyerhardt, J. A. | 2003 | USA | Prospective | 3549 | Hospital-based | 1988–1992 | 61.92 | 2936/613 | 287 | Medical records | colon cancer | adjusted HROS, HRRFS and unadjusted HRDFS; 5-year at OS, 5-year DFS and 5-year RFS | age, BMI, gender, race, baseline performance status, bowel obstruction, bowel perforation, stage of disease, presence of peritoneal implants, and completion of chemotherapy | 9 |
DM: diabetes mellitus; HROS: HR on overall survival; HRCSS: HR on cancer-specific survival; HRCVDS: HR on cardiovascular disease specific survival; HRDFS: HR on disease-free survival; HRRFS: HR on recurrence-free survival; 5-year at OS: the 5-year overall survival rate; 5-year at CSS: the 5-year cancer-specific survival rate; 5-year at CVDS: the 5-year cardiovascular disease specific survival rate; 5-year at DFS: the 5-year disease-free survival rate; RFS: the 5-year recurrence-free survival rate; BMI: body mass index; AJCC stage: the American Joint Committee on Cancer; CRC: colorectal cancer; ASA score: American Society of Anesthesiologists Score; AL: anastomotic leakage; SES: the socioeconomic status; TNM: tumor-node-metastasis; CVD: cardiovascular disease; CVA: old cardiovascular accident; CEA: carcinoembryonic antigen; WBC: white blood cell; CRP: C-reactive protein.
The pooled survival rate for the effect of DM on CRC prognosis
In colorectal cancer, the pooled 5-year OS rate in patients with DM was 49.8%, and that in patients without DM was 53.6%; the pooled 5-year CVDS rate in patients with DM was 90.5%, and that in patients without DM was 94.3%; the pooled 5-year CSS rate in patients with DM was 65.6%, and that in patients without DM was 69.0%; the pooled 5-year DFS rate in patients with DM was 60.9%, and that in patients without DM was 70.0%; the pooled 5-year RFS rate in patients with DM was 63.4%, and that in patients without DM was 68.5%. Similar results were also found in colon and rectal cancer. The detailed results on the pooled survival rate for the effect of DM on CRC Prognosis were shown in Table 2.
Table 2. The pooled survival rate for the effect of DM on CRC Prognosis.
| Colorectal cancer (%) | Colon cancer (%) | Rectal cancer (%) | |
|---|---|---|---|
| OS | |||
| Patients with DM | 49.8 (45.9, 53.6) | 49.9 (21.5, 78.2) | 50.9 (46.0, 55.8) |
| Patients without DM | 58.1 (53.5, 62.6) | 56.5 (44.1, 68.9) | 64.1 (62.0, 66.3) |
| CVDS | |||
| Patients with DM | 90.5 (85.9, 95.1) | — | — |
| Patients without DM | 94.3 (89.1, 99.5) | — | — |
| CSS | |||
| Patients with DM | 65.6 (61.3, 69.8) | 71.7 (55.1, 88.3) | 67.0 (64.8, 69.2) |
| Patients without DM | 69.0 (63.3, 74.7) | 75.4 (59.4, 91.3) | 74.8 (74.0, 75.7) |
| DFS | |||
| Patients with DM | 60.9 (46.2, 75.5) | 59.3 (37.2, 81.5) | 65.9 (63.0, 68.8) |
| Patients without DM | 70.0 (56.8, 83.3) | 69.5 (48.9, 90.1) | 68.2 (67.2, 69.2) |
| RFS | |||
| Patients with DM | 63.4 (51.9, 74.9) | 57.0 (51.3, 62.7) | — |
| Patients without DM | 68.5 (64.8, 72.3) | 65.0 (63.4, 66.6) | — |
DM: diabetes mellitus; OS: overall survival; CSS: cancer-specific survival; CVDS: cardiovascular disease—specific survival; DFS: disease-free survival; RFS: recurrence-free survival.
The overall pooled HRs for the effect of DM on CRC prognosis
In our meta-analysis, the number of studies on the colorectal cancer data provided was 23[6–10, 13–20, 27, 29, 30, 33, 36–39, 42, 43], the pooled HRs on OS and CVDS were statistically significant (HR on OS: 1.18, 95%CI: 1.12–1.24; HR on CVDS: 1.40, 95%CI: 1.29–1.52), the pooled HRs indicated that there were no significant difference on CSS, DFS and RFS. No publication bias was found in OS, CVDS, CSS and DFS.
The number of studies on the colon cancer data provided was 18[6, 8, 10–13, 28, 31, 40, 41, 44–47]. There was only one study on CVDS, and the pooled HR on CVDS was not analyzed. The pooled HRs on OS and DFS were statistically significant (HR on OS: 1.19, 95%CI: 1.10–1.27; HR on DFS: 1.35, 95%CI: 1.12–1.58), the pooled HRs indicated that there were no significant difference on CSS and RFS. Publication bias might exist in OS and CSS (OS: P for Begger test = 0.049, P for Egger test = 0.115; CSS: P for Begger test = 0.260, P for Egger test = 0.012), we used “trim and fill” analysis to deduce the potential unpublished studies, the results of OS and CSS(HR on OS: 1.19, 95%CI: 1.11–1.28; HR on CSS: 1.06, 95%CI: 0.98–1.14) were similar to the overall results, respectively.
The number of studies on the rectal cancer data provided was 10[6, 8, 10, 11, 13, 28, 40, 44, 45], there was only one study on CVDS, DFS and RFS, the pooled HRs on CVDS, DFS or RFS were not analyzed. The pooled HR on OS was statistically significant (HR on OS: 1.16, 95%CI: 1.04–1.29), the pooled HR indicated that there were no significant difference on CSS. No publication bias was found in OS and CSS.
The detailed results on the relationship between DM and CRC Prognosis are shown in Table 3.
Table 3. The overall pooled HR on the effect of DM on CRC Prognosis.
| Number of study | Model for meta-analysis | HR (95%CI) | I2 (%) | P for heterogeneity | P for Begger’s test | P for Egger’s test | |
|---|---|---|---|---|---|---|---|
| Colorectal cancer | |||||||
| OS | 23[6–10, 13–20, 27, 29, 30, 33, 36–39, 42, 43] | R | 1.18(1.12, 1.24) | 64.8 | <0.001 | 0.492 | 0.740 |
| CVDS | 3[13, 15, 30] | F | 1.40(1.29, 1.52) | 31.6 | 0.232 | 0.296 | 0.193 |
| CSS | 8[6–8, 13, 15, 29, 30, 39] | R | 1.03(0.93, 1.12) | 63.3 | 0.008 | 0.711 | 0.225 |
| DFS | 4[8, 9, 20, 38] | R | 1.14(0.71, 1.58) | 80.0 | 0.002 | 0.734 | 0.893 |
| RFS | 2[8, 36] | F | 1.08(0.84, 1.23) | 0.0 | 0.771 | — | — |
| Colon cancer | |||||||
| OS | 18[6, 8, 10–13, 28, 31, 32, 34, 35, 40, 41, 44–47] | R | 1.19(1.10, 1.27) | 86.9 | <0.001 | 0.049 | 0.115 |
| CVDS | 1[13] | — | 1.35(1.26, 1.45) | — | — | — | — |
| CSS | 6[6, 8, 12, 13, 28, 35] | F | 1.07(0.98, 1.16) | 38.9 | 0.146 | 0.260 | 0.012 |
| DFS | 2[8, 46] | F | 1.35(1.12, 1.58) | 0 | 0.447 | — | — |
| RFS | 2[8, 46] | F | 1.24(1.04, 1.44) | 0 | 0.634 | — | — |
| Rectal cancer | |||||||
| OS | 10[6, 8, 10, 11, 13, 28, 40, 44, 45] | R | 1.16(1.04, 1.29) | 61.9 | 0.005 | 0.474 | 0.529 |
| CVDS | 1[13] | — | 1.48(1.04, 1.29) | — | — | — | — |
| CSS | 4[6, 8, 13, 28] | R | 1.12(0.91, 1.32) | 55.2 | 0.082 | 0.308 | 0.389 |
| DFS | 1[8] | — | 0.98(0.76, 1.25) | — | — | — | — |
| RFS | 1[8] | — | 0.96(0.72, 1.28) | — | — | — | — |
R: the random effects model; F: the fixed effects model; DM: diabetes mellitus; OS: overall survival; CSS: cancer-specific survival; CVDS: cardiovascular disease—specific survival; DFS: disease-free survival; RFS: recurrence-free survival.
Subgroup analysis
Because of fewer studies on CVDS, CSS, DFS, and RFS, we used subgroup analysis on OS by the potential confounding factors, including region, type of study, sample size, population source, DM ascertainment, quality of studies and adjusted variables. In colorectal cancer, we found that the relationship between DM and CRC prognosis was significant in all groups, but not in Asian or blood glucose test groups. We found similar results in colon and rectal cancer. The detailed results on the subgroup analysis on OS for the effect of DM on CRC Prognosis were shown in Table 4.
Table 4. The subgroup analysis on OS for the effect of DM on CRC Prognosis.
| Colorectal cancer | Colon cancer | Rectal cancer | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of study | Model for meta-analysis | HR (95%CI) | I2 (%) | P for heterogeneity | Number of study | Model for meta-analysis | HR (95%CI) | I2 (%) | P for heterogeneity | Number of study | Model for meta-analysis | HR (95%CI) | I2 (%) | P for heterogeneity | |
| Region | |||||||||||||||
| America | 11[7, 13–15, 18, 19, 27, 30, 33, 37, 42] | R | 1.19(1.11, 1.27) | 78.0 | <0.001 | 5[13, 41, 45, 46] | F | 1.21(1.14, 1.29) | 33.5 | 0.198 | 3[13, 45] | F | 1.16(1.01, 1.32) | 24.1 | 0.268 |
| Europe | 4[6, 16, 17, 39] | F | 1.25(1.12, 1.37) | 5.3 | 0.366 | 6[6, 11, 28, 32, 40, 44] | F | 1.16(1.09 1.24) | 1.7 | 0.406 | 5[6, 11, 28, 40, 44] | R | 1.26(1.03, 1.49) | 74.8 | 0.003 |
| Asia | 8[8–10, 20, 29, 36, 38, 43] | F | 1.06(0.91, 1.22) | 26.1 | 0.220 | 6[8, 10, 12, 34, 35, 47] | F | 1.25(1.12, 1.39) | 31.7 | 0.198 | 2[8, 10] | F | 0.91(0.56, 1.25) | 7.6 | 0.298 |
| Oceania | 0 | — | — | — | — | 1[31] | — | 1.00(0.98, 1.02) | — | — | 0 | — | — | — | — |
| Type of study | |||||||||||||||
| Retrospective | 12[9, 10, 14, 16–20, 33, 36, 37, 39] | F | 1.14(1.09, 1.19) | 2.5 | 0.420 | 3[10, 41, 47] | F | 0.98(0.72, 1.18) | 0.0 | 0.416 | 1[10] | — | 0.32(0.04, 2.39) | — | — |
| Prospective | 11[6–8, 13, 15, 27, 29, 30, 38, 42, 43] | R | 1.22(1.12, 1.33) | 78.6 | <0.001 | 15[6, 8, 11–13, 28, 31, 32, 34, 35, 40, 44–46] | R | 1.21(1.12, 1.29) | 89.0 | <0.001 | 9[6, 8, 11, 13, 28, 40, 44, 45] | R | 1.17(1.05, 1.30) | 62.9 | <0.001 |
| Sample size | |||||||||||||||
| ≥ 10000 | 8[10, 13–15, 17, 18, 27, 43] | R | 1.14(1.07, 1.20) | 70.0 | 0.001 | 7[10, 11, 13, 31, 32, 45] | R | 1.14(1.01, 1.26) | 93.3 | <0.001 | 4[10, 13, 45] | F | 1.10(0.89, 1.32) | 37.7 | 0.186 |
| <10000 | 15[6–9, 16, 19, 20, 29, 30, 33, 36–39, 42] | R | 1.21(1.08, 1.33) | 55.6 | 0.005 | 11[6, 8, 12, 28, 34, 35, 40, 41, 44, 46, 47] | F | 1.22(1.13, 1.31) | 45.2 | 0.051 | 6[6, 8, 11, 28, 40, 44] | R | 1.21(1.01, 1.41) | 72.5 | 0.003 |
| Population source | |||||||||||||||
| Population-based | 10[7, 13–15, 18, 27, 30, 33, 42, 43] | R | 1.20(1.12, 1.28) | 79.8 | <0.001 | 8[10–13, 32, 44, 45] | F | 1.20(1.17, 1.23) | 0.0 | 0.456 | 6[10, 11, 13, 44, 45] | F | 1.09(0.95, 1.23) | 49.1 | <0.001 |
| Hospital-based | 13[6, 8–10, 16, 17, 19, 20, 29, 36–39] | F | 1.14(1.02, 1.25) | 31.8 | 0.129 | 10[6, 8, 28, 31, 34, 35, 40, 41, 46, 47] | R | 1.18(1.06, 1.30) | 80.7 | <0.001 | 4[6, 8, 28, 40] | R | 1.30(1.02, 1.58) | 75.2 | 0.007 |
| DM ascertainment | |||||||||||||||
| Medical records | 14[6, 8, 9, 13–15, 18, 19, 29, 33, 36, 37, 39, 42] | R | 1.18(1.11, 1.24) | 70.7 | <0.001 | 15[6, 8, 11–13, 28, 31, 34, 35, 40, 41, 44–46] | R | 1.20(1.11, 1.28) | 89.0 | <0.001 | 9[6, 8, 11, 13, 28, 40, 44, 45] | R | 1.17(1.05, 1.30) | 62.9 | 0.006 |
| Self-reported | 5[7, 10, 17, 27, 30] | F | 1.29(1.08, 1.51) | 49.5 | 0.095 | 2[10, 32] | F | 1.02(0.30, 1.73) | 0.0 | 0.504 | 1[10] | — | 0.32(0.04, 2.39) | — | — |
| Blood glucose test | 4[16, 20, 38, 43] | F | 0.95(0.65, 1.25) | 27.2 | 0.249 | 1[47] | — | 0.57(0.22, 1.47) | — | — | 0 | — | — | — | — |
| Quality of studies | |||||||||||||||
| Moderate | 5[9, 14–16, 30] | R | 1.16(1.03, 1.28) | 75.5 | 0.003 | 1[41] | — | 1.00(0.77, 1.30) | — | — | 0 | — | — | — | — |
| High | 18[6–8, 10, 13, 17–20, 27, 29, 33, 36–39, 42, 43] | R | 1.19(1.11, 1.27) | 47.4 | 0.014 | 17[6, 8, 10–13, 28, 31, 32, 34, 35, 40, 44–47] | R | 1.19(1.11, 1.28) | 87.6 | <0.001 | 10[6, 8, 10, 11, 13, 28, 40, 44, 45] | R | 1.16(1.04, 1.29) | 61.9 | 0.005 |
| Adjusted variables | |||||||||||||||
| no | 3[9, 15, 16] | F | 1.03(0.96, .10) | 0.0 | 0.823 | 1[41] | — | 1.00(0.77, 1.30) | — | — | 0 | — | — | — | — |
| yes | 20[6–8, 10, 13, 14, 17–20, 27, 29, 30, 33, 36–39, 42, 43] | R | 1.20(1.14, 1.26) | 57.8 | 0.001 | 17[6, 8, 10–13, 28, 31, 32, 34, 35, 40, 44–47] | R | 1.19(1.11, 1.28) | 87.6 | <0.001 | 0 | 10[6, 8, 10, 11, 13, 28, 40, 44, 45] | R | 1.16(1.04, 1.29) | 61.9 |
R: the random effects model; F: the fixed effects model; DM: diabetes mellitus; OS: overall survival; CSS: cancer-specific survival; CVDS: cardiovascular disease—specific survival; DFS: disease-free survival; RFS: recurrence-free survival.
Sensitivity analysis
The pooled HRs and their 95%CIs of sensitivity analysis were calculated by excluding one study at a time in colorectal cancer, colon cancer and rectal cancer, and the results indicated that the overall result was dependable. The results of sensitivity analysis were shown in Table 5.
Table 5. The sensitivity analysis of the overall pooled HR on the effect of DM on CRC Prognosis.
| The lowest HR (95%CI) | The highest HR (95%CI) | |
|---|---|---|
| Colorectal cancer | ||
| OS | 1.18(1.12, 1.24) | 1.38(1.31, 1.46) |
| CVDS | 1.38(1.31, 1.46) | 1.66(1.11, 2.51) |
| CSS | 1.00(0.92, 1.09) | 1.11(0.97, 1.27) |
| DFS | 1.03(0.68, 1.58) | 1.37(1.03, 1.83) |
| Colon cancer | ||
| OS | 1.18(1.10, 1.27) | 1.22(1.17, 1.26) |
| CSS | 1.03(0.97, 1.11) | 1.13(1.04, 1.23) |
| Rectal cancer | ||
| OS | 1.15(1.02, 1.28) | 1.22(1.09, 1.38) |
| CSS | 1.08(0.91, 1.29) | 1.24(0.93, 1.67) |
DM: diabetes mellitus; OS: overall survival; CSS: cancer-specific survival; CVDS: cardiovascular disease—specific survival; DFS: disease-free survival; RFS: recurrence-free survival.
Discussion
Our meta-analysis first analyzed both the 5-year survival rate and survival risk, which reflected the effect of DM on CRC prognosis. The results indicated that compared to patients without DM, patients with DM will have a 5-year shorter survival rate in colorectal, colon and rectal cancer, showed 18%, 19% and 16% decreased in OS, respectively. We also found similar results in CVDS, CSS, DFS and RFS. Due to the heterogeneity, we performed the subgroup analysis and sensitivity analysis to find the source of heterogeneity and make our results robust and credible. In subgroup analysis, though few results showed no statistical significance, we found that the results of subgroup analysis were generally similar to the overall results. When we carried out subgroup analysis by region, in Europe, patients with DM significantly have shorter OS in colorectal cancer, colon cancer and rectal cancer. In Asia, patients with DM significantly have shorter OS in colon cancer; there was no significance in colorectal cancer and rectal cancer, this may be the small sample size due to subgroup analysis. When we carried out subgroup analysis by type of study, there were significant differences in the results, except for that in prospective studies of colon cancer. When we carried out subgroup analysis by sample size and population source, the subgroup results were consistent with the overall results in colorectal and colon cancer, the results in size ≥ 10000 and population-based group did not show statistical significant in rectal cancer. When we carried out subgroup analysis by DM ascertainment, the results were consistent with the overall results in the group of medical records, except for that in the group of self-reported and blood glucose test. The sensitivity analysis also showed that the results of our meta-analysis were robust and credible.
Currently, the biological mechanism linkage between DM and CRC prognosis is still uncertain. This association may be mainly based on the effect of hyperinsulinemia, insulin resistance and cancer pathogenesis on the insulin/ insulin-like growth factor (IGF) system, which plays a critical role in the pathogenesis, progression, and prognosis of CRC. On the one hand, the insulin-like effects of IGF-1 interacting with associated receptors, such as IGF-1R, IR or hybrid receptors, play an important role in the maintenance of normal glucose homeostasis and etiopathogenesis of DM[48]. In DM patients, insulin resistance leads to a compensatory increase in insulin secretion, and by inhibition of IGF binding proteins, this hyperinsulinemia may increase the biological activity of IGF-1, which is an antiapoptotic and mitogenic factor[49]. On the other hand, insulin-like growth factors activate the IGF-1R, make it over expressed in cancer cells, and then trigger a number of intracellular signaling cascades that enhance cell cycle progression and inhibit apoptosis. Zhang et al indicated that IGF-1 and its receptor promoted both the growth and malignant transformation of adenomatous polyps[50]. Over expression of IGF-1, IGF-1R and IR were found in CRC group with DM than that in without DM[51]. The activation of insulin/IGF-dependent pathways has been also identified as a critical step contributing to several mechanisms of CRC resistance to both conventional and targeted therapeutic agents, leading to increased PI3K/Akt signaling that hinders the apoptotic signals triggered by chemotherapeutic drugs and desensitizes CRC cells to the effect of anti-EGFR antibodies[52]. Scartozzi et al. had reported that high IGF-1 expression correlated with poor clinical outcome in wild-type KRAS metastatic CRC patients treated with cetuximab and irinotecan. Their results indicated that engaging the IGF-1/IGF-1R system might enable tumor cells to escape anti-EGFR-mediated treatment as a consequence of IGF-1-driven stimulation of the PI3K–Akt pathway[53]. In recent years, some evidence suggested that IGF-1/IGF-1R polymorphisms are potential predictive/prognostic markers for cetuximab efficacy in metastatic CRC patients presenting wild- type KRAS[54].
In order to make our results more robust and credible, we made efforts in several ways. First of all, we not only searched the relevant studies in the four commonly used electronic databases, but also searched in Google Scholar, and tried our best not to miss the relevant studies. We also extracted the data on OS, CSS, CVDS, DFS and RFS, and used these indicators to evaluate the effect of DM on CRC prognosis. So far, our meta-analysis is the most comprehensive study of collecting indicators on the effect of DM on CRC prognosis. Second, we performed the quality assessment by NOS, which was widely used in meta-analysis and systematic reviews, and all the included studies were evaluated as high quality, which made our extracted data reliable. Third, we found that only one result in CSS of colon cancer existed publication bias, there were no publication bias in all other results. We used the “trim and fill” analysis to assess the possible effect of publication bias, but there was no significant change in the CSS result of colon cancer. The results of subgroup analysis and sensitivity analysis has also shown that our results were robust and credible. Finally, and most importantly, compared to previous studies[5], we not only routinely performed the pooled analysis on HR of OS, CSS, CVDS, DFS and RFS, which comprehensively reflect the difference of CRC prognosis between diabetic patients and nondiabetic patients; but also first extracted the 5-year survival rate from the included studies, and made the pooled analysis. Meanwhile, for collecting more useful data, we used the professional software to extract survival rate from the Kaplan-Meier curves[24, 25]. This would make the results stable, and give the researchers more intuitive impression on the effect of DM on prognosis in the fifth year.
There were several limitations in our meta-analysis. First, in order to collect the literatures more extensively, we searched the relevant articles in Google Scholar. If we found the relevant articles in Google Scholar, we purchased the article or sought help online[55].Second, in the included studies, we found that more studies focused on OS, compared to CSS, CVDS, DFS and RFS. In OS, the number of studies on colorectal, colon and rectal cancer was twenty-three, seventeen and ten. In CSS, CVDS, DFS and RFS, the maximum number of relevant studies was only eight. This might make the results unstable. In our meta-analysis, we analyzed both the 5-year survival rate and survival risk, and found their results were consistent. This indicated that our results were stable. Third, the results of our meta-analysis had a certain degree of heterogeneity. We performed subgroup analysis by the confounding factors, which might be the potential source of heterogeneity, and the results of subgroup analysis were similar to the overall results. We also performed the analysis of the effect of each study on the overall results sensitively, and did not find significant changes in the overall results.
In conclusion, our meta-analysis showed that DM could significantly decrease OS in CRC patients, but not CSS, CVDS, DFS and RFS. In future, to provide more evidence of clinical treatment, more high quality prospective cohort studies are needed to comprehensively analyze the effect of DM on CRC prognosis by CSS, CVDS, DFS and RFS.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
This work was supported by General Foundation of scientific research in the Department of Education in Liaoning (L2015592).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by General Foundation of scientific research in the Department of Education in Liaoning (L2015592).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87–108. Epub 2015/02/06. [DOI] [PubMed] [Google Scholar]
- 2.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010;87(1):4–14. Epub 2009/11/10. doi: 10.1016/j.diabres.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nature reviews Endocrinology. 2011;8(4):228–36. Epub 2011/11/09. doi: 10.1038/nrendo.2011.183 [DOI] [PubMed] [Google Scholar]
- 4.Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nature reviews Clinical oncology. 2016. Epub 2016/10/26. [DOI] [PubMed] [Google Scholar]
- 5.Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Diseases of the colon and rectum. 2013;56(11):1304–19. Epub 2013/10/10. PubMed Central PMCID: PMCPMC3800045. doi: 10.1097/DCR.0b013e3182a479f9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bella F, Minicozzi P, Giacomin A, Crocetti E, Federico M, Ponz de Leon M, et al. Impact of diabetes on overall and cancer-specific mortality in colorectal cancer patients. Journal of cancer research and clinical oncology. 2013;139(8):1303–10. Epub 2013/05/02. doi: 10.1007/s00432-013-1439-8 [DOI] [PubMed] [Google Scholar]
- 7.Cossor FI, Adams-Campbell LL, Chlebowski RT, Gunter MJ, Johnson K, Martell RE, et al. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer epidemiology. 2013;37(5):742–9. Epub 2013/06/19. PubMed Central PMCID: PMCPMC3769471. doi: 10.1016/j.canep.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon JY, Jeong DH, Park MG, Lee JW, Chu SH, Park JH, et al. Impact of diabetes on oncologic outcome of colorectal cancer patients: colon vs. rectal cancer. PloS one. 2013;8(2):e55196 Epub 2013/02/14. PubMed Central PMCID: PMCPMC3566217. doi: 10.1371/journal.pone.0055196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Li Q, Yang Z, Hu X, Qian W, Du Y, et al. Association of body mass index and smoking on outcome of Chinese patients with colorectal cancer. World journal of surgical oncology. 2013;11:271 Epub 2013/10/15. PubMed Central PMCID: PMCPMC3853928. doi: 10.1186/1477-7819-11-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison DS, Parr CL, Lam TH, Ueshima H, Kim HC, Jee SH, et al. Behavioural and metabolic risk factors for mortality from colon and rectum cancer: analysis of data from the Asia-Pacific Cohort Studies Collaboration. Asian Pacific journal of cancer prevention: APJCP. 2013;14(2):1083–7. Epub 2013/04/30. [DOI] [PubMed] [Google Scholar]
- 11.Walker JJ, Brewster DH, Colhoun HM, Fischbacher CM, Lindsay RS, Wild SH. Cause-specific mortality in Scottish patients with colorectal cancer with and without type 2 diabetes (2000–2007). Diabetologia. 2013;56(7):1531–41. Epub 2013/04/30. doi: 10.1007/s00125-013-2917-x [DOI] [PubMed] [Google Scholar]
- 12.Chen KH, Shao YY, Lin ZZ, Yeh YC, Shau WY, Kuo RN, et al. Type 2 diabetes mellitus is associated with increased mortality in Chinese patients receiving curative surgery for colon cancer. The oncologist. 2014;19(9):951–8. Epub 2014/07/26. PubMed Central PMCID: PMCPMC4153450. doi: 10.1634/theoncologist.2013-0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, Lin HC, He K, Hendryx M. Diabetes and prognosis in older persons with colorectal cancer. British journal of cancer. 2014;110(7):1847–54. Epub 2014/02/27. PubMed Central PMCID: PMCPMC3974085. doi: 10.1038/bjc.2014.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong L, Ahn C, Symanski E, Lai D, Du XL. Temporal trends in the leading causes of death among a large national cohort of patients with colorectal cancer from 1975 to 2009 in the United States. Annals of epidemiology. 2014;24(6):411–7. Epub 2014/02/18. doi: 10.1016/j.annepidem.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 15.Waheed S, Azad N, Waheed S, Yeh HC. Racial disparities and colorectal cancer survival in older adults with and without diabetes mellitus. Journal of gastroenterology and hepatology. 2014;29(12):1963–8. Epub 2014/06/10. PubMed Central PMCID: PMCPMC4612638. doi: 10.1111/jgh.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui G, Zhang T, Ren F, Feng WM, Yao Y, Cui J, et al. High Blood Glucose Levels Correlate with Tumor Malignancy in Colorectal Cancer Patients. Medical science monitor: international medical journal of experimental and clinical research. 2015;21:3825–33. Epub 2015/12/09. PubMed Central PMCID: PMCPMC4677694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fransgaard T, Thygesen LC, Gogenur I. Increased 30-day mortality in patients with diabetes undergoing surgery for colorectal cancer. Colorectal disease: the official journal of the Association of Coloproctology of Great Britain and Ireland. 2016;18(1):O22–9. Epub 2015/10/16. [DOI] [PubMed] [Google Scholar]
- 18.Paulus JK, Williams CD, Cossor FI, Kelley MJ, Martell RE. Metformin, Diabetes, and Survival among U.S. Veterans with Colorectal Cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;25(10):1418–25. Epub 2016/08/09. PubMed Central PMCID: PMCPMC5050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramjeesingh R, Orr C, Bricks CS, Hopman WM, Hammad N. A retrospective study on the role of diabetes and metformin in colorectal cancer disease survival. Current oncology (Toronto, Ont). 2016;23(2):e116–22. Epub 2016/04/29. PubMed Central PMCID: PMCPMC4835004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang IP, Tsai HL, Huang CW, Lu CY, Miao ZF, Chang SF, et al. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016;7(14):18837–50. Epub 2016/03/05. PubMed Central PMCID: PMCPMC4951333. doi: 10.18632/oncotarget.7719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L, Dong C, Cao Y, Fang X, Zhong C, Li D, et al. Prognostic Role of BRAF Mutation in Stage II/III Colorectal Cancer Receiving Curative Resection and Adjuvant Chemotherapy: A Meta-Analysis Based on Randomized Clinical Trials. PloS one. 2016;11(5):e0154795 Epub 2016/05/04. PubMed Central PMCID: PMCPMC4854379. doi: 10.1371/journal.pone.0154795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. Epub 2010/07/24. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 23.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ (Clinical research ed). 2016;355:i5953. Epub 2016/11/25. PubMed Central PMCID: PMCPMC5121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 Epub 2007/06/09. PubMed Central PMCID: PMCPMC1920534. doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815–34. Epub 1999/01/28. [DOI] [PubMed] [Google Scholar]
- 26.Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PloS one. 2013;8(8):e71930 Epub 2013/08/13. PubMed Central PMCID: PMCPMC3732238. doi: 10.1371/journal.pone.0071930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh HC, Platz EA, Wang NY, Visvanathan K, Helzlsouer KJ, Brancati FL. A prospective study of the associations between treated diabetes and cancer outcomes. Diabetes care. 2012;35(1):113–8. Epub 2011/11/22. PubMed Central PMCID: PMCPMC3241297. doi: 10.2337/dc11-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Poll-Franse LV, Haak HR, Coebergh JW, Janssen-Heijnen ML, Lemmens VE. Disease-specific mortality among stage I-III colorectal cancer patients with diabetes: a large population-based analysis. Diabetologia. 2012;55(8):2163–72. Epub 2012/04/25. PubMed Central PMCID: PMCPMC3390707. doi: 10.1007/s00125-012-2555-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang CW, Sun LC, Shih YL, Tsai HL, Chen CW, Yeh YS, et al. The impact on clinical outcome of high prevalence of diabetes mellitus in Taiwanese patients with colorectal cancer. World journal of surgical oncology. 2012;10:76 Epub 2012/05/05. PubMed Central PMCID: PMCPMC3533895. doi: 10.1186/1477-7819-10-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(1):53–9. Epub 2011/11/30. [DOI] [PubMed] [Google Scholar]
- 31.Sarfati D, Tan L, Blakely T, Pearce N. Comorbidity among patients with colon cancer in New Zealand. The New Zealand medical journal. 2011;124(1338):76–88. Epub 2011/09/29. [PubMed] [Google Scholar]
- 32.Morrison DS, Batty GD, Kivimaki M, Davey Smith G, Marmot M, Shipley M. Risk factors for colonic and rectal cancer mortality: evidence from 40 years' follow-up in the Whitehall I study. Journal of epidemiology and community health. 2011;65(11):1053–8. Epub 2011/03/11. doi: 10.1136/jech.2010.127555 [DOI] [PubMed] [Google Scholar]
- 33.Lieffers JR, Baracos VE, Winget M, Fassbender K. A comparison of Charlson and Elixhauser comorbidity measures to predict colorectal cancer survival using administrative health data. Cancer. 2011;117(9):1957–65. Epub 2011/04/22. doi: 10.1002/cncr.25653 [DOI] [PubMed] [Google Scholar]
- 34.Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, et al. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. International journal of colorectal disease. 2011;26(4):473–81. Epub 2010/12/31. doi: 10.1007/s00384-010-1113-4 [DOI] [PubMed] [Google Scholar]
- 35.Huang YC, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, et al. Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. Journal of cancer research and clinical oncology. 2011;137(2):211–20. Epub 2010/04/14. doi: 10.1007/s00432-010-0879-7 [DOI] [PubMed] [Google Scholar]
- 36.Noh GY, Hwang DY, Choi YH, Lee YY. Effect of diabetes mellitus on outcomes of colorectal cancer. Journal of the Korean Society of Coloproctology. 2010;26(6):424–8. Epub 2011/01/12. PubMed Central PMCID: PMCPMC3017979. doi: 10.3393/jksc.2010.26.6.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiao EY, Nambi PV, Naik AD. The impact of diabetes process and outcome quality measures on overall survival in patients with co-morbid colorectal cancer. Journal of cancer survivorship: research and practice. 2010;4(4):381–7. Epub 2010/08/20. PubMed Central PMCID: PMCPMC3175493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CQ, Fang LK, Cai SR, Ma JP, Yang GX, Yang W, et al. Effects of diabetes mellitus on prognosis of the patients with colorectal cancer undergoing resection: a cohort study with 945 patients. Chinese medical journal. 2010;123(21):3084–8. Epub 2010/12/18. [PubMed] [Google Scholar]
- 39.Jullumstro E, Kollind M, Lydersen S, Edna TH. Diabetes mellitus and outcomes of colorectal cancer. Acta oncologica (Stockholm, Sweden). 2009;48(3):361–7. Epub 2008/12/23. [DOI] [PubMed] [Google Scholar]
- 40.van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. International journal of cancer. 2007;120(9):1986–92. Epub 2007/01/19. doi: 10.1002/ijc.22532 [DOI] [PubMed] [Google Scholar]
- 41.Shonka NA, Anderson JR, Panwalkar AW, Reed EC, Steen PD, Ganti AK. Effect of diabetes mellitus on the epidemiology and outcomes of colon cancer. Medical oncology (Northwood, London, England). 2006;23(4):515–9. Epub 2007/02/17. [DOI] [PubMed] [Google Scholar]
- 42.Polednak AP. Comorbid diabetes mellitus and risk of death after diagnosis of colorectal cancer: a population-based study. Cancer detection and prevention. 2006;30(5):466–72. Epub 2006/10/31. doi: 10.1016/j.cdp.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 43.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(31):5017–24. Epub 2006/11/01. [DOI] [PubMed] [Google Scholar]
- 44.Lemmens VE, Janssen-Heijnen ML, Verheij CD, Houterman S, Repelaer van Driel OJ, Coebergh JW. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. The British journal of surgery. 2005;92(5):615–23. Epub 2005/03/22. doi: 10.1002/bjs.4913 [DOI] [PubMed] [Google Scholar]
- 45.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. American journal of epidemiology. 2004;159(12):1160–7. Epub 2004/06/12. doi: 10.1093/aje/kwh161 [DOI] [PubMed] [Google Scholar]
- 46.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003;21(3):433–40. Epub 2003/02/01. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Kim JH, Park SJ, Ock SY, Kwon SK, Choi YS, et al. Optimal glycemic target level for colon cancer patients with diabetes. Diabetes research and clinical practice. 2016;124:66–71. Epub 2017/01/21. doi: 10.1016/j.diabres.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 48.Rajpathak SN, Gunter MJ, Wylie-Rosett J, Ho GY, Kaplan RC, Muzumdar R, et al. The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes/metabolism research and reviews. 2009;25(1):3–12. Epub 2009/01/16. PubMed Central PMCID: PMCPMC4153414. doi: 10.1002/dmrr.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11(4):385–91. Epub 2002/04/03. [PubMed] [Google Scholar]
- 50.Zhang R, Xu GL, Li Y, He LJ, Chen LM, Wang GB, et al. The role of insulin-like growth factor 1 and its receptor in the formation and development of colorectal carcinoma. The Journal of international medical research. 2013;41(4):1228–35. Epub 2013/06/27. doi: 10.1177/0300060513487631 [DOI] [PubMed] [Google Scholar]
- 51.Ding J, Li C, Tang J, Yi C, Liu JY, Qiu M. Higher Expression of Proteins in IGF/IR Axes in Colorectal Cancer is Associated with Type 2 Diabetes Mellitus. Pathology oncology research: POR. 2016;22(4):773–9. Epub 2016/05/04. doi: 10.1007/s12253-016-0065-6 [DOI] [PubMed] [Google Scholar]
- 52.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G 2nd, et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer research. 2009;69(5):1951–7. Epub 2009/02/27. PubMed Central PMCID: PMCPMC3198868. doi: 10.1158/0008-5472.CAN-08-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scartozzi M, Mandolesi A, Giampieri R, Pierantoni C, Loupakis F, Zaniboni A, et al. Insulin-like growth factor 1 expression correlates with clinical outcome in K-RAS wild type colorectal cancer patients treated with cetuximab and irinotecan. International journal of cancer. 2010;127(8):1941–7. Epub 2010/01/26. doi: 10.1002/ijc.25193 [DOI] [PubMed] [Google Scholar]
- 54.Winder T, Zhang W, Yang D, Ning Y, Bohanes P, Gerger A, et al. Germline polymorphisms in genes involved in the IGF1 pathway predict efficacy of cetuximab in wild-type KRAS mCRC patients. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(22):5591–602. Epub 2010/10/12. PubMed Central PMCID: PMCPMC2982939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu B, Wu X, Bi Y, Yang Y. Effect of bilirubin concentration on the risk of diabetic complications: A meta-analysis of epidemiologic studies. Scientific reports. 2017;7:41681 Epub 2017/01/31. PubMed Central PMCID: PMCPMC5278382. doi: 10.1038/srep41681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.