Abstract
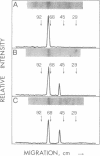
The proteolytically activated form of protein kinase C has been identified in human neutrophils by using a monoclonal antibody that recognizes both the native kinase and the catalytically active proteolytic fragment (protein kinase M). Stimulation with fMet-Leu-Phe results in the conversion of approximately 30% of native protein kinase C to protein kinase M, with little evidence of further degradation. Stimulation with phorbol 12-myristate 13-acetate, on the other hand, causes only a transient formation of protein kinase M, with complete loss of total kinase activity. These differences are related to the differences in biochemical responses, reported earlier, in neutrophils exposed to these two activators.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ase K., Berry N., Kikkawa U., Kishimoto A., Nishizuka Y. Differential down-regulation of protein kinase C subspecies in KM3 cells. FEBS Lett. 1988 Aug 29;236(2):396–400. doi: 10.1016/0014-5793(88)80064-4. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Carpenter D., Jackson T., Hanley M. R. Protein kinase Cs. Coping with a growing family. Nature. 1987 Jan 8;325(7000):107–108. doi: 10.1038/325107a0. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Corte G., Moretta L., Damiani G., Mingari M. C., Bargellesi A. Surface antigens specifically expressed by activated T cells in humans. Eur J Immunol. 1981 Feb;11(2):162–164. doi: 10.1002/eji.1830110220. [DOI] [PubMed] [Google Scholar]
- Inoue M., Kishimoto A., Takai Y., Nishizuka Y. Studies on a cyclic nucleotide-independent protein kinase and its proenzyme in mammalian tissues. II. Proenzyme and its activation by calcium-dependent protease from rat brain. J Biol Chem. 1977 Nov 10;252(21):7610–7616. [PubMed] [Google Scholar]
- Kakinuma K., Yamaguchi T., Kaneda M., Shimada K., Tomita Y., Chance B. A determination of H2O2 release by the treatment of human blood polymorphonuclear leukocytes with myristate. J Biochem. 1979 Jul;86(1):87–95. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989 Mar 5;264(7):4088–4092. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- McPhail L. C., Snyderman R. Activation of the respiratory burst enzyme in human polymorphonuclear leukocytes by chemoattractants and other soluble stimuli. Evidence that the same oxidase is activated by different transductional mechanisms. J Clin Invest. 1983 Jul;72(1):192–200. doi: 10.1172/JCI110957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Horecker B. L. The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem. 1986 Mar 25;261(9):4101–4105. [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Salamino F., Horecker B. L. Binding of protein kinase C to neutrophil membranes in the presence of Ca2+ and its activation by a Ca2+-requiring proteinase. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6435–6439. doi: 10.1073/pnas.82.19.6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Molski T. F., Borgeat P., White J. R., Sha'afi R. I. Phorbol esters inhibit the fMet-Leu-Phe- and leukotriene B4-stimulated calcium mobilization and enzyme secretion in rabbit neutrophils. J Biol Chem. 1985 Feb 25;260(4):2125–2131. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Damiani G., Salamino F., Sparatore B., Michetti M., Horecker B. L. Effects of a monoclonal anti-calpain antibody on responses of stimulated human neutrophils. Evidence for a role for proteolytically modified protein kinase C. J Biol Chem. 1988 Feb 5;263(4):1915–1919. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Michetti M., Sacco O., Salamino F., Sparatore B., Horecker B. L. Biochemical responses in activated human neutrophils mediated by protein kinase C and a Ca2+-requiring proteinase. J Biol Chem. 1986 Jun 25;261(18):8309–8313. [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Patrone M., Michetti M., Horecker B. L. Activation of neutrophil calpain following its translocation to the plasma membrane induced by phorbol ester or fMet-Leu-Phe. Biochem Biophys Res Commun. 1989 Apr 28;160(2):737–743. doi: 10.1016/0006-291x(89)92495-9. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Sparatore B., Michetti M., Salamino F., Horecker B. L. Isozymes of protein kinase C in human neutrophils and their modification by two endogenous proteinases. J Biol Chem. 1990 Jan 15;265(2):706–712. [PubMed] [Google Scholar]
- Pontremoli S., Sparatore B., Salamino F., Michetti M., Sacco O., Melloni E. Reversible activation of human neutrophil calpain promoted by interaction with plasma membranes. Biochem Int. 1985 Jul;11(1):35–44. [PubMed] [Google Scholar]
- Stabel S., Rodriguez-Pena A., Young S., Rozengurt E., Parker P. J. Quantitation of protein kinase C by immunoblot--expression in different cell lines and response to phorbol esters. J Cell Physiol. 1987 Jan;130(1):111–117. doi: 10.1002/jcp.1041300116. [DOI] [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Zollinger W. D., Dalrymple J. M., Artenstein M. S. Analysis of parameters affecting the solid phase radioimmunoassay quantitation of antibody to meningococcal antigens. J Immunol. 1976 Nov;117(5 PT2):1788–1798. [PubMed] [Google Scholar]