Highlights
-
•
Eloquent biosynthesis of AgNPs using green seaweed Enteromorpha compressa.
-
•
Characterization of AgNPs was done by UV–vis, XRD, FTIR, HRTEM, SAED pattern and EDX.
-
•
Effective antibacterial activity against different clinical bacterial and fungal pathogens and cytotoxic assay on EAC cells.
Keywords: Seaweed, Enteromorpha compressa, AgNPs, Characterization, Biomedical applications
Abstract
Green synthesis of nanoparticles using seaweeds are fascinating high research attention nowadays and also gaining center of attention in biomedical applications. In this work, we have synthesized biocompatible and functionalized silver nanoparticles using an aqueous extract of seaweed Enteromorpha compressa as a reducing as well as stabilizing agent and their efficient antimicrobial and anticancer activity are reported here. The UV–vis spectra of AgNPs showed the characteristics SPR absorption band at 421 nm. The chemical interaction and crystalline nature of the AgNPs were evaluated by FT-IR and XRD studies. The XRD result of AgNPs shows typical Ag reflection peaks at 38.1°, 44.2°, 64.4° and 77.1° corresponding to (111), (200), (220) and (311) Bragg’s planes. The surface morphology and composition of the samples were observed by HRTEM, EDS and SAED pattern analyses. Spherical shaped Ag nano structures were observed in the size ranges between 4 and 24 nm with clear lattice fringes in the HRTEM image. This report reveals that seaweed mediated synthesis of AgNPs and sustained delivery of Ag ions to the bacterial and fungal surface have been reducing their growth rate which was evaluated by well diffusion assay. The synthesized AgNPs showed favorable cytotoxicity against Ehlrich Ascites Carcinoma (EAC) cells with IC50 value was recorded at 95.35 μg mL−1. This study showed cost effective silver nanoparticles synthesis with excellent biocompatibility and thus could potentially be utilized in biomedical and pharmaceutical applications.
1. Introduction
Nanotechnology, a magic formula to influence every dimension of science, economy and all walks of life, which includes design, synthesis and manipulation of nanosized materials for range of applications such as in biology and medicine. Nanoparticles are considered as important in past decades and currently used in various applications such as catalytic activity, water purification, chemical and biological sensors, wireless electronic logic and memory schemes [1], [2], [3]. Recently biomimetic nanoparticles have been recognised as important nanomaterial for its broad spectrum of antimicrobial and anticancer activities. Metallic nanoparticles such as silver, gold and platinum have been used in the sector of bioelectronics, medicine and pharmaceuticals [4], [5]. Biological synthesis of nanoparticles is an emerging technical tool to address eco-friendly, cost effective, energy efficient and reliable production method of metal nanoparticles [6]. Among these silver nanoparticles (AgNPs) are one of the most widely used commercial and significance interest in recent decades based on the size, shape and their applications are classified [2], [5], [7]. The application of the nanoparticles have been applied in a wide variety of fields medicinal field, drug delivery, antibacterial properties such as anticancer, anticoagulant, anti-inflammatory and wound dressing [3], [8]. Biomimetic synthesis is a basic mechanism could help human to recognize the wonderful nanofactory found in living organism, primary and secondary metabolites richly available in plant and microbes in point of fact acts as reducing and stabilizing agents [9]. Plants, bacteria, fungi, yeast and algae have been extensively used to synthesise Ag, Au, Pd, Pt and Se nanoparticles and they have been used as antimicrobial, anticancer, larvicidal and anthelmintic agents recently [2], [10]. Chemical synthesis of metal nanoparticles requires reducing and capping agents customarily some surfactants. However, biological synthesis requires only extract, which is having reducing agent, capping agent, energy and everything required for the synthesis [10].
Seaweed is a class of marine algae reported to produce numerous bioactive principles with broad spectrum of biological activities. Antimicrobial, antiviral, antioxidant, antibiotic, anti-neoplastic, antifouling, anti-inflammatory, cytotoxic and antimitotic activities have been detected in brown, red and green algae. Therefore, marine algae can also be utilized effectively to synthesise inorganic nanoparticles [11], [12], [13]. Literatures witnesses Enteromorpha compressa has not been employed much to synthesise metal nanoparticles hitherto. Thus, present study was aimed to investigate the biosynthesis of AgNPs using the aqueous extract of seaweed E. compressa and characterize their biomedical activities.
2. Materials and methods
2.1. Collection of seaweed
The green seaweed, Enteromorpha compressa (Linnaeus), was collected from Pudumadam coastal region, Tamil Nadu (Lat 9°27′ N; Long 79°99′ E, Southeast coast of India) and transported to the laboratory in an ice box. Seaweed was washed thoroughly to remove adhesive particles and dust with running tap water followed by using distilled water and then they were dried for 3–4 days under shade. The dried materials were ground to fine powder using mixer grinder.
2.2. Preparation aqueous extract and synthesis of AgNPs
Seaweed powder of E. compressa (2 g) was boiled with 100 mL of sterilized Milli Q water for 10–15 min and filtered through Whatman No. 1 filter paper. The filtrate was treated with 90 mL aqueous solution of AgNO3 (1 mM) and incubated at room temperature for 1 h and color change in the reaction solution was noted by visual observation.
2.3. Characterization of AgNPs
Surface plasmon resonance (SPR) absorption bands were measured using a UV–vis spectrophotometer (UV-1800 Shimadzu, Japan) operated at a resolution of 1 nm, between 200 and 800 nm. The crystal structure of AgNPs were characterized by X-ray Diffraction (XRD) pattern using X’ Pert PRO Analytical X-ray diffractometer with X’ Pert High Score Plus Software operating at a voltage of 45 kV and current of 40 mA with Cu Kα radiation. The Fourier Transform Infrared (FT-IR-Nicolet Thermo spectrophotometer iS5, USA) spectra of seaweed extract and synthesized AgNPs colloidal solutions were analyzed in the range between 4000 and 400 cm−1 with a resolution of 4 cm−1 to arrive at the possible functional groups responsible for reduction and capping behaviour of bio-molecules present in the seaweed extracts. A drop of colloidal AgNPs coated on a copper plate and dried in a hot air oven and examined under Field Emission Scanning Electron Microscopy (FESEM) (Carl Zeiss, Germany) equipped with Energy Dispersive X-ray spectroscopy (EDX-JEOL, JSM-5610) analysis. The morphology and size of the nanoparticles were further authenticated by the HRTEM image. A drop of AgNPs were coated on carbon layered copper grids of 200 mesh size and dried for 5 min prior observation in a High Resolution Transmission Electron Microscope (HRTEM) (JEOL JEM 2100 HRTEM) operated at the accelerating voltage of 200 kV. The selected area electron diffraction (SAED) pattern was also recorded.
2.4. Antimicrobial assay
Antimicrobial activity of the synthesized AgNPs was evaluated against five human bacterial pathogens (Escherichia coli, Klebsiella pneumoniae, Pseudomonas sp., Staphylococcus aureus and Salmonella paratyphi) and fungal pathogens (Aspergillus flavus, A. niger, A. ochraceus, A. terreus and Fusarium moniliforme) using the agar well diffusion method [13]. The test organisms with the inoculums size of 108 cells mL−1 for bacteria and 105 cells mL−1 for fungal spores were streaked on the surface of the media using sterile cotton swab. Mueller-Hinton Agar and Potato Dextrose Agar (HiMedia, India) were used for bacteria and fungi respectively. The wells were loaded with 100 μL concentration of colloidal AgNPs. The sterilized distilled water was used as a negative control and the plates were incubated at 37 °C for 24 h for bacteria and at 30 °C for 48 to 96 h for fungi. The zone of inhibition was measured and expressed in mm. All the assays were performed in triplicate.
2.5. Cancer cell lines and culture conditions
Ehlrich Ascites Carcinoma (EAC) cell lines were obtained from the National Centre for Cell Science (NCCS), Pune, India and supplemented with RPMI medium for culture maintenance. EAC cells were treated with different concentrations of AgNPs (0–200 μg mL−1 for 24 h at 37 °C and 50% of Inhibitory Concentration was used for the Cytomorphological observation. After completion of the exposure period, the cells (control and exposed) were washed with PBS and fixed in 1:1 ratio of methanol and glacial acetic acid for 1 h at room temperature. Then, the cells were treated with ethidium bromide/acridine orange at 1:1 ratio. Stained cells were visualized under UV illumination using 40 × objectives (Nikon, fluorescence microscope) and their digitized images were analyzed for apoptotic morphology.
2.6. Statistical analysis
All data were expressed as mean ± standard deviations (SD) were presented. One-way analysis of variance (ANOVA; P < 0.05) was performed and the significance of each mean value was determined with the Duncan’s multiple range tests using the SPSS statistical analysis computer program (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. UV–vis spectrum of AgNPs
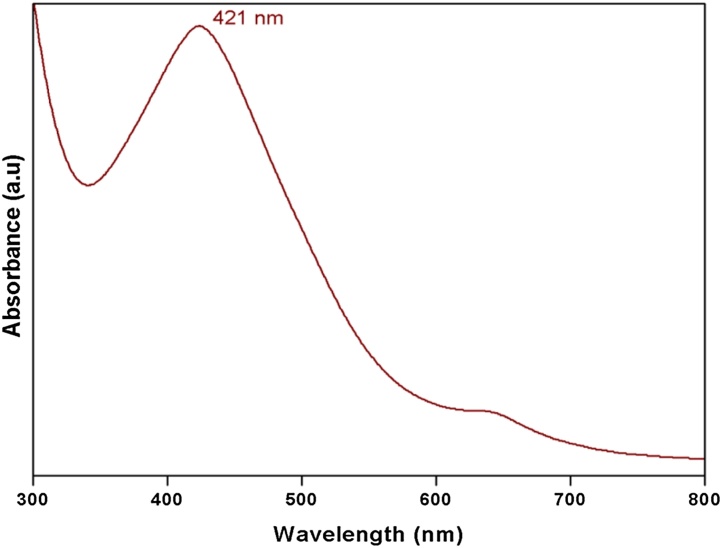
The Surface Plasmon Resonance vibration observed at 421 nm for the confirmation of AgNPs synthesis (Fig. 1). After the addition of aqueous extract of E. compressa, the colourless silver nitrate solution was changed to characteristic reddish brown color provided a convenient excitation of SPR vibrations, which indicates the formation of AgNPs. Several other researchers have also reported the absorption of Ag NPs between 410 and 440 nm, which was assigned to SPR of AgNPs [14].
Fig. 1.
UV–vis spectrum of AgNPs synthesized using aqueous extract of seaweed E. compressa.
3.2. XRD pattern of AgNPs
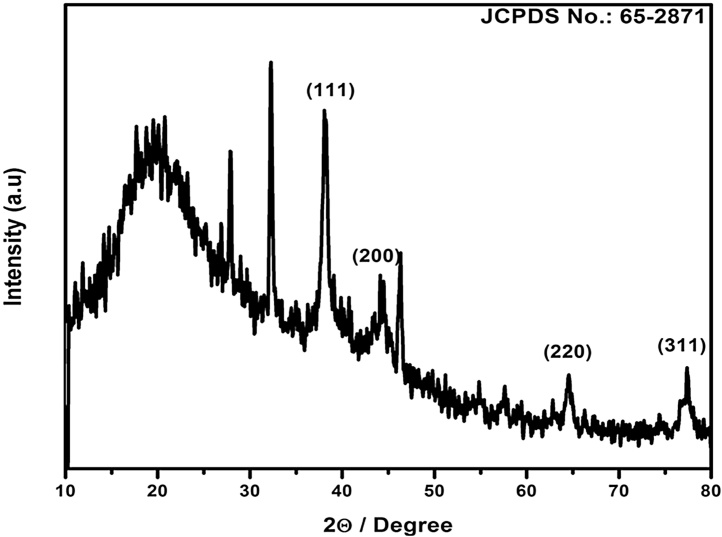
Furthermore, the XRD patterns of synthesized AgNPs was characterized by four distinct diffraction peaks at 38.1, 44.2, 64.4 and 77.1, which indexed the planes (111), (200), (220) and (311) of the face centered cubic silver (Fig. 2). A number of Bragg’s reflections corresponding to the lattice planes were observed which may be indexed based on the face centered cubic structures of Ag matched with the database of JCPDS. The standard XRD pattern of Ag was similar with JCPDS No. 65–2871. The XRD result was clearly shown that the silver NPs formed by the reduction of Ag+ ions by seaweed extract were crystalline in nature.
Fig. 2.
XRD pattern of AgNPs synthesized using aqueous extract of seaweed E. compressa.
3.3. FT-IR analysis of aqueous extract of E. compressa and synthesized AgNPs
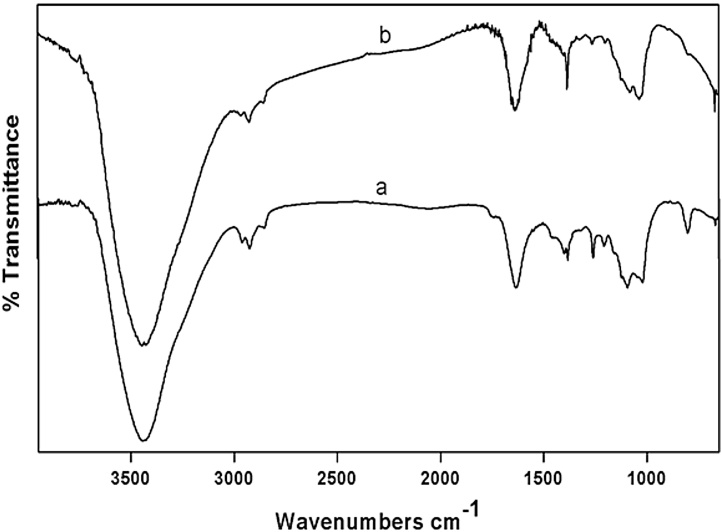
The FT-IR spectra of aqueous extract of seaweed E. compressa revealed the presence of different functional groups. The transmission peaks observed at 3443.52 and 1634.20 cm−1 represents O—H and C O stretching modes for the hydroxyl group and stretching bands of the carboxylic acid group respectively. Likewise, another band at 1021.46 cm−1 could be assigned the C—OH vibrations of the primary strong alcohol groups. When all the three transmission peaks were compared, the suppressed/increased peaks in the AgNPs were due to the consequence of metal nanoparticles bound to bioorganic molecules (Fig. 3). Thus, FT-IR spectra evidenced the presence of different functional groups played a significant role in the formation of AgNPs and its stability [15], [16].
Fig. 3.
FTIR spectra of aqueous extract of seaweed E. compressa (a) and AgNPs synthesized using aqueous extract of seaweed E. compressa (b).
3.4. HRTEM analysis of synthesized AgNPs
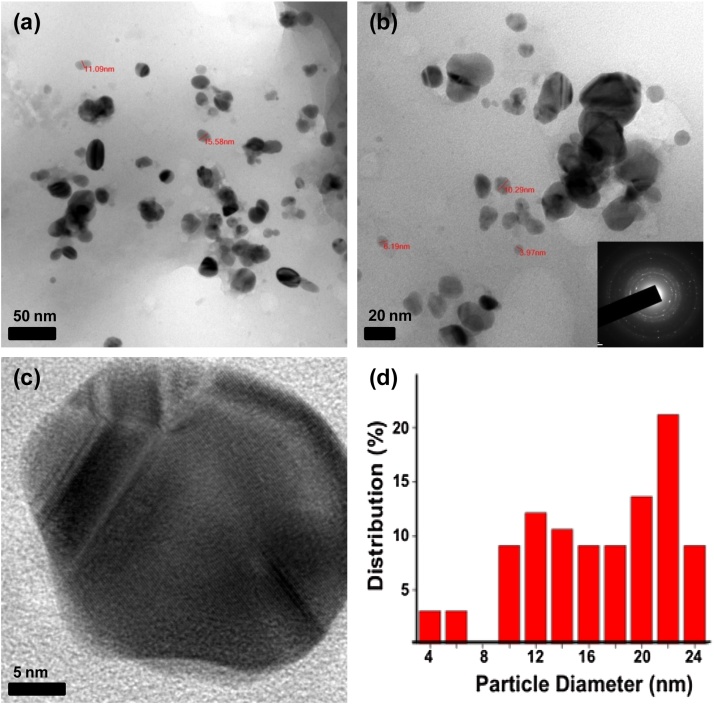
HRTEM provided further insight in the morphology and size details of the AgNPs. The AgNPs were spherical in shape, well separated and no agglomeration was noticed (Fig. 4a–c). The particles were formed in different sizes, ranging from 4 to 24 nm in size (Fig. 4d). These results were in agreement with the shape of the SPR vibrations (Fig. 1). The HRTEM investigation indicate good crystalline structure of the synthesized NPs and the distance of 0.23 nm between lattice planes was in agreement with the (111) lattice of face-centered cubic Ag [17], [18]. Crystalline structure of the NPs was further evidenced by the SAED pattern (Inset Fig. 4b) with bright circular rings corresponding to (111), (200), (220) and (311) Bragg’s reflection planes [13], [19].
Fig. 4.
HRTEM images shows different magnifications of AgNPs (a–c); (Inset (b) shows SAED pattern of AgNPs); and histogram of AgNPs (d).
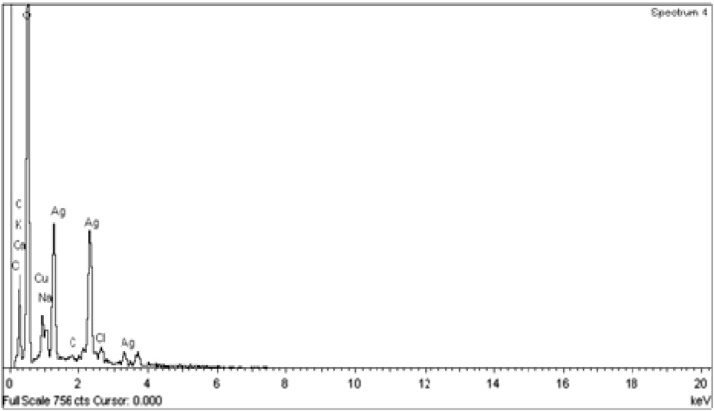
3.5. EDX analysis of synthesized AgNPs
Quantitative and qualitative determination of Ag through EDX analysis revealed the highest proportion of elemental Ag signals (Fig. 5). Minor peaks of the carbon, copper and others were observed which confirmed the existence of the biomolecules of E. compressa on the surface of synthesized AgNPs. However, the peak of copper was used for coating the sample.
Fig. 5.
EDX spectroscopy of AgNPs synthesized using aqueous extract of seaweed E. compressa.
3.6. Antimicrobial activity of AgNPs
The AgNPs exhibited good antimicrobial property against the pathogenic bacterial and fungal strains (Table 1). The zone of inhibition against bacterial and fungal strains was ranged between 10.5 ± 0.42 mm and 12.0 ± 0.41 mm and 9.2 ± 0.47 and 10.2 ± 0.47 mm respectively. The maximum zone of inhibition was registered against the bacterium E. coli, followed by K. pnemoniae > P. aeruginosa > S. aureus > S. paratyphi whereas in fungi A. niger registered maximum zone of inhibition followed by A. flavus, A. ochraceus, F. moniliforme and A. terreus. The AgNPs significantly inhibited the growth of bacteria and fungi at the selected concentration. Silver nanoparticles may attach to the surface of the cell wall and disturb the permeability and respiration. It is logical to state that the binding of the particles to the bacteria and fungi depends on the surface area available for electrostatic interaction [20]. Smaller particles having the larger surface area available for interaction will give more antimicrobial effect than the bulk counterparts [21]. Similarly, Patel et al. [19] reported that three cyanobacteria and one microalga mediated AgNPs were effectively inhibit the different bacterial strains. In the present study, the biosynthesized AgNPs showed potential antimicrobial activity with various bacterial and fungal pathogens which could be further used as a potential antimicrobial agents.
Table 1.
Antimicrobial potential of AgNPs synthesized using aqueous extract of seaweed E. compressa against different human pathogenic bacterial and fungal strains.
| Bacteria | Zone of inhibition (mm) | Fungi | Zone of inhibition (mm) |
|---|---|---|---|
| Escherichia coli | 12.0 ± 0.41 | Aspergillus terreus | 9.2 ± 0.47 |
| Pseudomonas aeruginosa | 11.4 ± 0.82 | Aspergillus flavus | 10.0 ± 0.82 |
| Klebsiella pneumoniae | 11.5 ± 0.62 | Aspergillus ochraceus | 9.5 ± 0.14 |
| Stapylococcus aureus | 11.1 ± 0.47 | Aspergillus niger | 10.2 ± 0.47 |
| Salmonella paratyphi | 10.5 ± 0.42 | Fusarium moniliforme | 9.4 ± 0.84 |
Results are the mean values of three independent experiments and SDs are shown. All the values are statistically significant (P < 0.001).
3.7. Cytotoxicity of AgNPs on EAC cells
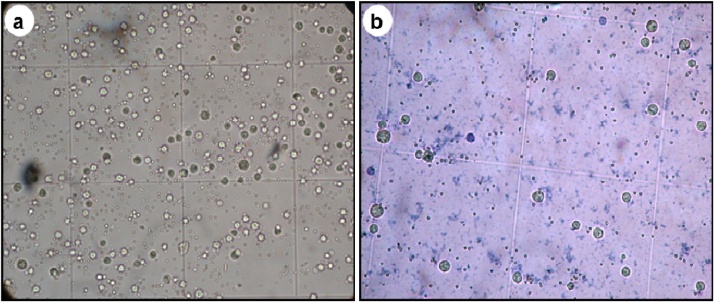
An IC50 value of AgNPs was recorded at 95.35 μg mL−1. The inhibitory effect was found to be directly proportional to the increasing concentration of AgNPs. The present study suggests that the seaweeds mediated AgNPs was effective in controlling the growth of EAC cells by activating the mitochondrian dependent apoptotic program. Most recently, cytotoxic effect of AgNPs synthesized using various plants sources on HeLa cell lines (Melia azedarach), human cervical carcinoma cell lines (Podophyllum hexandrum) and human colon cancer cell lines HCT15 (Vitex negundo) were reported by various authors [22], [23], [24]. Furthermore, Cytomorphology observations of treated cells showed distinct cellular morphological changes representing unhealthy cells, whereas control cells were irregular convergent aggregates with rounded and polygonal cells (Fig. 6a and b). This is due to the disruption of the cellular composition by surface interactions of nanoparticles on cells. The present study is coherent with the earlier report of AgNPs synthesized using the aqueous extract of Cassia fistula flower and their apoptosis was evaluated by AO/EB fluorescence staining showed effective cytotoxic potential against MCF7 cells at 7.19 mg mL−1 (IC50) [25]. To the best of our knowledge, the potential effect of AgNPs synthesized using the aqueous extract of seaweed E. compressa on EAC cells has been investigated for the first time in this study and further detailed investigation is necessary to evaluate the application of AgNPs in cancer therapy.
Fig. 6.
Cytotoxicity of AgNPs on Ehlrich Ascites Carcinoma cells. Control (a) and effect of synthesized AgNPs on Ehlrich Ascites Carcinoma cells (b).
4. Conclusion
The aqueous extract of seaweed E. compressa was prepared and conveniently employed for eco-friendly and single-pot biosynthesis of AgNPs. The diversified functional groups present in the seaweed extract are responsible for the reduction of Ag metal ions. Spherical shaped AgNPs were observed through HRTEM analysis, it ranged from 4 to 24 nm and the average size was 14 nm. The results clearly indicated that synthesized AgNPs had effective inhibition against different bacterial and fungal pathogenic strains. The synthesized AgNPs showed in vitro cytotoxic activity against Ehlrich Ascites Carcinoma cells. Furthermore, in vivo investigations are necessary to understand the complete action of seaweed-mediated AgNPs and to evaluate its potential applications in biomedical field.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
The first author (VSR) thank to University Grants Commission, New Delhi, India for the financial support through Dr. D.S. Kothari Post-Doctoral Fellowship Scheme (No. F.4-2/2006 (BSR)/BL/13-14/0312, Dt.: 19th-May-2014).
References
- 1.Sun Y., Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298:2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 2.Vijayan S.R., Santhiyagu P., Ramasamy R., Arivalagan P., Kumar G., Ethiraj K., Ramaswamy B.R. Seaweeds: a resource for marine bionanotechnology. Enzyme Microb. Technol. 2016;95:45–57. doi: 10.1016/j.enzmictec.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Pugazhendhi S., Kirubha E., Palanisamy P.K., Gopalakrishnan R. Synthesis and characterization of silver nanoparticles from Alpinia calcarata by Green approach and its applications in bactericidal and nonlinear optics. Appl. Surf. Sci. 2015;357:1801–1808. [Google Scholar]
- 4.Patra C.R., Bhattacharya R., Mukhopadhyay D., Mukherjee P. Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv. Drug Deliv. Rev. 2010;62:346–361. doi: 10.1016/j.addr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankar P.D., Shobana S., Karuppusamy I., Pugazhendhi A., Ramkumar V.S., Arvindnarayan S., Kumar G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzyme Microb. Technol. 2016;95:28–44. doi: 10.1016/j.enzmictec.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Thirumurugan A., Aswitha P., Kiruthika C., Nagarajan S., Christy A.N. Green synthesis of platinum nanoparticles using Azadirachta indica—an eco-friendly approach. Mater. Lett. 2016;170:175–178. [Google Scholar]
- 7.Pugazhendhi S., Sathya P., Palanisamy P.K., Gopalakrishnan R. Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B. 2016;159:155–160. doi: 10.1016/j.jphotobiol.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Singh P., Singh H., Kim Y.J., Mathiyalagan R., Wang C., Yang D.C. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzyme Microb. Technol. 2016;86:75–83. doi: 10.1016/j.enzmictec.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- 10.Narayanan K.B., Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Inter. Sci. 2010;156:1–13. doi: 10.1016/j.cis.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Asmathunisha N., Kathiresan K. A review on biosynthesis of nanoparticles by marine organisms. Colloids Surf. B. 2013;103:283–287. doi: 10.1016/j.colsurfb.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Singaravelu G., Arockiamary J.S., Kumar V.G., Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B. 2007;57:97–101. doi: 10.1016/j.colsurfb.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Vijayan S.R., Santhiyagu P., Singamuthu M., Kumari Ahila N., Jayaraman R., Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014:e938272. doi: 10.1155/2014/938272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankar S.S., Rai A., Ahmad A., Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interf. Sci. 2004;275:496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Govindaraju K., Kiruthiga V., Kumar V.G., Singaravelu G. Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii grevilli and their antibacterial effects. J. Nanosci. Nanotechnol. 2009;9:5497–5501. doi: 10.1166/jnn.2009.1199. [DOI] [PubMed] [Google Scholar]
- 16.Bharathi Raja S., Suriya J., Sekar V., Rajasekaran R. Biomimetic of silver nanoparticles by Ulva lactuca seaweed and evaluation of its antibacterial activity. Int. J. Pharm. Pharm. Sci. 2012;4:139–143. [Google Scholar]
- 17.Philip D., Unni C. Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Physica. 2011;43:1318–1322. [Google Scholar]
- 18.Abdul Razack S., Duraiarasan S., Mani V. Biosynthesis of silver nanoparticle and its application in cell wall disruption to release carbohydrate and lipid from C. vulgaris for biofuel production. Biotechnol. Rep. 2016;11:70–76. doi: 10.1016/j.btre.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel V., Berthold D., Puranik P., Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015;5:112–119. doi: 10.1016/j.btre.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sondi I., Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Gnanadesigan M., Anand M., Ravikumar S., Maruthupandy M., Ali M.S., Vijayakumar V., Kumaraguru A.K. Antibacterial potential of biosynthesised silver nanoparticles using Avicennia marina mangrove plant. Appl. Nanosci. 2011;2:143–147. [Google Scholar]
- 22.Sukirtha R., Priyanka K.M., Antony J.J., Kamalakkannan S., Thangam R., Gunasekaran P., Krishnan M., Achiraman S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012;47:273–279. [Google Scholar]
- 23.Prabhu D., Arulvasu C., Babu G., Manikandan R., Srinivasan P. Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochem. 2013;48:317–324. [Google Scholar]
- 24.Jeyaraj M., Rajesh M., Arun R., MubarakAli D., Sathishkumar G., Sivanandhan G., Dev G.K., Manickavasagam M., Premkumar K., Thajuddin N., Ganapathi A. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllum hexandrum on human cervical carcinoma cells. Colloids Surf. B. 2013;102:708–717. doi: 10.1016/j.colsurfb.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Remya R.R., Rajasree S.R.R., Aranganathan L., Suman T.Y. An investigation on cytotoxic effect of bioactive AgNPs synthesized using Cassia fistula flower extract on breast cancer cell MCF-7. Biotechnol. Rep. 2015;8:110–115. doi: 10.1016/j.btre.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]