Graphical abstract
Keywords: Lipase, Immobilization, Chitosan, Fatty acid ethyl ester, Esterification, Supercritical fluids
Highlights
-
•
Lipase B from Candida antarctica immobilized on activated chitosan.
-
•
The loading capacity of the new biocatalyst is around 20 mg/g of support.
-
•
Biocatalyst was higher than that of the CALB-Octyl (by a 53-fold factor).
-
•
Enzymatic esterification reaction in supercritical or near-critical CO2.
-
•
Molecular sieves promoted 16.0% increase in enzymatic esterification reaction.
Abstract
The objective of this new paper was to evaluate the enzymatic esterification reaction conducted in supercritical or near-critical CO2, catalyzed by immobilized lipase B from Candida antarctica (CALB). The biocatalyst was prepared through the immobilization of CALB by covalent attachment using chitosan sequentially activated with Glycidol, ethylenediamine (EDA) and glutaraldehyde as support. In order to determine the best operational conditions of the esterification reaction (1: 1 (alcohol–acid); biocatalyst content, 10% (by substrate mass); 45 °C), an experimental design (23) was conducted to evaluate the effects of the following parameters: alcohol to oil molar ratios, reaction time and temperature. The maximum loading of chitosan was 20 mg protein/g support, and the thermal and solvent stability of the new biocatalyst was higher than that of the CALB-GX (by a 26-fold factor), CALB-OC (by a 53-fold factor) and Novozym 435 (by a 3-fold factor). The maximum conversion was 46.9% at a temperature of 29.9 °C, ethanol to oleic acid molar ratio equal to 4.50:1, and a reaction time of 6.5 h. Additionally, the removal of water from the medium, by using molecular sieves, promoted a 16.0% increase in the conversion of oleic acid into ethyl esters.
1. Introduction
Over the past three decades, the use of carbon dioxide as supercritical media (ScCO2) has been pointed out as an alternative to the use of conventional solvents in the production of compounds of high economic value [1], [2], [3], [4]. Carbon dioxide presents interesting characteristics, such as being nontoxic, nonflammable, low cost, available in large quantities, tunable between solvent properties and solvation, and relative low critical properties [5], [6]. Additionally, ScCO2 has a high diffusivity, for this reason it could be easily separated from the reaction media [7], [8].
Esters are compounds obtained usually by esterification reaction from a carboxylic acid (organic or inorganic) and an alcohol (low or high molecular weight) [9]. Another important way to obtain these compounds is by transesterification process, e.g., the most popular method of producing biodiesel (mixture of mono-alkyl esters) from vegetable oils (triglycerides) [10]. Esterification and transesterification processes use chemical or enzymatic methods to obtain esters. In this work, the enzymatic method was analyzed, as this permitted to label these as green products.
Esters production is widely distributed in many fields, like fragrances and flavors used in the food [11], cosmetic [12], pharmaceutical (acetylsalicylic acid, benzocaine, etc.), resin, solvents and biolubricant industries [13].
The yield of production of esters via esterification by enzymatic protocols depends of several parameters, such as, the enzyme itself (determine the feasibility of the process), the type of support and immobilization method, as well as medium reaction conditions [14]. Main reaction conditions are temperature, pH, acid-alcohol ratio, enzyme concentration and water content (subproduct of the reaction).
The enzymatic synthesis of esters of fatty acids using supercritical CO2 is an environmentally friendly strategy [15]. With this in mind, in this work, the synthesis of ethyl-oleate, which is a model system [16], [17], was investigated using lipases. Lipases are one of the most important enzymes in biocatalysis [18], since they can be used in a wide range of industrial applications: modification of oils and fats, synthesis of organic compounds[19], supplements and detergents [20], in analytical procedures [21], foods and pharmaceutical processing [22], [23], and in the production of biofuels (transesterification and/or esterification processes) [24].
It is important to mention that the use of enzymes in industrial applications could be enhanced by immobilization onto a solid supports [25], [26], [27]. A proper immobilization may improve enzyme stability, and also some other parameters like activity, selectivity, etc., [28], [29], [30], sometimes coupling immobilization to purification [31].
For these goals, the support surface is very relevant to determine the final properties of the immobilized enzyme [32]. In light of this, lipase B from Candida antarctica (CALB) has been selected, due to its spread use in processes involving enzymatic synthesis [33], [34]. The enzyme has a small lid that does not fully seclude the active center from the medium [35].
In this work, we have explored the possibility of using the chitosan (partially deacetylated chitin) as a support of enzyme immobilization[36], [37], [38], [39]. Chitin is the second most important natural polymer in the world [40]. The main sources of this polymer are two marine crustaceans, shrimp and crabs [40].
As immobilization method, we have selected the activation of the primary amino groups of the support with glutaraldehyde. This is a very popular and versatile method for enzyme immobilization, mainly in the case of lipases [41], [42]. We have compared this biocatalyst with two others. The first one, the support was octyl agarose, a support that immobilizes lipases via interfacial activation [43] and provides a simple method for the immobilization, purification, stabilization and hyperactivation of the lipases [44]. Finally, glyoxyl-agarose was utilized. This support immobilizes protein via their amino groups and requires the immobilization at alkaline pH values [45], being recognized as one of the most effective for enzyme stabilization via multipoint covalent attachment [46].
The resulting biocatalyst was then evaluated in the production of ethyl oleate in ScCO2. In order to develop a sustainable and technologically efficient process, the response surface methodology (RSM) was used to investigate the effect of operational parameters (temperature, substrates molar ratios and reaction time) in the enzymatic synthesis. This statistical method is specially adequate when some interactions between the different variables are expected [47], [48], [49].
2. Materials and methods
2.1. Materials
Soluble lipase B from Candida antarctica (CALB) (10.9 mg of protein/mL) was purchased from Codexis (Redwood, USA). Powdered chitosan, 85.2% deacetylation degree, was purchased from Polymar Ind. Ltda (Ceará, Brazil). Glycidol 96% GC (2,3-epoxy-1-propanol), p-nitrophenyl butyrate (pNPB) and p-nitrophenol (pNP), 4BCL agarose beads and octyl-agarose beads were purchased from Sigma–Aldrich (St. Louis,USA). Oleic acid and anhydrous ethanol 99.9% were used as substrates, ethylenediamine and glutaraldehyde solution Grade II 25% were purchased from Vetec (São Paulo, Brazil). Molecular sieve 4A (Na2O[Al2O3(5·OSiO2)]12H2O) was purchased from W.R. Grace & Co, (Massachusetts, USA). Carbon dioxide (99.9% purity in the liquid phase) was purchased from White Martins S.A. (Ceará, Brazil). All reagents and solvents were of analytical grade, and it they have been used without any further purification.
2.2. Preparation and modification of chitosan support for enzyme immobilization
In this work, 4.0 wt.% chitosan was used for CALB immobilization [50], after being activated with glycidol, oxidized with sodium periodate, modified with ethylenediamine (EDA) and finally activated with glutaraldehyde (Silva et al., 2012; Neta et al., 2012). The support prepared by using this procedure was here named chitosan–glyoxyl–EDA–glu. Activation of chitosan with glycidol was carried out by etherification and further oxidation with sodium periodate [51]. Then, 10 g of Chitosan–Glyoxyl gel was reacted with 40 mL of a 2 M ethylenediamine solution, pH 10.0 [52]. Finally, 9 mL of sodium bicarbonate buffer, pH 10.0 [38], containing 5% (v/v) of glutaraldehyde [38], was added to 1 g of chitosan–Glyoxyl–EDA. The mixture was kept under agitation for 60 min at 25 °C. After this time, the support was washed with distilled water to remove the excess of activating agent.
2.3. Preparation of glyoxyl-agarose beads
The activation of agarose beads was performed according to the procedure described in [45]. The gel was suspended in 1 M NaOH and 0.5 M NaBH4 2:1 (v/v). These reducing conditions prevent uncontrolled oxidation of the gel by the alkaline conditions. While keeping this mixture in an ice bucket, glycidol was added drop wise in order to reach a final concentration of 2 M. The obtained suspension was gently stirred overnight at 22 °C. The modified gel was then washed with abundant distilled water, filtered and then incubated in an aqueous solution (300 mL) containing 60 μmol NaIO4/g gel in order to achieve glyoxyl groups. This oxidative reaction was allowed to proceed for 2–3 h under mild stirring at room temperature [53]. Finally, the support was washed with distilled water and stored at 4 °C.
2.4. Immobilization of CALB on chitosan-GLYOXYL-EDA-GLU
CALB immobilization onto chitosan–GLYOXYL-–EDA-GLU (CALB-CH) was carried out in a batch reactor, under gentle agitation, at 25 °C, by contacting the enzyme in 100 mM bicarbonate buffer, pH 10.0, and the previously activated support, for 5 h. Initially, the immobilization was carried out using 20 U/g of support (1 mg of protein per g of wet support) to prevent diffusion limitations that could alter the results. Immobilization parameters were calculated by determining hydrolytic activities of supernatant during the process. A blank assay was also conducted to evaluate a possible enzyme deactivation under the immobilization conditions. For this purpose, a solution of CALB was placed in a reactor under the same conditions of immobilization, but in the absence of support.
After measuring the initial (Ati) and the final (Atf) enzyme activity in the supernatant, the immobilization yield was calculated by using Eq. (1), as indicated by [38].
| (1) |
The theoretical activity (Att) of immobilized lipase on the support could be calculated by using the amount of enzyme offered/g of support (Atoff − U/g support) and the immobilization yield, as can be seen in Eq. (2). After measuring the activity of the immobilized enzyme (Atd − U/g support), the recovery activity was calculated by using Eq. (3):
| (2) |
| (3) |
2.5. Immobilization on glyoxyl-agarose beads (GLX-support)
A 10 g of support was suspended in 100 mL of CALB solution (20 U/g support) (1 mg of protein per g of wet support) in 100 mM sodium carbonate at pH 10 and 25 °C for 72 h [54]. Derivatives were then reduced by addition of solid NaBH4 (0.1% (m/v)) [54]. After gentle stirring for 30 min at room temperature, the resulting derivatives were washed with abundant distilled water to eliminate residual sodium borohydride.
2.6. Immobilization of CALB on octyl-agarose beads (OC-support)
The immobilization was performed using 1 mg of protein per g of wet support as previous described [44]. The commercial samples of the enzymes were diluted in the corresponding volume of 5 mM sodium phosphate at pH 7. Then, the support was added. The activity of both supernatant and suspension was followed using pNPB. After immobilization the suspension was filtered and the supported enzyme was washed several times with distilled water.
2.7. Lipase activity and protein concentration
The hydrolytic activity of soluble and immobilized CALB was determined according to a methodology previously described by Bhatnagar [55] with slight modifications pointed below. Assays were performed in a spectrophotometer (Thermo Scientific–Analytics) at 400 nm with temperature and agitation control. Activity measurements were performed in 25 mM sodium phosphate at pH 7.0 and 25 °C, determining the released p-nitrophenol in the hydrolysis of 0.4 mM pNPB (ε = 10.052 M−1cm−1 under these conditions). To start the reaction, 30–200 μL of lipase solution or suspension was added to 2.5 mL of substrate solution. One international unit of activity (U) was defined as the amount of enzyme that hydrolyzes 1 μmol of pNPB per minute under the conditions described previously. Protein concentration was determined using Bradford's method [56] and bovine serum albumin was used as the reference.
2.8. Thermal inactivation of derivatives
To check the stability of the different enzyme derivatives, 1 g of biocatalyst was suspended in 5 mL of 10 mM of sodium acetate at pH 5, sodium phosphate at pH 7 or sodium carbonate at pH 9 at different temperatures. Periodically, samples were withdrawn and the activity was measured using pNPB. The deactivation constant and half-life time for each immobilized derivative were calculated according to the Sadana and Henley model [57] using Microcal Origin version 8.1.
2.9. Stability assays of derivatives in the presence of dioxane
Enzyme preparations were incubated in mixtures of 70% dioxane/30% 100 mM Tris at pH 7 and at different temperatures to proceed to their inactivation. Periodically, samples were with-drawn and the activity was measured using pNPB. The deactivation constant and half-life time for each immobilized derivative was calculated according to the Sadana and Henley model [57] using Microcal Origin version 8.1. The dioxane present in the measurement samples had not a significant effect on enzyme activity determination.
2.10. Support CALB loading capacity
The loading capacity of the support was investigated by offering different amounts of protein per g of support. The amount of protein ranged from 0.5 to 25 mg protein/g support.
2.11. Supports scanning electron micrographs
The morphology surface of the supports were observed by scanning electron microscopy (SEM) using the JEOL equipment (JSM 35C model). In this stage, drops of suspension in water of each sample were placed on sample holders (metallic stubs) and dried within silica gel dryer at room temperature for 24 h, followed by coating with gold using a standard sputtering technique.
2.12. Esterification reactions in supercritical carbon dioxide
The enzymatic production of ethyl oleate from oleic acid and ethanol in supercritical carbon dioxide was conduceted in batch mode, using a 13 mL stainless steel reactor placed in a thermostatic bath. For each run, the reactor was packed with 10% w/w of immobilezed enzyme, based on the mass of fatty acid used [6], 10 g of oleic acid and the desired amount of ethanol. First, the system was pressurized to an initial pressure of 100 bar at 25 °C, using CO2. After that, the temperature was adjusted according to the desired reaction conditions and the reaction was initiated. Samples were withdrawn from the reactor at the end of pre-determined times in order to calculate oleic acid conversion.
A central composite rotatable design (CCRD) was used to evaluate the effects of temperature, molar ratio of substrates and reaction time on fatty acid ethyl ester conversion (response). The variables and their levels were selected based on the literature [58], [59], [60], [61], as it could be observed in Table 1.
Table 1.
Experiments design. Experiments were performed as described in Section 2.
| Variables | Code | − 1.68 | − 1 | 0 | + 1 | + 1.68 |
|---|---|---|---|---|---|---|
| Temperature (°C) | x1 | 29.9 | 35 | 42.5 | 50 | 55.1 |
| Reaction time (hours) | x2 | 0.6 | 3 | 6.5 | 10 | 12.4 |
| Molar ratio ethanol: fatty acid | x3 | 1.1 | 2.5:1 | 4.5:1 | 6.5:1 | 7.9 |
The removal of water during the esterification of oleic acid by CALB immobilized on chitosan was also evaluated under the optimized reaction conditions. The synthesis of ethyl oleate in the presence or absence of the molecular sieve as a function of time was compared.
2.13. Analysis
2.13.1. Physical and chemical characterization of oleic acid and ethtyl oleate
The characterization of oleic acid (substrate) and ethyl esters (product) obtained by esterification was carried out by different analytical methods, suggested by the American Oil Chemists Society (AOCS) and the American Society for Testing Materials (ASTM), as well as by gas chromatography (GC–MS).
The analysis of GC–MS was performed with a Thermo Trace GC Ultra instrument coupled with a Thermo DSQ II mass spectrometer Saturn 2000 MS/MS, equipped with a flame ionization detector (Thermo Fisher Scientific, Texas, USA). A capillary column BPX5 SGE (30 m × 0.25 mm × 0.25 μm) was used to separate the fatty acid ethyl esters. The injector and detector temperature was set to 250 °C and the column temperature was programmed to increase from 110 °C to 215 °C (at rate of 5 °C min−1) remaining at 215 °C for 24 min.
Free fatty acids were determined by using the AOCS official method Ca-5a-40 [62]. The determination of the iodine index was conducted by Winkler's Bromate Method, AOCS Cd1-25 [62]. Density and viscosity was determined according to ASTM D-4052 [63], while moisture has been determined in according to ASTM D-1744A [63]. Acidity was determined by the method AOCS Cd3d-63 [62].
2.13.2. Substrate conversion
The acidity index is an indication of the reaction performance, corresponding to the decrease of fatty acids concentration in the oil, due to the esterification[61].Therefore, substrate conversion was calculated using this index, determined by the AOCS method described in 2.4.1, see Eq. (4).
| (4) |
where IA0 and IAF are the initial and final acidity indexes, respectively.
2.14. Reuse of the immobilized enzyme
After the esterification reaction in ScCO2, the immobilized enzymes were separated from the reaction medium by vacuum filtration using a sintered glass funnel. The biocatalyst was washed 3 times with hexane to dissolve and remove any residual reaction product from the support, and allowed to stay in the vacuum system for 30 min to ensure its dryness.
3. Results and discussion
3.1. Scanning electron microscopy of supports
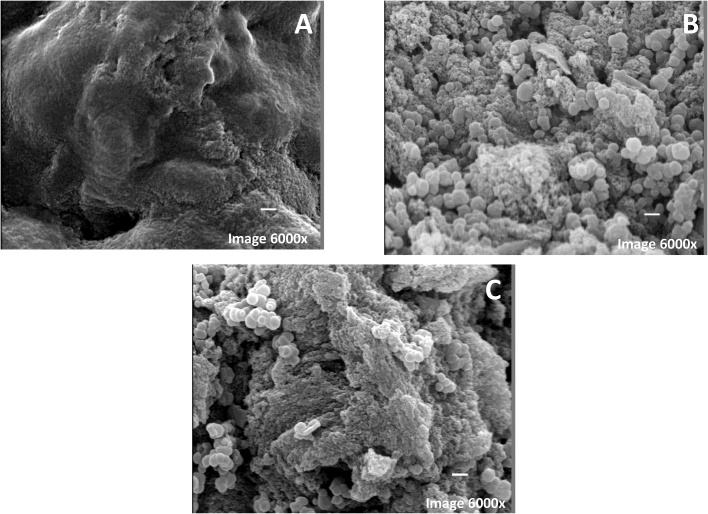
The analysis in scanning electron microscopy highlights the details of the surface texture of support before and after treatment and CH-CALB preparation as can be seen in Fig. 1. In panel A, it is possible to notice an irregular surface of the initial chitosan support, with few pores. After treatment with glycidol, EDA and glutaraldehyde, panel B, there is a distinct surface with larger pores. After enzyme immobilization, panel C, the support surface is covered with small beads, very likely formed by protein aggregates confirming the presence of high amounts of immobilized enzyme on the support.
Fig. 1.
Scanning electron micrographs of support. (A) Support chitosan no treatment; (B) Support chitosan–glyoxyl–EDA–glu. (C) Preparation Chitosan-glyoxyl–EDA–glu-CALB. Experiments were performed as described in Section 2.
3.2. Biocatalyst preparation: lipase immobilization
In this work, different biocatalysts were prepared by the immobilization of soluble enzymes. The advantage of the immobilization of the enzymes for the heterogeneous catalysis is a probable increase of the catalytic area.
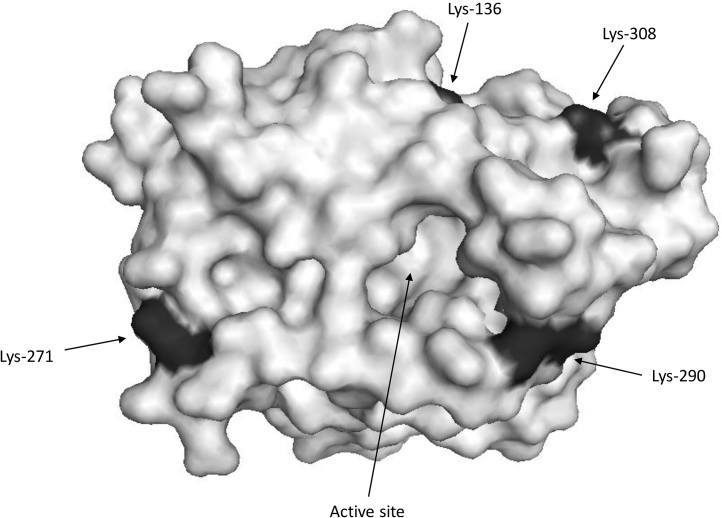
CALB was immobilized onto chitosan-GLYOXYL-EDA-GLU (CALB-CH) and the results obtained in the immobilization process are shown in Table 2. A high immobilization yield was achieved (94.7%). According to the literature [41], glutaraldehyde activated supports may immobilize CALB via a triple mechanism: ion exchange, interfacial activation and covalent attachment and, so a large amount of enzyme rapidly binds to the support, promoting an increase in the IY and in the activity of the immobilized enzyme. Nevertheless, it is important to stress that a moderate value of recovered activity (30.2%) was obtained. Several possibilities may be used to explain this result. For instance, the enzyme, by binding to the CALB-CH, may suffer distortions in its structure (Fig. 2), leading to a decrease of catalytic activity [64]. Fig. 2 shows the model structure of the open form of CALB in the area surrounding the active center, with all the reactive groups marked. It is clear that CALB has many reactive groups on this area that may provide the enzyme covalent immobilization. On the other hand, it is possible that some protein molecules may still be desorbed during washes from the support by not reacting in a covalent way with the glutaraldehyde molecules.
Table 2.
Immobilization parameters of CALB–chitosan derivatives: recovery activity (Atr), immobilization yield (IY), enzyme activities of the supernatant (soluble enzyme) before (Ati) and after (Atf) immobilization, and derivative activity (Atd). Experiments were performed as described in Section 2.
| Ati (U/g) | Atf (U/g) | Atd (U/g) | IY (%) | Atr (%) |
|---|---|---|---|---|
| 18.95 ± 0.92 | 0.85 ± 0.07 | 5.73 ± 0.59 | 94.70 ± 1.37 | 30.20 ± 2.86 |
Fig. 2.
3D surface structure model of open form of CALB (PDB code 1TCA). The 3D surface structure was obtained using PyMol vs 0.99.
The use of GX-agarose to immobilize proteins requires the simultaneous involvement of several amino groups of the protein [45], [51]. Using this support to immobilize CALB, around 45% of the offered activity was immobilized after 72 h, while the activity of the suspension remained almost unaltered. Therefore, GLX-CALB was prepared for further comparisons with chitosan biocatalysts.
Using OC-agarose beads, immobilization was very fast [44]. CALB activity remained almost unaltered after immobilization on octyl, very likely due to the very small lid that does not fully seclude the active center from the reaction media [35].
The properties of CALB-CH preparation were compared to those of CALB-OC and CALB-GX derivatives.
3.3. Thermal stability of different CALB preparations under different experimental conditions
Table 3 shows the half-lives of soluble CALB, CALB-CH, CALB-OC, CALB-GX and Novozym 435 at pH5.0, pH7.0 and pH9.0 at 65 °C. All immobilized preparations are more stable than the soluble enzyme.
Table 3.
Half-lives (expressed in minutes) of the different CALB preparations under different inactivation conditions. Experiments were performed as described in Section 2.
| Preparation | pH 5.0, 65 °C | pH 7.0, 65 °C | pH 9.0, 65 °C | 70% Dioxane, 25 °C, pH 7.0 |
|---|---|---|---|---|
| Soluble enzyme | 5.0 ± 1.0 | 3.0 ± 0.5 | 2.0 ± 0.5 | 1.5 ± 0.5 |
| CALB- CH | 42 ± 2.0 | 23 ± 1.5 | 12 ± 3.0 | 8.0 ± 1.0 |
| CALB-GX | 10 ± 1.5 | 11 ± 1.5 | 12 ± 1.5 | 0.28 ± 0.03 |
| CALB-OC | 77 ± 3.0 | 47 ± 2.0 | 15 ± 1.0 | 0.15 ± 0.03 |
The covalently immobilized preparations were less thermostable than CALB-OC, this fact is associated with the high stability of the open form of the immobilized enzyme stabilized by adsorption [65], [66] and the low density of lysine on the surface of the enzyme (Fig. 2). Similar results were found using this lipase and supports activated with divinylsulfone, even although an intense multipoint covalent attachment could be expected using this support [67], [68].
Focusing on the chitosan preparations, at pH 5 the CALB-CH preparations were more stable than CALB-GX one by a 4-fold factor, however, as can be seen in Table 3, they were two times less stable than CALB-OC preparation. When compared with Novozym 435 they were three times more stable. At pH7, the CALB-CH preparations were twice more stable than the CALB-GX, but when compared with CALB-OC preparations, this preparation was less stable by a factor of 2. When compared with Novozym 435 they have similar stability. The CALB-CH preparations showed similar stability when comparing the preparations of CALB-GX and CALB-OC at pH 9. When compared with Novozym 435 they were four times more stable.
These differences in relative stability may be related to the implication of different areas of the enzyme in the immobilization on the different supports, most considering that at each pH value the conformational changes involved in enzyme inactivation have been shown to be very diverse [69].
The different immobilization strategies must be evaluated for stability results. Focusing on the data Novozym 435, is a commercially available heterogeneous biocatalyst system that consists of (CALB) immobilized within a macroporous resin (Lewatit VP OC 1600) poly(methyl methacrylate-co-divinylbenzene). Comparing with the biocatalyst prepared in our manuscript, first, the immobilization strategy that we use in this work, the multi-point covalent bond to stabilize the enzyme in the support, and this technique is considered as the best strategy of immobilization of enzymes by many authors [28], [29], [70], [71], since it allows the stabilization of the enzyme in the open conformation. More active, providing greater rigidification in the enzyme in the support, avoids leaching, keeps the enzyme active for longer, this is important for the reuse of the biocatalyst.
3.4. Stability in organic solvents
Table 3 shows the stability of soluble CALB, CALB-CH, CALB-OC and CALB-GX in 70% dioxane. The soluble enzyme exhibits very low stability, and after about 1 min under the experimental conditions the activity was full destroyed.
Focusing on the chitosan-preparations, CALB-CH was more 26 times more stable than CALB-GX. When compared to CALB-OC, CALB-CH was more stable by a factor of 53 times, and was more stable by a factor of three times than Novozym 435. The low stability of OC-CALB and Novozym 435 in organic medium has been explained by the weakening of adsorption interactions between enzyme and support in the presence of the organic medium, that may permit the enzyme desorption [54].
3.5. Loading capacity
Fig. 3 shows that CH allows immobilizing approximately 20.0 mg of CALB/g of support. Using enzyme loading lower than 20 mg/g support of the enzyme, the enzyme is almost fully immobilized. The loading capacity of the new support was the same than using octyl agarose support (20 mg per wet gram using CALB). In any case, the loading capacity of the chitosan is satisfactory.
Fig. 3.
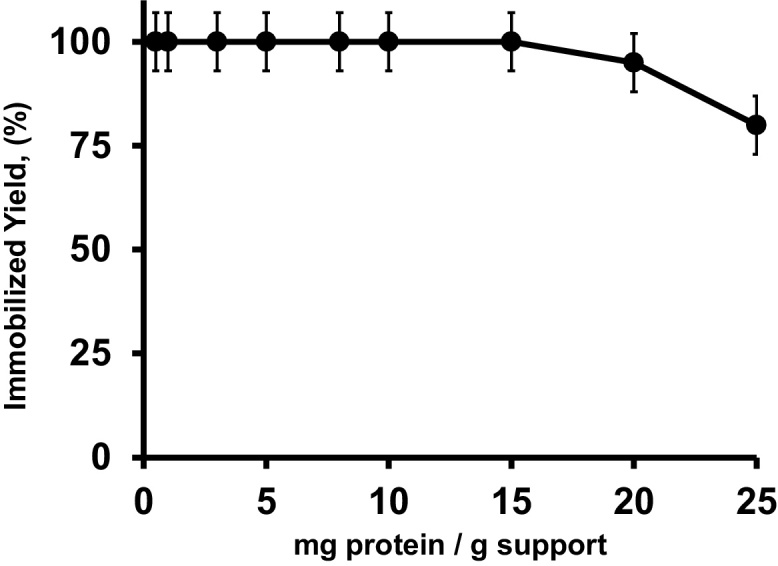
Loading capacity of CHITOSAN–GLYOXYL–EDA–GLU support. Experiments were performed as described in Section 2.
3.6. Reactions in ScCO2
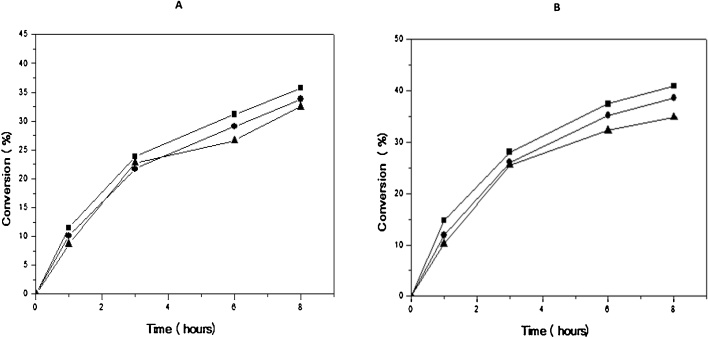
The literature reports different temperatures for the use of lipases in the synthesis of ethyl oleate: 37 °C [38], 40 °C [72], among others. Therefore, the reaction was conducted at a two different temperatures (30 and 50 °C). The conversion values for the synthesis of ethyl oleate are given in Fig. 4. A negligible influence of temperature on the ethyl oleate synthesis rate could be observed. In addition, after 10 h of reaction, when the reaction seems to be in equilibrium, the conversion achieved at 50 °C was only 1.13-fold higher than the one achieve at 30 °C.
Fig. 4.
Influence of temperature on enzymatic esterification of oleic acid with ethanol by supercritical alcohol molar ratio: 6,5:1 oil. Legends: (■) 35 °C and (●) 50 °C. Experiments were performed as described in Section 2.
Afterwards, the effect of ethanol to oleic acid molar ratio (2.5:1, 4.5:1, 6.5:1) was investigated at the two temperatures (35 and 50 °C) and results are depicted in Fig. 5(A) and (B). It is important to mention that alcohol in excess (stoichiometric ratio of 3:1) has been used to ensure a high reaction rate and minimize the limitations of diffusion using organic solvent or solvent free medium [73], [74]. However, it could be observed that conversion values are very close, almost independent of the molar ratio using supercritical medium. In fact, the lowest molar ratios allow achieving the highest conversion rates, suggesting a negative effect of the alcohol on enzyme activity under these conditions. This result could indicate that there is no need for a stoichiometric excess in order to promote the reaction. Similar results were observed in the literature. For instance, Madras et al. [75] observed that as the amount of alcohol increased, the conversion into esters progressed more slowly in the balance and the conversion increased. These authors also found the existence of an optimum molar ratio and above that value, the initial rate of reaction was lower. Wang [76] studying the enzymatic production of biodiesel from waste oil acids, also found that an excessive amount of alcohol decreased the initial rate of reaction. This behavior could be explained by the alcohol inhibition effect [77], [78], [79]. Due to high polarity of short-chain alcohols, such as ethanol, the alcohol can be adsorbed on the carrier on which the enzyme was immobilized, removing the layer of hydration of the enzyme, which can promote its inactivation [58].
Fig. 5.
Panel (A): Influence of molar ratio alcohol: acid in the enzymatic esterification of oleic acid with ethanol by means of supercritical fluid at a temperature of 35 °C. Panel (B): Influence of molar ratio alcohol: acid in the enzymatic esterification of oleic acid with ethanol by means of supercritical fluid at a temperature of 50 °C. Legends: molar ratio (■) 2.5:1, (●) 4.5:1 and (▲) 6.5:1. Experiments were performed as described in Section 2.
3.7. Effect of the operational conditions (temperature, molar ratio and reaction time) on the enzymatic esterification of oleic acid using ethanol in ScCO2
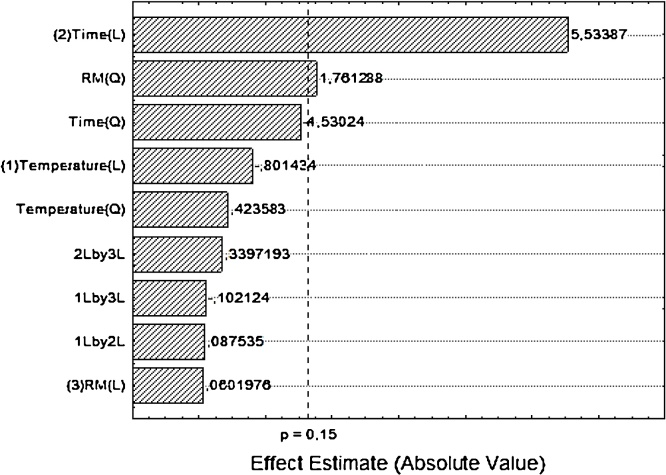
In order to find out the influence of the operational conditions (temperature, molar ratio and reaction time) on the enzymatic esterification of oleic acid using ethanol in ScCO2, a factorial design was conducted and the complete design matrix and the results are shown in Table 4. A Pareto chart is given in Fig. 6. The influence of the variables and their interactions at a significance level of 85% could be seen. It could be observed that the reaction time (x2) and quadratic molar ratio (x32) has significantly influence in the conversion. Reaction time (x2) is the most significantly variable among the evaluated ones for the production ethyl esters in relation to the molar ratio (x3). In the absence of an interaction effect, the variables can be analyzed separately, so the application of statistical method revealed that the interactions x1x2, x1x3, x2x3, are not significant. By multiple regression analysis, using the experimental results of the full factorial design, a model was set up for representing the production of ethyl esters, as a function of the variables, see Eq. (8). In this model, (x1), (x2) and (x3), represents temperature, reaction time and molar ratio, respectively, as follows:
| YEthylesters = 32.8x1 − 1.47x1 + 0.94x12 + 10.16x2 − 3.41x22 + 0.11x3 +3.9x32 + 0.21x1x2 − 0.24x1x3 + 0.81x2x3 | (8) |
Table 4.
Experimental design and results. Experiments were performed as described in Section 2.
| Run | Coded Variables |
Responses | ||
|---|---|---|---|---|
| x1 | x2 | x3 | Ethyl esters (%) | |
| 1 | −1 | −1 | −1 | 23.82 ± 1.7 |
| 2 | −1 | −1 | 1 | 22.74 ± 1.6 |
| 3 | −1 | 1 | −1 | 39.44 ± 1.6 |
| 4 | −1 | 1 | 1 | 41.17 ± 1.4 |
| 5 | 1 | −1 | −1 | 28.08 ± 1.7 |
| 6 | 1 | −1 | 1 | 25.57 ± 1.7 |
| 7 | 1 | 1 | −1 | 44.09 ± 1.8 |
| 8 | 1 | 1 | 1 | 45.29 ± 1.9 |
| 9 | −1,68 | 0 | 0 | 46.87 ± 1.9 |
| 10 | 1,68 | 0 | 0 | 25.49 ± 1.7 |
| 11 | 0 | −1,68 | 0 | 3.35 ± 1.8 |
| 12 | 0 | 1,68 | 0 | 44.37 ± 1.7 |
| 13 | 0 | 0 | −1,68 | 43.97 ± 1.6 |
| 14 | 0 | 0 | 1,68 | 45.26 ±1.4 |
| 15 | 0 | 0 | 0 | 32.43 ± 1.2 |
| 16 | 0 | 0 | 0 | 32.95 ± 1.2 |
Fig. 6.
Pareto chart of standardized effects on ethyl esters production. (L) is the linear and (Q) is the quadratic interaction of variables. Experiments were performed as described in Section 2.
The model presented a reasonable coefficient of determination (R2 = 0.87), indicating that 87% of the variability of response may be explained by the previous model. The F value of 122.6 is much larger than F (2.11), indicating that the model is significant at confidence level of 85%. The results of the Analysis of Variance (ANOVA) are given in Table 5. It is possible to see that the probability p-value is low (0.0001), indicating the accuracy of the model.
Table 5.
Statistical analysis. Experiments were performed as described in Section 2.
| Sources of variation | Sum of squares | Degrees of freedom | Mean squares | Fvalue | Probability P |
|---|---|---|---|---|---|
| Model | 1885.27 | 9 | 209.47 | 4.55 | <0.0001 |
| Residual | 276.26 | 6 | 46.04 | ||
| Total | 2161.53 | 15 |
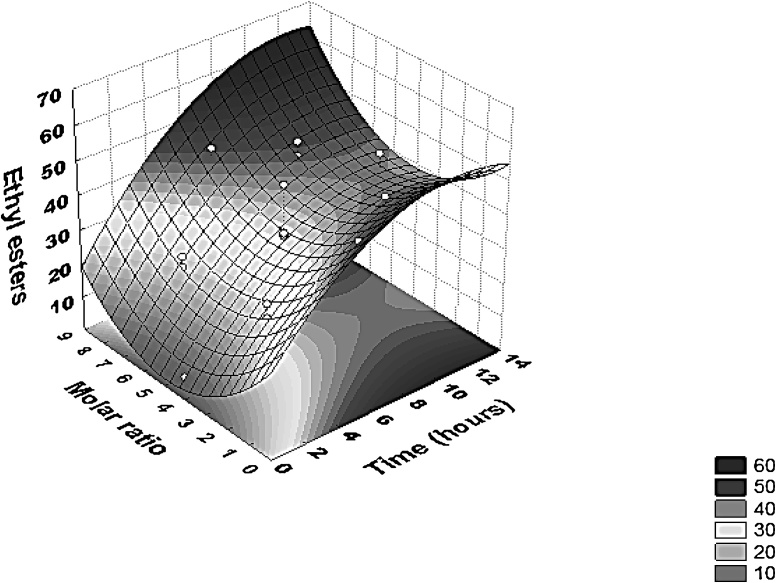
The model was then used to plot the surface response (Fig. 7) representing the dependent variable conversion as a function of reaction time and molar ratio at a fixed temperature. By analyzing the response surface, it can be seen that, independent of the molar ratio, enhancing reaction time, conversion is enhanced up to 10 h, where it could be supposed that a steady state reaction was reached. Regarding molar ratio, it is possible to see that higher values of conversion were achieved at low (more than 50%) or at high levels of oil/alcohol molar ratio (more than 60%). Therefore, considering the results obtained, the optimized conditions were defined as being: temperature of 29.9 °C, molar ratio ethanol: oil equal to 4.5:1 and reaction time of 10 h. These results showed a satisfactory representation of the process model and a good correlation between the experimental results and the theoretical values predicted by the model equation.
Fig. 7.
Response surface contour for interaction on ethyl esters production between time and molar ratio. Experiments were performed as described in Section 2.
3.8. Influence of water removal
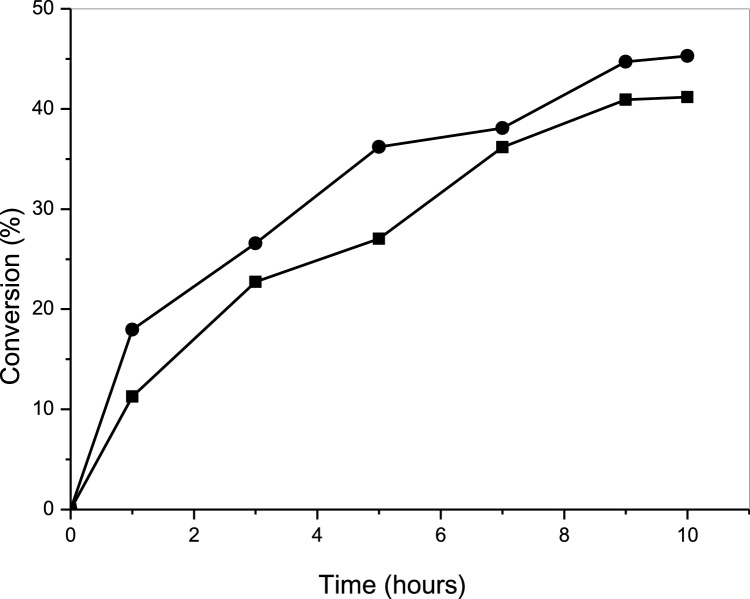
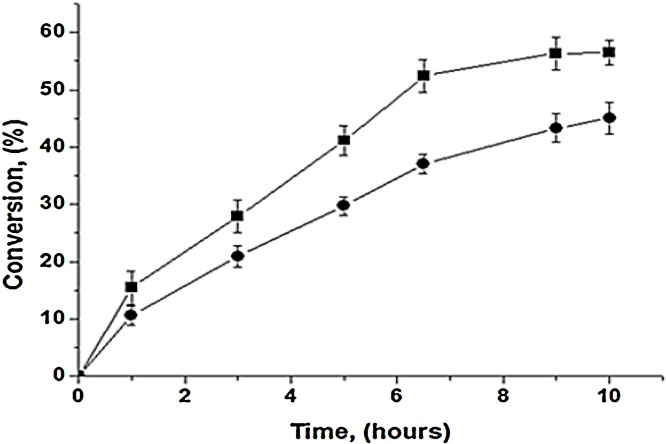
Aiming to improve the conversion, esterification reaction was conducted in the absence and in the presence of zeolite, which has been used for water removal [61], [80]. Experiments were performed using the optimized conditions (29.9 °C, and molar ratio equal to 4.5:1) and reaction was monitored up to 6.5 h (see Fig. 8). It is possible to notice an increase in conversion when the zeolite was used, from 46.9% to 56.5% (more than 16%). This result thus confirms the efficiency of the use of zeolite as an agent to remove water from the reaction medium, so contributing to increase of conversion on ethyl esters.
Fig. 8.
Comparison of conversion values obtained with the use and without the use of zeolite type A. The tests were carried out varying reaction times between 1 and 10 h, with a temperature of 29.9 °C, molar ratio ethanol: oil equal to 4.5: 1. Legends: with zeolite (■) and without zeolite (●). Experiments were performed as described in Section 2.
3.9. Reuse of the immobilized enzyme
The preparation immobilized was reused for five cycles in the performing of synthesis of ethyl oleate in supercritical carbon dioxide, in the conditions of substrate molar ratio, 1: 1 (alcohol–acid); biocatalyst content, 10% (by substrate mass); 45 °C; molecular sieves, 20% (by oleic acid mass). The lipase was vacuum filtered using a vacuum system and washed thoroughly with hexane at the end of each reaction cycle.
The reuse of the immobilized preparation at the optimal conditions is shown in Fig. 9. It was possible to reuse the biocatalyst for 1 cycle keeping 90% of its initial activity, fast losing activity in the following cycles, suggesting the very negative effect of supercritical CO2 on enzyme stability [81], [82], [83], [84]. In fact, some authors suggest the combined use of supercritical media and ionic liquids to prevent these problems [85], [86].
Fig. 9.
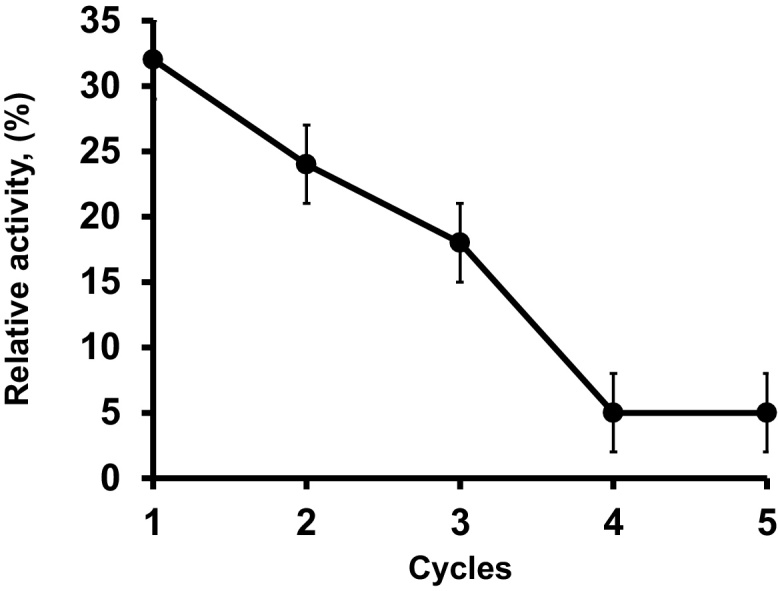
Reuse of the immobilized enzyme in the synthesis of ethyl oleate in supercritical carbon dioxide. Reaction conditions: substrate molar ratio, 1: 1 (alcohol–acid); biocatalyst content, 10% (by substrate mass); 45 °C; molecular sieves, 20% (by substrate mass) oleic acid. Experiments were performed as described in Section 2.
4. Conclusions
The support CH has shown to be very useful to immobilize CALB. This support produces a biocatalyst that is more thermostable and stable in the presence of organic solvents than the soluble CALB (8-folds), or GLX-CALB, while improve the performance of OC-CALB in organic media. The new biocatalyst showed a maximum loading of 20 mg protein/g support, similar to octyl-agarose.
This catalyst was employed in the esterification of ethanol and oleic acid in supercritical CO2. Temperature and molar ration, on the tested domain, present a negligible influence on the enzymatic synthesis of ethyl oleate in using supercritical carbon dioxide. Nevertheless, we suggest the following operational conditions: T = 30 °C and molar ratio (ethanol: fatty acid) of 2.5:1. From experimental design analysis, reaction time and quadratic molar ratio influence the conversion of fatty acid to ethyl oleate, with an optimal operational condition of 46.87% at T = 29.9 °C, molar ratio ethanol: fatty acid equals 4.5:1 and reaction time of 6.5 h. The use of zeolite could enhance the conversion by removing water from the medium. This new strategy to obtain ethyl oleate using a biocatalyst from renewable polymeric matrixes can be considered environmentally benign, low cost, commercially available, and stable. The low stability of the immobilized CALB under the experimental conditions constitutes a break to the implementation of this medium, even although it may have some environmental advantages. Strategies to improve enzyme stability in these conditions are under development in our research group.
Acknowledgments
The financial support by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil), FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico) and MINECO from Spanish Government, (project number CTQ2013-41507-R) is gratefully acknowledged.
References
- 1.Slostowski C., Marre S., Bassat J.-M., Aymonier C. Synthesis of cerium oxide-based nanostructures in near- and supercritical fluids. J. Supercrit. Fluids. 2013;84:89–97. [Google Scholar]
- 2.Brunner G. Supercritical fluids: technology and application to food processing. J. Food Eng. 2005;67:21–33. [Google Scholar]
- 3.Lozano P., Garcia-Verdugo E., Luis S.V., Pucheault M., Vaultier M. (Bio)catalytic continuous flow processes in scCO2 and/or ILs: towards sustainable (Bio)catalytic synthetic platforms. Curr. Org. Synth. 2011;8:810–823. [Google Scholar]
- 4.Filatova E.V., Turova O.V., Kuchurov I.V., Kostenko A.A., Nigmatov A.G., Zlotin S.G. Asymmetric catalytic synthesis of functionalized tetrahydroquinolines in supercritical fluids. J. Supercrit. Fluids. 2016;109:35–42. [Google Scholar]
- 5.Beckman E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids. 2004;28:121–191. [Google Scholar]
- 6.Varma M.N., Deshpande P.A., Madras G. Synthesis of biodiesel in supercritical alcohols and supercritical carbon dioxide. Fuel. 2010;89:1641–1646. [Google Scholar]
- 7.Li F.-W., Suo Q.-L., Hong H.-L., Zhu N., Wang Y.-Q., Guo L.-L. Copper(I)-catalyzed homocoupling reaction of terminal alkynes in supercritical CO2. J. Supercrit. Fluids. 2014;92:70–74. [Google Scholar]
- 8.Erdmenger T., Guerrero-Sanchez C., Vitz J., Hoogenboom R., Schubert U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010;39:3317–33333. doi: 10.1039/b909964f. [DOI] [PubMed] [Google Scholar]
- 9.Kiss M.A., Sefanovits-Bányai É., Tóth Á., Boross L. Extractive synthesis of ethyl-oleate using alginate gel co-entrapped yeast cells and lipase enzyme. Eng. Life Sci. 2004;4:460–464. [Google Scholar]
- 10.Ranganathan S.V., Narasimhan S.L., Muthukumar K. An overview of enzymatic production of biodiesel. Bioresour. Technol. 2008;99:3975–3981. doi: 10.1016/j.biortech.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Ozyilmaz G., Gezer E. Production of aroma esters by immobilized Candida rugosa and porcine pancreatic lipase into calcium alginate gel. J. Mol. Catal. B Enzym. 2010;64:140–145. [Google Scholar]
- 12.Khan N.R., Rathod V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: a review. Process Biochem. 2015;50:1793–1806. [Google Scholar]
- 13.Åkerman C.O., Hagström A.E.V., Mollaahmad M.A., Karlsson S., Hatti-Kaul R. Biolubricant synthesis using immobilised lipase: process optimisation of trimethylolpropane oleate production. Process Biochem. 2011;46:2225–2231. [Google Scholar]
- 14.Pires-Cabral P., da Fonseca M.M.R., Ferreira-Dias S. Esterification activity and operational stability of Candida rugosa lipase immobilized in polyurethane foams in the production of ethyl butyrate. Biochem. Eng. J. 2010;48:246–252. [Google Scholar]
- 15.Taher H., Al-Zuhair S., Al-Marzouqi A.H., Haik Y., Farid M. Enzymatic biodiesel production of microalgae lipids under supercritical carbon dioxide: process optimization and integration. Biochem. Eng. J. 2014;90:103–113. [Google Scholar]
- 16.Dutra Madalozzo A., Sanvido Muniz L., Baron A.M., Piovan L., Alexander Mitchell D., Krieger N. Characterization of an immobilized recombinant lipase from Rhizopus oryzae: synthesis of ethyl-oleate. Biocatal. Agric. Biotechnol. 2014;3:13–19. [Google Scholar]
- 17.Guauque Torres M.P., Foresti M.L., Ferreira M.L. CLEAs of Candida antarctica lipase B (CALB) with a bovine serum albumin (BSA) cofeeder core: study of their catalytic activity. Biochem. Eng. J. 2014;90:36–43. [Google Scholar]
- 18.Jaeger K.-E., Eggert T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002;13:390–397. doi: 10.1016/s0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi Ziarani G., Gholamzadeh P., Asadiatouei P., Lashgari N. The role of Pseudomonas cepacia lipase in the asymmetric synthesis of heterocyclic based compounds. J. Mol. Catal. B Enzym. 2015;122:93–116. [Google Scholar]
- 20.Hasan F., Shah A.A., Hameed A. Industrial applications of microbial lipases. Enzyme Microb. Technol. 2006;39:235–251. [Google Scholar]
- 21.Teena Momsia P.M. A review on microbial lipase-Versatile tool for industrial applications. Intenational J. Life Sci. Biotechnol. Pharma Res. 2013;2:1–9. ( http://new.ijlbpr.com/jlbpradmin/upload/ijlbpr_51d452a2abcec.pdf) [Google Scholar]
- 22.Sharma R., Chisti Y., Banerjee U.C. Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 2001;19:627–662. doi: 10.1016/s0734-9750(01)00086-6. [DOI] [PubMed] [Google Scholar]
- 23.de Souza T.C., de S. Fonseca T., da Costa J.A., Rocha M.V.P., de Mattos M.C., Fernandez-Lafuente R., Gonçalves L.R.B., dos Santos J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida antarctica: application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016;130:58–69. [Google Scholar]
- 24.Lozano P. Enzymes in neoteric solvents: from one-phase to multiphase systems. Green Chem. 2010;12:555. [Google Scholar]
- 25.Sheldon R.A., van Pelt S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev. 2013;42:6223–6235. doi: 10.1039/c3cs60075k. [DOI] [PubMed] [Google Scholar]
- 26.DiCosimo R., McAuliffe J., Poulose A.J., Bohlmann G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013;42:6437. doi: 10.1039/c3cs35506c. [DOI] [PubMed] [Google Scholar]
- 27.Liese A., Hilterhaus L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013;42:6236. doi: 10.1039/c3cs35511j. [DOI] [PubMed] [Google Scholar]
- 28.Mateo C., Palomo J.M., Fernandez-Lorente G., Guisan J.M., Fernandez-Lafuente R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007;40:1451–1463. [Google Scholar]
- 29.Garcia-Galan C., Berenguer-Murcia Á., Fernandez-Lafuente R., Rodrigues R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011;353:2885–2904. [Google Scholar]
- 30.Rodrigues R.C., Ortiz C., Berenguer-Murcia Á., Torres R., Fernández-Lafuente R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013;42:6290–6307. doi: 10.1039/c2cs35231a. [DOI] [PubMed] [Google Scholar]
- 31.Barbosa O., Ortiz C., Berenguer-Murcia Á., Torres R., Rodrigues R.C., Fernandez-Lafuente R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015;33:435–456. doi: 10.1016/j.biotechadv.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 32.dos Santos J.C.S., Barbosa O., Ortiz C., Berenguer-Murcia A., Rodrigues R.C., Fernandez-Lafuente R. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem. 2015;7:2413–2432. [Google Scholar]
- 33.Anderson E.M., Larsson K.M., Kirk O. One biocatalyst-many applications: the use of Candida Antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998;16:181–204. [Google Scholar]
- 34.Kirk O., Christensen M.W. Lipases from Candida antarctica: unique biocatalysts from a unique origin. Org. Process Res. Dev. 2002;6:446–451. [Google Scholar]
- 35.Uppenberg J., Hansen M.T., Patkar S., Jones T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure. 1994;2:293–308. doi: 10.1016/s0969-2126(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 36.Bezerra C.S., de Farias Lemos C.M.G., de Sousa M., Gonçalves L.R.B. Enzyme immobilization onto renewable polymeric matrixes: past, present, and future trends. J. Appl. Polym. Sci. 2015;132:1–15. [Google Scholar]
- 37.Krajewska B. Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb. Technol. 2004;35:126–139. [Google Scholar]
- 38.Silva J.A., Macedo G.P., Rodrigues D.S., Giordano R.L.C., Gonçalves L.R.B. Immobilization of Candida antarctica lipase B by covalent attachment on chitosan-based hydrogels using different support activation strategies. Biochem. Eng. J. 2012;60:16–24. [Google Scholar]
- 39.do N., Neta A.S., dos Santos J.C.S., de S., Sancho O., Rodrigues S., Gonçalves L.R.B., Rodrigues L.R. Enzymatic synthesis of sugar esters and their potential as surface-active stabilizers of coconut milk emulsions. Food Hydrocoll. 2012;27:324–331. [Google Scholar]
- 40.Schiffman J.D., Schauer C.L. One-step electrospinning of cross-linked Chitosan fibers. Biomacromolecules. 2007;8:2665–2667. doi: 10.1021/bm7006983. [DOI] [PubMed] [Google Scholar]
- 41.Barbosa O., Ortiz C., Berenguer-Murcia Á., Torres R., Rodrigues R.C., Fernandez-Lafuente R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014;4:1583. [Google Scholar]
- 42.Barbosa O., Torres R., Ortiz C., Fernandez-Lafuente R. Versatility of glutaraldehyde to immobilize lipases: effect of the immobilization protocol on the properties of lipase B from Candida antarctica. Process Biochem. 2012;47:1220–1227. [Google Scholar]
- 43.Manoel E.A., dos Santos J.C.S., Freire D.M.G., Rueda N., Fernandez-Lafuente R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015;71:53–57. doi: 10.1016/j.enzmictec.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Lafuente R., Armisén P., Sabuquillo P., Fernández-Lorente G., Guisán J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids. 1998;93:185–197. doi: 10.1016/s0009-3084(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 45.Mateo C., Abian O., Bernedo M., Cuenca E., Fuentes M., Fernandez-Lorente G. Some special features of glyoxyl supports to immobilize proteins. Enzyme Microb. Technol. 2005;37:456–462. [Google Scholar]
- 46.Mateo C., Palomo J.M., Fuentes M., Betancor L., Grazu V., López-Gallego F. Glyoxyl agarose: a fully inert and hydrophilic support for immobilization and high stabilization of proteins. Enzyme Microb. Technol. 2006;39:274–280. [Google Scholar]
- 47.Rocha M.V.P., de Matos L.J.B.L., de Lima L.P., Figueiredo P.M. da S., Lucena I.L., Fernandes F.A.N. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014;167:343–348. doi: 10.1016/j.biortech.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Santos F.F.P., Malveira J.Q., Cruz M.G.A., Fernandes F.A.N. Production of biodiesel by ultrasound assisted esterification of Oreochromis niloticus oil. Fuel. 2010;89:275–279. [Google Scholar]
- 49.Myers R.H., Montgomery D.C., Anderson-Cook C. Response surface methodology: process and product optimization using designed experiments. Wiley Ser. Probab. Stat. 2009;704 [Google Scholar]
- 50.Rodrigues D.S., Mendes A.A., Adriano W.S., Gonçalves L.R.B., Giordano R.L.C. Multipoint covalent immobilization of microbial lipase on chitosan and agarose activated by different methods. J. Mol. Catal. B Enzym. 2008;51:100–109. [Google Scholar]
- 51.Guisán J.M. Aldehyde-agarose gels as activated supports for immobilization-stabilization of enzymes. Enzyme Microb. Technol. 1988;10:375–382. [Google Scholar]
- 52.Fernandez-Lafuente R., Rosell C.M., Rodriguez V., Santana C., Soler G., Bastida A. Preparation of activated supports containing low pK amino groups. A new tool for protein immobilization via the carboxyl coupling method. Enzyme Microb. Technol. 1993;15:546–550. doi: 10.1016/0141-0229(93)90016-u. [DOI] [PubMed] [Google Scholar]
- 53.F. Schierbaum, Whistler, Roy. L., undM. L. Wolfrom: Methods in Carbohydrate Chemistry. Vol. II. Reactions of carbohydrates. Herausgeg. unter Assistenz v.James N. BeMiller. Academic Press, New York und London, 1963. 572 Seiten, Gr.−8°, Ganzleinen, Preis $ 19,50, Starch − Stärke. 15 (1963) 423-423. 10.1002/star.19630151111.
- 54.Rueda N., dos Santos J.C.S., Torres R., Ortiz C., Barbosa O., Fernandez-Lafuente R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015;5:11212–11222. [Google Scholar]
- 55.Bhatnagar T., Boutaiba S., Hacene H., Cayol J.-L., Fardeau M.-L., Ollivier B. Lipolytic activity from Halobacteria: screening and hydrolase production. FEMS Microbiol. Lett. 2005;248:133–140. doi: 10.1016/j.femsle.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 56.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 57.Sadana A., Henley J.P. Analysis of enzyme deactivations by a series-type mechanism: influence of modification on the activity and stability of enzymes. Ann. N. Y. Acad. Sci. 1987;501:73–79. doi: 10.1111/j.1749-6632.1987.tb45687.x. ( http://www.scopus.com/inward/record.url?eid=2-s2.0-0023077096&partnerID=tZOtx3y1) [DOI] [PubMed] [Google Scholar]
- 58.Köse Ö., Tüter M., Aksoy H.A. Immobilized Candida antarctica lipase-catalyzed alcoholysis of cotton seed oil in a solvent-free medium. Bioresour. Technol. 2002;83:125–129. doi: 10.1016/s0960-8524(01)00203-6. [DOI] [PubMed] [Google Scholar]
- 59.Rathore V., Madras G. Synthesis of biodiesel from edible and non-edible oils in supercritical alcohols and enzymatic synthesis in supercritical carbon dioxide. Fuel. 2007;86:2650–2659. [Google Scholar]
- 60.Lozano P., Víllora G., Gómez D., Gayo A.B., Sánchez-Conesa J.A., Rubio M. Membrane reactor with immobilized Candida antarctica lipase B for ester synthesis in supercritical carbon dioxide. J. Supercrit. Fluids. 2004;29:121–128. [Google Scholar]
- 61.Oliveira J.F.G., Lucena I.L., Saboya R.M.A., Rodrigues M.L., Torres A.E.B., Fernandes F.A.N. Biodiesel production from waste coconut oil by esterification with ethanol: the effect of water removal by adsorption. Renew. Energy. 2010;35:2581–2584. [Google Scholar]
- 62.AOCS, Official Methods and Recommended Practices of the AOCS, 6th Edition, 2nd Printing, in: Determ. Cis-, Trans-, Satur. Monounsaturated Polyunsaturated Fat. Acids Veg. or Non-Ruminant Anim. Oils Fats by Capill. GLC (Ce 1h-05), AOCS, 2009.
- 63.American Society for Testing and Materials Annual, Annual Book of ASTM Standards., Philadelphia, PA, USA. Sec., 4 (1992) 04-08. 10.1520/C1530_C1530M-04R10E01.
- 64.Mendes A.A., de Castro H.F., de S. Rodrigues D., Adriano W.S., Tardioli P.W., Mammarella E.J. Multipoint covalent immobilization of lipase on chitosan hybrid hydrogels: influence of the polyelectrolyte complex type and chemical modification on the catalytic properties of the biocatalysts. J. Ind. Microbiol. Biotechnol. 2011;38:1055–1066. doi: 10.1007/s10295-010-0880-9. [DOI] [PubMed] [Google Scholar]
- 65.Jaeger K.E., Ransac S., Koch H.B., Ferrato F., Dijkstra B.W. Topological characterization and modeling of the 3D structure of lipase from Pseudomonas aeruginosa. FEBS Lett. 1993;332:143–149. doi: 10.1016/0014-5793(93)80501-k. [DOI] [PubMed] [Google Scholar]
- 66.Cygler M., Schrag J.D. Structure and conformational flexibility of Candida rugosa lipase. Biochim. Biophys. Acta. 1999;1441:205–214. doi: 10.1016/s1388-1981(99)00152-3. http://www.ncbi.nlm.nih.gov/pubmed/10570248, (Accessed 12 July 2015) [DOI] [PubMed] [Google Scholar]
- 67.dos Santos J.C.S., Rueda N., Torres R., Barbosa O., Gonçalves L.R.B., Fernandez-Lafuente R. Evaluation of divinylsulfone activated agarose to immobilize lipases and to tune their catalytic properties. Process Biochem. 2015;50:918–927. [Google Scholar]
- 68.dos Santos J.C.S., Rueda N., Gonçalves L.R.B., Fernandez-Lafuente R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzyme Microb. Technol. 2015;77:1–7. doi: 10.1016/j.enzmictec.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez A., Cruz J., Rueda N., dos Santos J.C.S., Torres R., Ortiz C. Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv. 2016;6:27329–27334. [Google Scholar]
- 70.Hernandez K., Fernandez-Lafuente R. Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011;48:107–122. doi: 10.1016/j.enzmictec.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Klibanov A.M. Enzyme stabilization by immobilization. Anal. Biochem. 1979;93:1–25. http://www.scopus.com/inward/record.url?eid=2-s2.0-0018408815&partnerID=tZOtx3y1, (Accessed 7 November 2014). [PubMed] [Google Scholar]
- 72.Gao Y., Tan T., Nie K., Wang F. Immobilization of lipase on macroporous resin and its application in synthesis of biodiesel in low aqueous media. Chin. J. Biotechnol. 2006;22:114–118. doi: 10.1016/s1872-2075(06)60008-3. [DOI] [PubMed] [Google Scholar]
- 73.Verdasco-Martín C.M., Villalba M., dos Santos J.C.S., Tobajas M., Fernandez-Lafuente R., Otero C. Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Biochem. Eng. J. 2016;111:75–86. [Google Scholar]
- 74.Villalba M., Verdasco-Martín C.M., dos Santos J.C.S., Fernandez-Lafuente R., Otero C. Operational stabilities of different chemical derivatives of Novozym 435 in an alcoholysis reaction. Enzyme Microb. Technol. 2016;90:35–44. doi: 10.1016/j.enzmictec.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 75.Madras G., Kolluru C., Kumar R. Synthesis of biodiesel in supercritical fluids. Fuel. 2004;83:2029–2033. [Google Scholar]
- 76.jiang X.W.W.A.N.G., HUANG Q.J.D., HUANG F.H. Lipase-catalyzed production of biodiesel from high acid value waste oil using ultrasonic assistant. Chin. J. Biotechnol. 2007;23:1121–1128. doi: 10.1016/s1872-2075(07)60067-3. [DOI] [PubMed] [Google Scholar]
- 77.Noureddini H., Gao X., Philkana R.S. Immobilized Pseudomonas cepacia lipase for biodiesel fuel production from soybean oil. Bioresour. Technol. 2005;96:769–777. doi: 10.1016/j.biortech.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 78.Romero M.D., Calvo L., Alba C., Daneshfar A., Ghaziaskar H.S. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in n-hexane. Enzyme Microb. Technol. 2005;37:42–48. [Google Scholar]
- 79.Chowdary G.V., Ramesh M.N., Prapulla S.G. Enzymic synthesis of isoamyl isovalerate using immobilized lipase from Rhizomucor miehei: a multivariate analysis. Process Biochem. 2000;36:331–339. [Google Scholar]
- 80.Nijhuis T.A., Beers A.E.W., Kapteijn F., Moulijn J.A. Water removal by reactive stripping for a solid-acid catalyzed esterification in a monolithic reactor. Chem. Eng. Sci. 2002;57:1627–1632. [Google Scholar]
- 81.Wimmer Z., Zarevucka M. A review on the effects of supercritical carbon dioxide on enzyme activity. Int. J. Mol. Sci. 2010;11:233–253. doi: 10.3390/ijms11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mesiano A.J., Beckman E.J., Russell A.J. Supercritical biocatalysis. Chem. Rev. 1999;99:623–634. doi: 10.1021/cr970040u. [DOI] [PubMed] [Google Scholar]
- 83.Romero M.D., Calvo L., Alba C., Habulin M., Primožič M., Knez Ž. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in supercritical carbon dioxide. J. Supercrit. Fluids. 2005;33:77–84. [Google Scholar]
- 84.Rezaei K., Temelli F., Jenab E. Effects of pressure and temperature on enzymatic reactions in supercritical fluids. Biotechnol. Adv. 2007;25:272–280. doi: 10.1016/j.biotechadv.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Bernal J.M., Lozano P., García-Verdugo E., Burguete M.I., Sánchez-Gómez G., López-López G. Supercritical synthesis of biodiesel. Molecules. 2012;17:8696–8719. doi: 10.3390/molecules17078696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lozano P., García-Verdugo E., Bernal J.M., Izquierdo D.F., Burguete M.I., Sánchez-Gómez G. Immobilised lipase on structured supports containing covalently attached ionic liquids for the continuous synthesis of biodiesel in scCO 2. ChemSusChem. 2012;5:790–798. doi: 10.1002/cssc.201100692. [DOI] [PubMed] [Google Scholar]