Abstract
Adipose tissue (AT) is a well-established target of growth hormone (GH) and is altered in clinical conditions associated with excess, deficiency and absence of GH action. Due to the difficulty in collecting AT from clinical populations, genetically modified mice have been useful in better understanding how GH affects this tissue. Recent findings in mice would suggest that the impact of GH on AT is beyond alterations of lipolysis, lipogenesis or proliferation/ differentiation. AT depot-specific alterations in immune cells, extracellular matrix, adipokines, and senescence indicate an expanded role for GH in AT physiology. This mouse data will guide additional studies necessary to evaluate the therapeutic potential and safety of GH for conditions associated with altering AT, such as obesity. In this review, we introduce several relatively new intricacies of GH’s effect on AT, focusing on recent studies in mice. Finally, we summarize the clinical implications of these findings.
Keywords: growth hormone, adipose tissue, Laron syndrome, acromegaly, growth hormone deficiency, obesity, bGH mice, GHR−/− mice, GHA mice, Ames dwarf
Adipose tissue
Our understanding of adipose tissue (AT) has evolved over the past few decades, revealing a much more complex and interactive tissue than once thought. Traditionally, AT was believed to serve mainly as an energy reservoir for triglycerides, armed to release or store energy in response to afferent signals. As such, many hormone receptors, such as the growth hormone receptor (GHR), insulin receptors and B-adrenergic receptors, are abundantly expressed on AT. More recent studies reveal additional characteristics of AT important to its function. For example, AT contains abundant non-adipocyte cells that contribute to tissue function, an extracellular matrix (ECM) and vascularity that regulate its expandability, an inherent endocrine role through the production and secretion of adipokines and a capacity to remodel and take on a thermogenic potential. Furthermore, AT depots from distinct anatomic locations have been shown to possess different properties and metabolic features. For these reasons, it is important to study AT from different sites, at the cellular as well as whole tissue level, and to consider other factors such as age and diet when describing the impact of a hormone, such as growth hormone (GH), on this tissue.
Properties of Adipose tissue
AT is a loose connective tissue consisting of adipocytes as well as a number of other cells collectively referred to as the stromal vascular fraction (SVF). SVF cells include endothelial cells, fibroblasts, preadipocytes, mesenchymal stem cells [1], and cells of the innate and adaptive immune systems. In total cell number, these nonadipocyte cells within AT can predominate and have been shown to be somewhat dynamic, depending on the state of the tissue [2, 3]. In particular, the diversity of the immune cells is now better appreciated and includes the more abundant macrophage population (of varying activation state and antigenicity), but also various T cell populations, natural killer (NK) cells, dendritic cells, B cells, eosinophils and mast cells [2, 4]. The shift from a lean to an obese state is accompanied by dynamic shift in these immune cells, which includes a loss of T regulatory cells [5] and eosinophils, but increases in neutrophils, specific T cell subsets, B cells, dendritic, NK and mast cells [6–8]. Besides infiltrating cells, SVF cells resident to AT, such as preadipocytes, can radically change in phenotype or function. Examples of this include the capacity for the preadipocyte, which can account for 15–50% of total cells in AT, to become a mature adipocyte as well as the tendency of preadipocytes to senescence with advancing age [9]. Collectively, the cell types and their functional state have a profound effect on the microenvironment and function of AT.
Furthermore, AT is also not a homogeneous tissue. That is, there are depot specific differences within an organism, and as a result, AT depots are sometimes considered mini-organs because of how they differ in secretory profile, function, and structure [10]. The factors, such as GH, that control these differences are of high significance since the regional distribution of AT is strongly associated with metabolic disorders, with visceral AT being most problematic. This heterogeneity is, in part, due to the different cellular composition, as noted in the previous paragraph but also influenced by other structural factors such as neural innervation, lymphatics or blood vessels within the tissue. For example, in the mesenteric depots, the lymphatics and lymph nodes are pronounced providing a means to transport ingested fat, which results in exposure of cells in this depot to the subset of exogenous fats that may contribute to a unique secretory profile [11]. There are also marked differences in the types of immune cells that infiltrate the tissue and the expandability of the individual depots. While the cause of depot specific differences could be structural or cellular, there is also evidence of genetic and epigenetic differences depot to depot [12]. While the factors responsible for establishing and maintaining depot specific differences are not fully elucidated, these data illustrate the need to sample multiple depots to understand how a hormone influences AT and to be cautious in extrapolating the findings from a single depot to the tissue as a whole.
Contributing to the complexity of AT are several other relatively recent advances. First, there are distinct classes of AT (brown/BAT and white/WAT) as well as adipocytes including white, brown and beige/brite adipocytes. These different adipocyte classes reside in specific depots and have very distinct functions with white adipocytes mainly functioning to store energy and the other two having more thermogenic capacity [reviewed in 13]. Increasing beige or brown adipocytes in response to exogenous or endogenous signals is associated with significant improvements in glucose and lipid metabolism [13]; as a result, these signals have garnered significant attention as a target for therapeutic intervention for obesity. Second, the extracellular matrix (ECM) plays a critical role in the structure and function of AT. In WAT, the ECM proteins, such as collagen VI, elastin, laminin, and fibronectin that crosslink with collagen, are needed for restructuring to accommodate increases and decreases in triglyceride storage [14, 15]. There are many fibrous proteins involved in the structure of the ECM, but collagen VI in particular plays an important role [16]. Healthy AT expansion appears to occur when the extracellular matrix is flexible; however, an imbalance in ECM synthesis and degradation may promote fibrosis in the tissue, limiting proper growth, contributing to tissue inflammation and dysfunction [17]. Fibrosis and ECM remodeling of AT has been implicated in metabolic dysregulation in many recent animal and human studies. Third, even the processes that control lipolysis, which was the focus of much of the earlier work on AT, have additional layers of complexity. The discovery of adipose triglyceride lipase (ATGL) in 2004 uncovered the main lipase involved in the triglyceride (TG) lipolysis while the better understood hormone sensitive lipase was determined to be more important for lipolysis of diglycerides. Furthermore, the perilipin family of proteins, along with other lipid droplet associated proteins, coat the surface of lipid droplets and actively regulate basal and stimulated lipolysis [18]. Fourth, while not as recent, AT is now acknowledged as an active endocrine tissue actively secreting adipokines. Classic examples of adipokines, such as leptin and adiponectin, have been studied for years and continue to be ascribed new physiological functions. For example, leptin has been shown to regulate both the number of somatotrophs as well as the secretion of GH [19]. In addition, newer adipokines are routinely uncovered with recent examples including chemerin [20, 21] and Metrnl/subfatin [22, 23], as well as the paracrine/autocrine secretions of immune and senescent cells (the so called senescence secretory phenotype or SASP [24]). While this is not an exhaustive list, these examples illustrate a relatively recent evolution in our understanding of the molecular/cellular complexity of this once simple tissue.
Growth Hormone
Growth hormone (GH) is a pleiotropic protein hormone that promotes growth, as its name implies, through the alteration of nutrient metabolism and partitioning. Overall, GH has anabolic effects on almost all tissues promoting the growth of that tissue. However, GH has a dramatic effect on AT. In AT, GH promotes the release of stored energy by increasing lipolysis and decreasing lipogenesis as well as influencing proliferation and differentiation of the preadipocytes [25]. Thus, GH can drastically alter lipid metabolism and decrease AT mass.
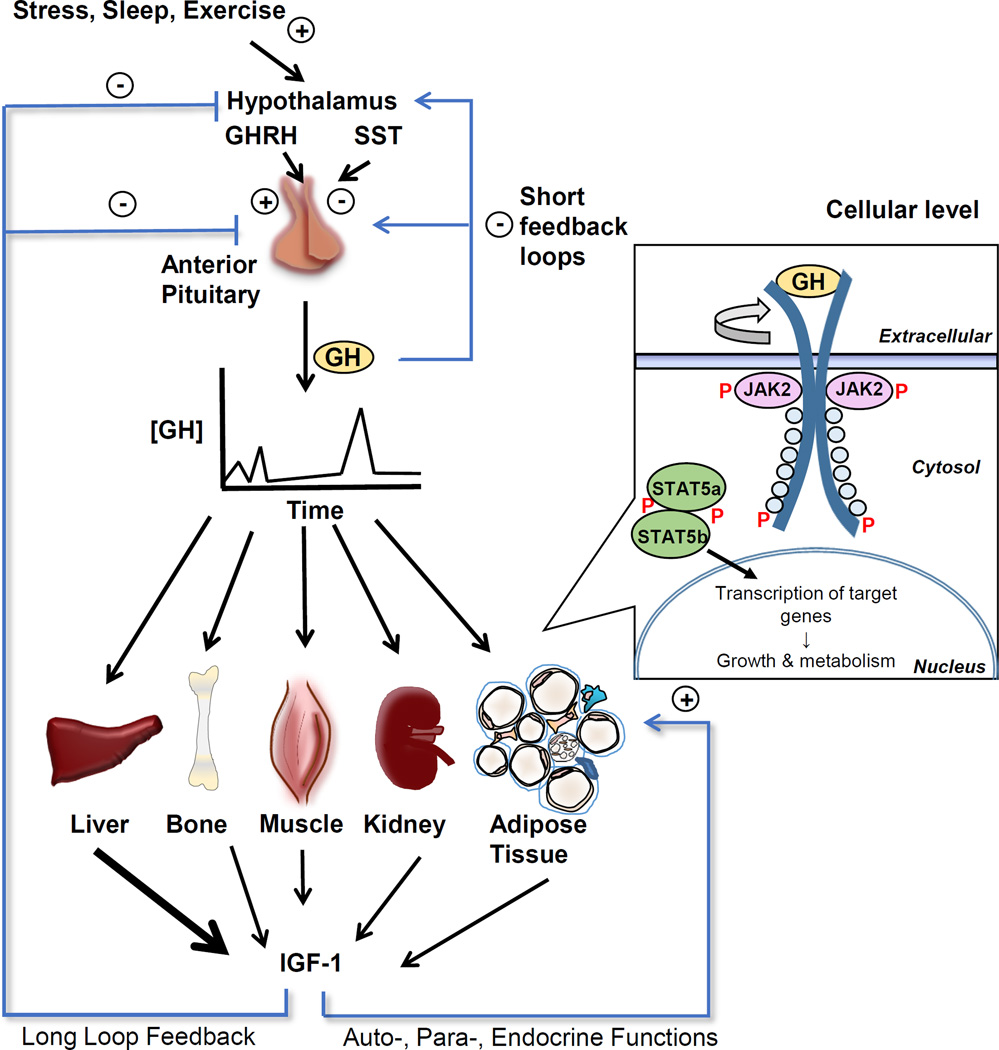
As illustrated in Figure 1, GH is secreted from the somatrophs of the anterior pituitary in a pulsatile manner in response mainly to the balance of GH releasing hormone and somatostatin. GH action is initiated with GH binding to the growth hormone receptor (GHR), a receptor which is ubiquitously expressed in most tissues including muscle, adipose, liver, kidney, heart, intestine, and lung. Proper binding of GH to its predimerized cognate receptor triggers a conformational change leading to transphosphorylation and activation of associated tyrosine kinases (JAK2). Activated JAK2 then phosphorylates tyrosines in the cytoplasmic domain of GHR, which results in the recruitment of additional intracellular signaling molecules [26]. As a result, signal transduction occurs though several pathways. One of the main pathways is via Signal Transducer and Activator of Transcription 5 (STAT5) a and b, which are phosphorylated and dimerize, allowing them to bind to STAT responsive elements of promoter/enhancer regions of genes related to metabolism, body growth, and insulin like growth factor-1 (IGF-1) production [26]. JAK2-independent signaling pathways are also activated utilizing the Src family kinases rather than JAK2 to stimulate p44/42 mitogen-activated protein kinase (MAPK) activity and the transcription activator Elk-1.
Figure 1. Target tissues and intracellular signaling of growth hormone (GH).
GH is released in a pulsatile fashion from the anterior pituitary. Its release is triggered by growth hormone releasing hormone (GHRH) from the hypothalamus while somatostatin (SST) inhibits GH release. GH action is induced by binding to the predimerized growth hormone receptor (GHR) on target tissues such as liver, bone, kidney, muscle and adipose tissue. Binding induces a rotation in the dimerized receptor resulting in a realigning of the associated Janus kinase 2 (JAK2) molecules, triggering autophosphorylation of tyrosine residues of JAK2 and then phosphorylation of the cytoplasmic domain of GHR. GH-activated JAK2 also phosphorylates and activates multiple signaling proteins and pathways including Signal Transducer and Activator of Transcription 5, IRS and PI-3 kinase and MAPK. The binding of GH to GHR may also activate Src tyrosine kinase, initiating other signaling pathways. Upon phosphorylation, STAT5 dimerizes and migrates to the nucleus where it increases transcription of a variety of genes involved in growth and metabolism, including IGF-1. IGF-1 acts in a negative feedback manner to decrease GH secretion and also acts on target tissues in autocrine/paracrine/endocrine manner. With kind permission from Springer Science+Business Media: Living Large: What Mouse Models Reveal about Growth Hormone and Obesity. 2015. Berryman DE, Householder L, Lesende V, List EO, Kopchick JJ. Figure 4.2.
As noted, one outcome of the GH-GHR signaling cascade is the production of IGF-1. IGF-1 is a critical growth factor released mainly from the liver but also extrahepatic tissues. Because of the tight association between GH induced signaling and IGF-1 production, it is often difficult to discern the effects of GH directly versus that of IGF-1. As will be described below, while there are some animal models that have allowed one to dissect the effect of GH versus IGF-1 (e.g. LiGHRKO), generally these two hormones are impacted in a coordinated manner.
Clinical Conditions Caused by Absence, Decrease, or Excess in GH action
The dramatic effects of GH on the AT can be observed in clinical conditions related to the absence, decrease, or excess of GH action. Laron Syndrome (LS), which is an autosomal recessive disorder characterized by insensitivity to GH, is caused by mutations to genes that encode molecules in the GHR signaling pathway with the majority of cases occurring in the GHR gene itself [27]. Individuals with LS have notable gains in fat mass yet are protected from cancer [28] and, at least in an Ecuadorian cohort, retain enhanced insulin sensitivity with a resistance to diabetes [29, 30]. LS also results in altered circulating adipokine levels with both high molecular weight and total adiponectin levels increased while leptin levels tend to be elevated in some but not all studies [30–32]. Besides LS, the well characterized Itabaianinha cohort of dwarfs in Brazil provide data regarding genetic isolated GH deficiency due to homozygous GH-releasing hormone receptor (GHRHR) mutations. This population has been reported to have elevated central or visceral obesity [33] or no significant alterations in fat mass [34] along with no change in lifespan [35] or no apparent protection from diabetes [36].
Likewise, patients with GH deficiency (GHD) exhibit marked increases in fat mass, primarily due to increased adipocyte volume, whereas cell numbers are lower [37]. GHD can result from genetic defects, traumatic brain injury, irradiation, or organ lesions, such as a pituitary or hypothalamic tumors, which cause a destruction of somatotrophs or GHRH-producing neurons. The clinical manifestations of GHD depend, in part, on whether it develops during childhood or during adult life. The patients have altered body composition with markedly decreased lean mass and increased fat mass, which is most pronounced in the abdominal area. They suffer from hypoglycemia and dyslipidemia and have altered adipokine protein expression pattern (usually elevated leptin, adiponectin and visfatin) [37–39].
At the opposite end of the spectrum is acromegaly, a condition of GH hypersecretion usually caused by a pituitary adenoma [40]. Acromegaly confers various physical and metabolic consequences, such as enlarged hands, feet, and facial bones, and insulin resistance [40] Relevant to this review, AT mass is dramatically reduced with acromegaly in both subQ and visceral depots with an unusual ectopic fat deposition in intermuscular AT [41]. As with LS and GHD, acromegaly is accompanied by altered adipokines, including lower leptin and adiponectin and higher visfatin levels [42, 43]. In fact, the important role of AT in disease progression is highlighted by recent evidence that visfatin may serve as an index of disease activity as well as AT dysfunction [42].
Because of the difficulty in collection AT samples from distinct depots from these clinical populations, many have turned to the use of animal models that closely mimic these conditions to better understand GH’s impact on AT. While there are obvious differences in mouse and man, these mouse lines have provided clues as to how GH may alter this tissue at the molecular or cellular level. These mouse lines will be described below with particular attention given to the newer complexities of AT alluded to above. Collectively, the findings from clinical studies and mouse lines have confirmed that GH has profound effects on AT, its effects are depot dependent, and GH impacts more than the lipolytic, lipogenic, and proliferative processes.
AT in mice with alterations in GH action
Many different mouse lines are available to better understand the relationship between GH and AT physiology. These different mice can be generally classified as having increased or decreased GH action. A summary of those that will be discussed in this review and their phenotype is outlined in Table 1; the mice are listed in order from no GH action to increased GH action. It is important to note that Ames and Snell dwarf mice have mutations that interfere with the proper development of the anterior pituitary and thus have primary deficiencies in GH, prolactin and thyroid-stimulating hormone. New technologies for genetic manipulation of mice has provided other means to manipulate GH action conditionally, usually either temporally or tissue-specifically to study its role in AT. For example, Luque et al. [44] describe an adult GH deficient mouse in which they ablate the somatotrophs with a Cre system that utilizes diphtheria toxin thus attempting to eliminate some of the confounding effects due to the dwarf size of the GHR−/− mice. Circulating levels of GH and IGF-1 are decreased but are still detectable in these mice. Interestingly, even partial GH deficiency, as shown in these mice, has a dramatic impact on AT. In addition, the effects of tissue-specific disruption of GHR have been reported by several groups using cre/lox technology. Only the liver- [45], adipose- [46] and macrophage- [47] specific disruptions of GHR will be discussed because of their relevance to this review.
Table 1.
Phenotypic comparison of genetically modified mice with altered GH action.
| Variable | GHR−/− [49, 50] |
Ames[51, 63, 88]* |
Snell[48, 63]* |
Lit/Lit[89] | GHA[49, 50] | AOiGHD[44] | FaGHRKO[46] | MacGHRKO[73 ] |
LiGHRKO[45] | bGH[50, 52] |
|---|---|---|---|---|---|---|---|---|---|---|
| GH action | absent | ↓↓ | ↓↓ | ↓↓ | ↓ | ↓ (adult) | ↔ | ↓ | ↑ | ↑↑ |
| Defect | Gene disrupti on of GHR gene |
homozygo us recessive mutation in prop-1 gene |
homozygo us recessive mutation in the Pit- 1 gene |
GH-defic ient due to recessive mutation in the Ghrhr gene |
Transgenic for GHR antagonist gene |
adult GH deficient mouse |
Fat-specific gene disruption of GHR gene |
Macrophage specific gene disruption of GHR gene |
Liver-specific gene disruption of the GHR gene |
Transgenic for bovine GH |
| GH | ↑ | ↓↓ | ↓↓ | ↓↓ | ↑ | ↓ | ↔ | ↑ | ↑↑ | |
| IGF-1 | ↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓ | ↓ | ↔/↑ | ↓ | ↑↑ | |
|
Body weight and growth |
↓↓ | ↓↓ | ↓↓ | ↓↓ | ↓ | ↔ | ↑ | ↔ | ↓ | ↑ |
|
Insulin sensitivity |
↑ | ↑ | ↑ | ↑ | ↔ (young) ↓(older) |
↑ | ↔ | ↓ | ↓ | ↓ |
| Lifespan | ↑ | ↑ | ↑ | ↑ | ↔ | No data | No data | ↔ | ↓ | |
|
Fat mass (Depot Differences) |
↑↑ (subQ mainly) |
↑ | ↑ (subQ mainly) |
↑ | ↑↑↑ (subQ mainly) |
↑ (subQ & retro) |
↑↑↑ (all depots) |
↑ (Epi) | ↑(young) ↓(older) (all depots) |
↑(young) ↓(older) (all depots) |
| Adipocyte Size | ↑ | ↓ (Epi) | No data | ↑ | No data | ↑ | ↑ | ↔ | ↓ | |
|
Adipokine Leptin Adiponectin Resistin |
↑ ↑↑ ↑ |
↔ ↑ No data |
↑ ↑ No data |
↔/↑ ↑ No data |
↑↑ ↑ ↑ |
↑ ↔ No data |
↔/↑ ↔/↓ ↔ |
No data No data No data |
↑(female) ↑ ↑ |
↓ ↓ ↓ |
| Senescence | ↓ | ↓ | No data | No data | ↔ | No data | No data | No data | No data | ↑ |
Ames dwarfs and Snell dwarf mice are deficient in GH, PRL, and TSH
As shown in Table 1, absence, reduction, and excess of GH-induced signaling has profound effects on adiposity, which is age- and depot-dependent. Mice with reductions in GH action are relatively obese throughout life [48, 49]. However, the increased adiposity in several of these lines, such as GH receptor gene distupted (GHR−/−), dwarf Lit/Lit, GH antagonist transgenic (GHA), Adult onset inducible GHD (AOiGHD), Ames dwarf and Snell dwarf mice, is not uniform with the subQ depot disproportionately enlarged and with minimal changes in the epididymal fat pad [44, 48, 50–52]. Because these mice have preferential enlargement of subQ AT and, as subQ AT is thought to be “healthier” than other adipose depots, it is tempting to speculate that the subQ enlargement is in part responsible for the favorable metabolic phenotype of these long-lived dwarf mice. However, there is also evidence of a benefit for the other fat pads in these dwarf mice. For example, removal of the same epididymal and retroperitoneal fat pads in GHR−/− and Ames dwarf mice significantly decreases circulating adiponectin and impairs glucose metabolism [53]. This further supports an inherent functional difference of these depots in the long-lived mice.
GHA transgenic mice are worthy of additional discussion as they are an interesting exception to the trend of increased lifespan and improved metabolic profile noted for the other dwarf lines. GHA mice have dramatic increases in the subQ depot at all ages, similar to the other lines, but eventually all depots become enlarged with advancing age [52]. The age at which the non-subQ depots increase corresponds with the transition to a more insulin resistant state, presumably a result of the marked adiposity gains. The more dramatic gains in fat mass and loss of insulin sensitivity likely counterbalance any positive effect that GH reduction may have on lifespan in these mice.
bGH transgenic mice show a change in AT as a function of age. More specifically, AT mass is increased at younger ages, but is dramatically reduced compared to controls with advancing age [54]. The depot differences in AT mass are not as apparent in bGH as all depots appear to be similarly decreased. However, specific molecular and cellular properties of the AT depots in bGH mice are still evident and become more dramatic with advancing age, as we will discuss in more detail below.
Metabolic effects of GH in adipose tissue
It is well accepted that GH decreases fat mass by stimulating lipolysis, reducing glucose uptake by counteracting insulin action thus inhibiting lipogenesis [25]. GH has been known to promote lipolysis via increasing hormone sensitive lipase (HSL) activity for many years [25, 55, 56]. However, our understanding of lipolysis has evolved and the direct impact of GH on the more recent key players in lipolysis, such as adipose tissue triglyceride lipase (ATGL) and perilipin, has not been systematically studied in whole animal models. GH likely alters these other molecules as well to promote lipolysis as GH increases the expression of ATGL in primary adipocytes [57] and perilipin and ATGL are increased in human omental AT explants treated with GH and dexamethasone (although though there is no significant effect of GH alone in this experimental system) [58]. Evaluating these key lipolytic proteins in the mouse lines would be of great interest.
GH also alters fat mass by influencing preadipocyte differentiation and proliferation although the results are mixed with some studies showing an increase, others a decrease and some pointing to IGF-1 as the main hormone responsible for this effect [59]. Importantly, there is evidence that the role of GH on these processes is depot dependent. For example, a difference in proliferation and differentiation potential has been reported for periovarian but not subQ depots of GHR−/− mice [60]. Likewise, in a recent study, isolated AT mesenchymal stem cells from GHR−/− mice are shown to have increased adipogenesis potential only in the subQ depot; this finding could partially explain the disproportionate increase in subQ fat in these mice. This same study also showed that GH’s ability to impair adipogenesis in bGH mice may involve activation of the Wnt/β-catenin signaling pathway [61]. Interestingly, some of the earlier studies focused on cell lines or primary cultures derived from a single depot [59], which may contribute to the discrepancy in the literature.
GH and endocrine properties of AT
GH greatly alters the endocrine output of AT. In general, leptin, adiponectin, and resistin are all reduced with increased GH action, which may be due to the direct action of GH, the indirect action of IGF-1, or a consequence of other features of these mice such as their reduction in fat mass. In contrast, these hormones are generally elevated in the mice with reduced GH action such as the GHR−/− mice [54, 62–64]. Evidence for a direct effect of GH on the expression of adiponectin expression has been provided by cell culture studies in which GH treatment of differentiated 3T3-L1 adipocytes results in a decrease in adiponectin levels [65].
A better understanding of the role of GH on the endocrine properties of AT have been provided by the fat- and liver specific gene-disrupted mouse lines (FaGHRKO and LiGHRKO, respectively) in which these adipokines are distinctly regulated and different from what has been reported with global GHR gene disruption (GHR−/− mice). FaGHRKO mice have higher levels of leptin in accordance with their extreme obesity and similar to the mice with global GHR disruption (GHR−/−) but low and normal levels of adiponectin and resistin, respectively [46]. This suggests that that the mass of AT itself or the action of GH on AT is not the major driver of at least adiponectin and resistin production, but indicate that there are other factor besides GH that influence the secretion pattern of these adipokines from AT. The levels of adipokines reported in the LiGHRKO mice would suggest that the liver plays a central role in altering these adipokines in AT. That is, the adipokine profile of LiGHRKO mice closely resembles that of the global GHR−/− mice [45].
GH and senescence of AT
Cellular senescence describes the process in which division competent cells enter a state of irreversible growth arrest. Importantly, senescent cells also become resistant to apoptotic cell death, which prohibits their clearance from the tissue. Senescent cells remain metabolically active, secreting a range of chemokines, cytokines, proteases and other factors – collectively called the senescence-associated secretory phenotype (SASP) - that can promote inflammation and impair tissue function [66]. Important to AT, when preadipocytes become senescent they can no longer differentiate into functional adipocytes; consequently, as senescent cells accumulate, adipogenesis is impaired and AT loses its ability to store lipid [66]. This phenomenon has been suggested to explain the decreased lipid storage capacity in subQ AT depots with advancing age and the lipid being redirected to visceral and ectopic depots over time resulting in lipotoxicity [67].
GH activity has been demonstrated to influence AT senescence. That is, increased GH action in bGH mice as well as in chronically GH-injected mice results in significantly greater senescent cell accumulation in AT [68]. Likewise, decreased GH action, as in Snell dwarf and GHR−/− mice, results in lower senescent cell burden [68]. Thus, the well-documented enlargement of the subQ AT depot in the GHR−/− and Snell dwarf mice may be a result of this phenomenon. Interestingly, GHA mice appear to be an exception to this trend as their AT senescence levels are comparable to control mice despite a decrease in GH action [69]. Perhaps the discrepancy is due to the fact that GHA mice have a more modest reduction in GH action than Snell or GHR−/− mice or that senescence reflects longevity and GHA mice do not have an increased lifespan. Regardless, the normal levels of senescent cell burden in GHA AT could be considered a positive outcome as these mice are also extremely obese, and obesity itself has been shown to accelerate AT senescence [70].
GH and AT immune cells
The GHR is expressed in a wide array of innate and adaptive immune cells [71] Thus, it is not surprising that GH has been shown to alter immune function with most published data focusing on the hormone’s ability to influence T cell development and function [71]. Several recent studies have focused on the role of GH on AT immune cells. Studies using mice with a targeted deletion of GHR in macrophages reveal the importance of GH action on macrophages in regulating adipogenesis [72] and AT inflammation during diet-induced obesity [73]. More recently, our laboratory demonstrated that GH action influences WAT immune cell distribution in a depot specific manner [74]. In this study, we report that bGH mice have an increase in the amount of total leukocytes in the mesenteric (visceral) depot as compared to control mice. Furthermore, macrophages represent a higher percentage of the SVF in bGH subQ and mesenteric depots relative to wild-type controls; these macrophages in bGH mice more commonly exhibit an anti-inflammatory or M2 phenotype. T helper cells are also more abundant in the subQ WAT of bGH mice relative to controls. Finally, transcriptome profiling of AT from GHR−/− mice relative to controls suggests increased dendritic cells in AT of these dwarf mice although quantification of cell populations in GHR−/− AT from these mice has not been reported [75]. To date, these are the only published studies that have attempted to address the role of GH on altering the immune cell population numbers or function in AT; however, these data are striking and represent a novel means by which GH could influence the health and function of this tissue and ultimately the organism.
GH and Fibrosis
AT fibrosis, or the buildup of extracellular matrix (ECM) proteins, primarily collagen, is a recently recognized hallmark of obesity. While high levels of GH have been shown to promote fibrosis in numerous other tissues such as muscle, bone, and skin [76, 77], the role of the GH in AT fibrosis has not been fully examined. However, preliminary data from our laboratory does suggest a significant role for GH in collagen deposition in AT (Householder et al, submitted). That is, bGH mice appear to have elevated collagen content/fibrosis in AT, with the subQ depot showing the most pronounced fibrosis. The depot difference is particularly interesting since the decrease in AT mass of bGH mice is similar in all depots, yet the fibrosis is highly depot dependent. Likewise, GHA and GHR−/− mice appear to have a decrease in ECM deposition, which again is mainly apparent in subQ depots. This interesting observation would suggest that in addition to GH directly altering proteins involved in lipolysis, lipogenesis and proliferation/differentiation, GH may also alter AT expansion by restricting adipocyte enlargement directly via a rigid ECM.
What does this mean for the clinical use of treatments that modify GH action?
Acromegaly
Acromegaly can be treated with surgery, radiotherapy, and/or pharmacologically, all with the goal of decreasing both GH and IGF-1 to within normal limits. Treatment is critical as GH hypersecretion leads to a multisystem disease state that result in several comordities, psychological alterations, physical disfigurement as well as premature death. With treatment, the decrease in GH action is accompanied by increases in subQ and visceral AT, albeit to a greater extent in men than in women, decreases in intramuscular fat deposition, and increases in ectopic fat deposition in liver [41]. The studies described in this review for bGH mice, which share many features with the clinical condition of acromegaly, would suggest that the decrease in AT mass with acromegaly is accompanied by a depot-specific increase in the flux of unfavorable immune cells [74], fibrosis (Householder et al, submitted) and preadipocyte senescence [68], all of which are hallmarks of unhealthy AT. Thus, while the acromegaly treatment methods may increase AT, the AT is likely healthier with treatment, or in other words, the quantity of AT may not reflect the overall quality of the tissue. Future studies in clinical populations that assess more than just AT mass and that are able to sample multiple depots are needed to confirm the results in bGH mice.
Growth disorders and GHD
Treatment of children and adults with GH deficiency consists of replacement therapy with recombinant human GH. GH treatment improves height, body composition, bone density, and overall quality of life. Importantly, it results in an overall reduction of fat mass, including a shift in adipocyte volume and size toward normal [78, 79]. Levels of insulin and adipokines are partly normalized, and especially in adult GHD, lipid metabolism is improved [39, 80]. Patients with LS show clinical manifestations that are similar, although not identical, to GHD. The patients are GH insensitive; thus, there is no shortage of GH. LS patients are currently treated with IGF-1 with or without IGF binding protein 3 [81]. Studies are limited as to the impact of this treatment on AT. While an initial reduction in fat mass is observed with recombinant IGF-1 treatment, long term gains in adiposity have been noted. A recent study reports that lower doses injected twice daily may be more beneficial as it results in less fat accumulation [82, 83].
Obesity
With a doubling in worldwide obesity and with nearly one-third of US adults now considered obese [84], alternative methods for obesity management are desperately needed. Human obesity, especially in the visceral depots, is associated with a markedly suppressed spontaneous GH secretion and a decreased response to GH stimuli, such as GH releasing hormone [85]. The suppression of GH is secondary to obesity and is, in part, explained by hyperinsulinemia and increased leptin, reduced ghrelin activity and elevated free fatty acids [86]. Circulating IGF-1 levels remain unaffected or slightly lowered, whereas free IGF-1 tends to increase, probably due to an insulin-mediated reduction in IGF binding protein (IGFBP)-1 and -2 [86]. Substantial weight loss and a decrease in insulin resistance are able to restore GH secretion.
Since the late 1980s, a large amount of attention has focused on recombinant human GH and its effects on body composition and metabolic disturbance in the setting of obesity [recently reviewed in 85]. In most clinical studies, GH at therapeutic levels has failed to demonstrate a striking impact on health. In these studies, generalized or abdominal obese individuals typically do not respond to recombinant human GH administration with weight loss. However, despite unaltered bodyweight, most studies show a reduction in visceral AT and an improvement in glucose and lipid profile, and some trials also demonstrated reductions in subQ mass [87]. It was suggested that the ability of GH to directly affect proteins involved in lipolysis and lipogenesis was the main cause of AT reduction. However, some of the recent findings in mice may suggest other mechanisms, such as fibrosis or senescence, as described elsewhere in this review. Importantly, the data in mice would suggest that the reduction in AT mass with GH therapy would not be accompanied by favorable changes in AT. Further, GH is a diabetogenic agent that may have ill-effects on glucose metabolism. Thus, its use as a therapeutic agent to reduce obesity is likely limited.
Expert commentary
For the past decades, the rate of overweight and obesity has increased dramatically posing a major challenge in clinical practice. GH, because of its favorable impact on body composition in decreasing AT - particular abdominal and visceral stores - and increasing lean mass, has been postulated to be a pharmacological method to complement lifestyle changes. In humans, it is well known that obesity reduces GH levels and that obese individuals with the lowest GH secretion exhibit the most severe metabolic consequences. Thus, GH appears to be a reasonable candidate for therapy. However, most studies with recombinant GH therapy show no or only modest change in body weight in obese patients although there is striking evidence of the direct ability of GH therapy to decrease AT and in particular abdominal/visceral AT stores. However, these studies are often limited by small sample size, short duration of follow-up, and the potential adverse effect of GH on glucose homeostasis.
This review describes the impact of GH on AT based on recent findings in animal models as well as the insight provided by clinical conditions associated with the absence (LS), decrease (GHD) or excess (acromegaly) in GH action. Collectively, these data from clinical populations and from animal models would suggest that overall health of AT is compromised with excess GH even though fat mass is reduced. The mechanisms for AT reductions are likely to be much more complex than first thought and go far beyond the ability of GH to regulate lipolysis, lipogenesis or preadipocyte differentiation/proliferation. As illustrated by the GH mouse lines, GH is able to affect adipocyte senescence, leading to impaired adipogenesis and reduced lipid accumulation in a depot specific manner. It alters AT immune cell population, perhaps by increasing the amount of leukocytes in visceral depots, as seen in the bGH mouse. Finally, GH increases collagen deposition and promotes fibrosis, which may restrict adipocyte expansion. These findings are striking and represent novel means by which GH influence AT and have profound effects on health and function of this tissue. Further, these mouse lines with modifications in GH-induced intracellular signaling suggest that clinical readouts related to immune function, adipokine shifts, senescence or fibrosis may serve as better indicators of AT health in the clinical setting than simple measures of mass. Finally, to understand the many effects of GH on AT, it is necessary to acknowledge the importance of cross talk and interaction among different tissues. This has become especially obvious from studies in mice with tissue-specific alterations in GH signaling. These concerns make it unlikely that GH will be of therapeutic value in obesity in the near foreseeable future
Five-year view
The increasing prevalence of obesity and obesity-related metabolic disorders will increase the search for alternative treatment strategies. Currently available drugs for the treatment of obesity show low or moderate efficacy, are expensive, and/or require long-term treatment to maintain a weight-loss. GH does decrease AT mass. Thus, the field of research related to AT and the GH/IGF system may well increase within future years. However, more research is warranted to better understand how GH influences AT at the molecular and cellular level to better understand its efficacy. Importantly, possible health benefits need to balance against safety concerns.
Two other important lessons gleaned from the studies on GH and AT are that 1) not all AT is created equal and 2) AT mass as an indicator of AT health is likely not appropriate. Because of this, there is a need to determine clinical markers of AT quality versus quantity as well as of the anatomical location of the AT. Although evaluating location and functional changes in AT is challenging in clinical populations, mouse lines with modifications in GH-induced intracellular signaling would suggest that clinical readouts related to immune function, adipokine shifts, senescence or fibrosis may serve as better indicators AT health and depot location in the clinical setting than simple measures of overall AT mass.
Key issues.
Adipose tissue (AT) is significantly more complex than previously thought, requiring one to reevaluate how a hormone, such as growth hormone (GH), alters the tissue.
Clinical disorders associated with excess, decrease or absence of GH action, such as acromegaly, growth hormone deficiency and Laron Syndrome, respectively, are associated with significant alterations in AT mass and adipokine profiles.
Mouse lines with altered GH action provide a useful means for a comparative analysis of GH action in obesity as well as clinical disorders associated with GH action (LS, GHD and acromegaly).
GH is an important regulator of AT metabolism well documented to decrease AT mass via stimulating lipolysis, inhibiting lipogenesis, and affecting adipocyte proliferation and differentiation. However, newer finding in mice reveal novel ways in which GH alters AT including endocrine regulation, senescence, immune cell modulation and extracellular matrix deposition.
Finding from clinical populations with altered GH action and animal models confirm that GH does not alter AT uniformly with subQ and visceral AT differentially impacted and that as GH decreases the quantity of fat, the quality of AT may be compromised.
Obese patients present with a markedly suppressed spontaneous as well as stimulated GH secretion. GH treatment of obese subjects has little effect on bodyweight. However, visceral depot size is decreased and metabolic profile is generally improved. Based on mouse data, the health of the AT with GH treatment is likely compromised.
Acknowledgments
This work was supported by NIH grant P01AG031736, by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by the AMVETS, and by the Diabetes Institute at Ohio University. D. Berryman and E. List received a Growth for Grant Innovation Grant from Merck Serano.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Contributor Information
Darlene E. Berryman, Executive Director, The Diabetes Institute at Ohio University, 108 Konneker Research Labs, Ohio University, (740) 593-9661 – phone, (740) 593-4795 - fax
Brooke Henry, 108 Konneker Research Labs, Ohio University, (740) 593-9665.
Rikke Hjortebjerg, Medical Research Laboratory, Department of Clinical Medicine, Aarhus University, Noerrebrogade 44, 8000 Aarhus C, Denmark, +45 6166 8045 - phone, +45 7846 2150 - fax.
Edward O. List, Senior Scientist, 218 Konneker Research Labs, Edison Biotechnology Institute, Ohio University, (740) 593-4620 – phone, (740) 593-4795 - fax
John J. Kopchick, Distinguished Professor, Goll Ohio Eminent Scholar, 172 Water Tower Drive, Ohio University, (740) 593-4534 – phone, (740) 593-4795 – fax
References
- 1.Prunet-Marcassus B, et al. From heterogeneity to plasticity in adipose tissues: site-specific differences. Experimental Cell Research. 2006;312(6):727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Huh JY, et al. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and Cells. 2014;37(5):365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazenbalk G, et al. Novel pathway of adipogenesis through cross-talk between adipose tissue macrophages, adipose stem cells and adipocytes: evidence of cell plasticity. PLoS One. 2011;6(3):e17834. doi: 10.1371/journal.pone.0017834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23(3):512–518. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deiuliis J, et al. Visceral adipose inflammation in obesity is associated with critical alterations in tregulatory cell numbers. PLoS One. 2011;6(1):e16376. doi: 10.1371/journal.pone.0016376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausman GJ, Dodson MV. Stromal Vascular Cells and Adipogenesis: Cells within Adipose Depots Regulate Adipogenesis. J Genomics. 2013;1:56–66. doi: 10.7150/jgen.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertola A, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61(9):2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nature Medicine. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepe A, et al. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57(1):66–75. doi: 10.1159/000279755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem cell reviews. 2012;8(1):55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 11.von der Weid PY, Rainey KJ. Review article: lymphatic system and associated adipose tissue in the development of inflammatory bowel disease. Alimentary Pharmacology and Therapeutics. 2010;32(6):697–711. doi: 10.1111/j.1365-2036.2010.04407.x. [DOI] [PubMed] [Google Scholar]
- 12.Gehrke S, et al. Epigenetic regulation of depot-specific gene expression in adipose tissue. PLoS One. 2013;8(12):e82516. doi: 10.1371/journal.pone.0082516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betz MJ, Enerback S. Human Brown Adipose Tissue: What We Have Learned So Far. Diabetes. 2015;64(7):2352–2360. doi: 10.2337/db15-0146. [DOI] [PubMed] [Google Scholar]
- 14.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divoux A, et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes. 2010;59(11):2817–2825. doi: 10.2337/db10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun K, et al. Fibrosis and adipose tissue dysfunction. Cell metabolism. 2013;18(4):470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Londos C, et al. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol. 1999;10(1):51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- 19.Childs GV, et al. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology. 2011;152(1):69–81. doi: 10.1210/en.2010-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts SW. Trash Talk by Fat: Chemerin as a Reactive Oxygen Species Provocateur in the Vasculature. Hypertension. 2015;66(3):466–468. doi: 10.1161/HYPERTENSIONAHA.115.05738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neves KB, et al. Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox. Hypertension. 2015;66(3):657–666. doi: 10.1161/HYPERTENSIONAHA.115.05616. [DOI] [PubMed] [Google Scholar]
- 22.Rao RR, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li ZY, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS neuroscience & therapeutics. 2014;20(4):344–354. doi: 10.1111/cns.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moller N, Jorgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]
- 26.Brooks AJ, et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783. doi: 10.1126/science.1249783. [DOI] [PubMed] [Google Scholar]
- 27.Laron Z, et al. Classification of growth hormone insensitivity syndrome [editorial] J Pediatr. 1993;122(2):241. doi: 10.1016/s0022-3476(06)80120-4. [DOI] [PubMed] [Google Scholar]
- 28.Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. European Journal of Endocrinology / European Federation of Endocrine Societies. 2011;164(4):485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- 29.Laron Z, et al. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity) Clin Endocrinol (Oxf) 2006;65(1):114–117. doi: 10.1111/j.1365-2265.2006.02558.x. [DOI] [PubMed] [Google Scholar]
- 30.Guevara-Aguirre J, et al. GH Receptor Deficiency in Ecuadorian Adults Is Associated With Obesity and Enhanced Insulin Sensitivity. The Journal of clinical endocrinology and metabolism. 2015;100(7):2589–2596. doi: 10.1210/jc.2015-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanety H, et al. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol. 2009;161(6):837–844. doi: 10.1530/EJE-09-0419. [DOI] [PubMed] [Google Scholar]
- 32.Laron Z, et al. Serum leptin in obese patients with Laron syndrome before and during IGF-I treatment. J Pediatr Endocrinol Metab. 1998;11(5):653–656. doi: 10.1515/JPEM.1998.11.5.653. [DOI] [PubMed] [Google Scholar]
- 33.Gomes-Santos E, et al. Increased visceral adiposity and cortisol to cortisone ratio in adults with congenital lifetime isolated GH deficiency. Journal of Clinical Endocrinology and Metabolism. 2014;99(9):3285–3289. doi: 10.1210/jc.2014-2132. [DOI] [PubMed] [Google Scholar]
- 34.Pereira RM, et al. Heterozygosity for a mutation in the growth hormone-releasing hormone receptor gene does not influence adult stature, but affects body composition. The Journal of clinical endocrinology and metabolism. 2007;92(6):2353–2357. doi: 10.1210/jc.2007-0092. [DOI] [PubMed] [Google Scholar]
- 35.Aguiar-Oliveira MH, et al. Longevity in untreated congenital growth hormone deficiency due to a homozygous mutation in the GHRH receptor gene. Journal of Clinical Endocrinology and Metabolism. 2010;95(2):714–721. doi: 10.1210/jc.2009-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vicente TA, et al. Lifetime congenital isolated GH deficiency does not protect from the development of diabetes. Endocr Connect. 2013;2(2):112–117. doi: 10.1530/EC-13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaves VE, Junior FM, Bertolini GL. The metabolic effects of growth hormone in adipose tissue. Endocrine. 2013;44(2):293–302. doi: 10.1007/s12020-013-9904-3. [DOI] [PubMed] [Google Scholar]
- 38.Ukropec J, et al. Adipokine protein expression pattern in growth hormone deficiency predisposes to the increased fat cell size and the whole body metabolic derangements. The Journal of clinical endocrinology and metabolism. 2008;93(6):2255–2262. doi: 10.1210/jc.2007-2188. [DOI] [PubMed] [Google Scholar]
- 39.Molitch ME, et al. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011;96(6):1587–1609. doi: 10.1210/jc.2011-0179. [DOI] [PubMed] [Google Scholar]
- 40.Melmed S. Acromegaly pathogenesis and treatment. The Journal of clinical investigation. 2009;119(11):3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes-Vidal CM, et al. Adipose Tissue Redistribution and Ectopic Lipid Deposition in Active Acromegaly and Effects of Surgical Treatment. The Journal of clinical endocrinology and metabolism. 2015;100(8):2946–2955. doi: 10.1210/jc.2015-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciresi A, et al. Serum visfatin levels in acromegaly: Correlation with disease activity and metabolic alterations. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2015;25(5):240–246. doi: 10.1016/j.ghir.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Olarescu NC, et al. Adipocytes as a source of increased circulating levels of nicotinamide phosphoribosyltransferase/visfatin in active acromegaly. The Journal of clinical endocrinology and metabolism. 2012;97(4):1355–1362. doi: 10.1210/jc.2011-2417. [DOI] [PubMed] [Google Scholar]
- 44.Luque RM, et al. Metabolic Impact of Adult-Onset, Isolated, Growth Hormone Deficiency (AOiGHD) Due to Destruction of Pituitary Somatotropes. PLoS One. 2011;6(1):e15767. doi: 10.1371/journal.pone.0015767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.List EO, et al. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.List EO, et al. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Molecular Endocrinology. 2013;27(3):524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu C, et al. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. The Journal of biological chemistry. 2013;288(22):15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flurkey K, et al. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berryman DE, et al. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147(6):2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- 50.Berryman DE, et al. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Wiesenborn DS, et al. The effect of calorie restriction on insulin signaling in skeletal muscle and adipose tissue of Ames dwarf mice. Aging. 2014;6(10):900–912. doi: 10.18632/aging.100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berryman DE, et al. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21(3):113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon V, et al. The contribution of visceral fat to improved insulin signaling in Ames dwarf mice. Aging Cell. 2014;13(3):497–506. doi: 10.1111/acel.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berryman DE, et al. Growth hormone and adipose tissue: beyond the adipocyte. Growth Hormone and IGF Research. 2011;21(3):113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richelsen B, et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000;49(7):906–911. doi: 10.1053/meta.2000.6738. [DOI] [PubMed] [Google Scholar]
- 56.Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. Journal of Lipid Research. 2002;43(10):1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- 57.Yang HL, Sun C, Qi RL. Effect of suppressor of cytokine signaling 2 (SOCS2) on fat metabolism induced by growth hormone (GH) in porcine primary adipocyte. Molecular Biology Reports. 2012;39(9):9113–9122. doi: 10.1007/s11033-012-1783-9. [DOI] [PubMed] [Google Scholar]
- 58.Fain JN, et al. Stimulation of human omental adipose tissue lipolysis by growth hormone plus dexamethasone. Molecular and Cellular Endocrinology. 2008;295(1–2):101–105. doi: 10.1016/j.mce.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Nam SY, Lobie PE. The mechanism of effect of growth hormone on preadipocyte and adipocyte function. Obes Rev. 2000;1(2):73–86. doi: 10.1046/j.1467-789x.2000.00015.x. [DOI] [PubMed] [Google Scholar]
- 60.Flint DJ, et al. Developmental aspects of adipose tissue in GH receptor and prolactin receptor gene disrupted mice: site-specific effects upon proliferation, differentiation and hormone sensitivity. J Endocrinol. 2006;191(1):101–111. doi: 10.1677/joe.1.06939. [DOI] [PubMed] [Google Scholar]
- 61.Olarescu NC, et al. GH action influences adipogenesis of mouse adipose tissue-derived mesenchymal stem cells. The Journal of endocrinology. 2015;226(1):13–23. doi: 10.1530/JOE-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masternak MM, et al. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. doi: 10.1111/j.1474-9726.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown-Borg HM, Bartke A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2012;67(6):652–660. doi: 10.1093/gerona/gls086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lubbers ER, et al. Adiponectin in mice with altered growth hormone action: links to insulin sensitivity and longevity? The Journal of Endocrinology. 2012 doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolfing B, et al. Interfering effects of insulin, growth hormone and glucose on adipokine secretion. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2008;116(1):47–52. doi: 10.1055/s-2007-990275. [DOI] [PubMed] [Google Scholar]
- 66.Tchkonia T, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell metabolism. 2013;17(5):644–656. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tchkonia T, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stout MB, et al. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 2014;6(7):575–586. doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comisford R, et al. Growth Hormone Receptor Antagonist Transgenic Mice Have Increased Subcutaneous Adipose Tissue Mass, Altered Glucose Homeostasis and No Change in White Adipose Tissue Cellular Senescence. Gerontology. 2015 doi: 10.1159/000439050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villaret A, et al. Adipose tissue endothelial cells from obese human subjects: differences among depots in angiogenic, metabolic, and inflammatory gene expression and cellular senescence. Diabetes. 2010;59(11):2755–2763. doi: 10.2337/db10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Hormone and IGF Research. 2009;19(3):187–197. doi: 10.1016/j.ghir.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Lu C, et al. A novel effect of growth hormone on macrophage modulates macrophage-dependent adipocyte differentiation. Endocrinology. 2010;151(5):2189–2199. doi: 10.1210/en.2009-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu C, et al. Targeted Deletion of Growth Hormone (GH) Receptor in Macrophage Reveals Novel Osteopontin-mediated Effects of GH on Glucose Homeostasis and Insulin Sensitivity in Diet-induced Obesity. Journal of Biological Chemistry. 2013;288(22):15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benencia F, et al. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology. 2014:en20141794. doi: 10.1210/en.2014-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stout MB, et al. Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget. 2015;6(29):26702–26715. doi: 10.18632/oncotarget.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doessing S, et al. GH and IGF1 levels are positively associated with musculotendinous collagen expression: experiments in acromegalic and GH deficiency patients. Eur J Endocrinol. 2010;163(6):853–862. doi: 10.1530/EJE-10-0818. [DOI] [PubMed] [Google Scholar]
- 77.Longobardi S, et al. Growth hormone (GH) effects on bone and collagen turnover in healthy adults and its potential as a marker of GH abuse in sports: a double blind, placebo-controlled study. The GH-2000 Study Group. The Journal of clinical endocrinology and metabolism. 2000;85(4):1505–1512. doi: 10.1210/jcem.85.4.6551. [DOI] [PubMed] [Google Scholar]
- 78.Amato G, et al. Recombinant growth hormone (GH) therapy in GH-deficient adults: a long-term controlled study on daily versus thrice weekly injections. J Clin Endocrinol Metab. 2000;85(10):3720–3725. doi: 10.1210/jcem.85.10.6881. [DOI] [PubMed] [Google Scholar]
- 79.Hazem A, et al. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. European Journal of Endocrinology / European Federation of Endocrine Societies. 2012;166(1):13–20. doi: 10.1530/EJE-11-0558. [DOI] [PubMed] [Google Scholar]
- 80.Meazza C, et al. Metabolic parameters and adipokine profile in growth hormone deficient (GHD) children before and after 12-month GH treatment. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2014;46(3):219–223. doi: 10.1055/s-0033-1358730. [DOI] [PubMed] [Google Scholar]
- 81.Rosenbloom AL. A half-century of studies of growth hormone insensitivity/Laron syndrome: A historical perspective. Growth Hormone and IGF Research. 2015 doi: 10.1016/j.ghir.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Laron Z, et al. Long-term IGF-I treatment of children with Laron syndrome increases adiposity. Growth Hormone and IGF Research. 2006;16(1):61–64. doi: 10.1016/j.ghir.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Guevara-Aguirre J, et al. Recommended IGF-I dosage causes greater fat accumulation and osseous maturation than lower dosage and may compromise long-term growth effects. Journal of Clinical Endocrinology and Metabolism. 2013;98(2):839–845. doi: 10.1210/jc.2012-3704. [DOI] [PubMed] [Google Scholar]
- 84.Ogden CL, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berryman DE, et al. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9(6):346–356. doi: 10.1038/nrendo.2013.64. [DOI] [PubMed] [Google Scholar]
- 86.Lewitt MS, Dent MS, Hall K. The Insulin-Like Growth Factor System in Obesity, Insulin Resistance and Type 2 Diabetes Mellitus. Journal of clinical medicine. 2014;3(4):1561–1574. doi: 10.3390/jcm3041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franco C, et al. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90(3):1466–1474. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 88.Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- 89.Fleenor D, et al. Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology. 2005;146(1):103–112. doi: 10.1210/en.2004-0744. [DOI] [PubMed] [Google Scholar]
- 90.Berryman DE, et al. Living Large: What Mouse Models Reveal about Growth Hormone and Obesity. Switzerland: Springer International Publishing; 2015. [Google Scholar]