ABSTRACT
Mobile genetic elements are near ubiquitous DNA segments that revealed a surprising variety of strategies for their propagation among prokaryotes and between eukaryotes. In bacteria, conjugative elements were shown to be key drivers of evolution and adaptation by efficiently disseminating genes involved in pathogenicity, symbiosis, metabolic pathways, and antibiotic resistance. Conjugative plasmids of the incompatibility groups A and C (A/C) are important vehicles for the dissemination of antibiotic resistance and the consequent global emergence and spread of multi-resistant pathogenic bacteria. Beyond their own mobility, A/C plasmids were also shown to drive the mobility of unrelated non-autonomous mobilizable genomic islands, which may also confer further advantageous traits. In this commentary, we summarize the current knowledge on different classes of A/C-dependent mobilizable genomic islands and we discuss other DNA hitchhikers and their implication in bacterial evolution. Furthermore, we glimpse at the complex genetic network linking autonomous and non-autonomous mobile genetic elements, and at the associated flow of genetic information between bacteria.
KEYWORDS: A/C, antibiotic resistance, genomic island, integrative conjugative element, mobilization, plasmid
Bacterial genomes are dynamic entities subjected to a constant flow of loss and gain of genetic material.1 Gene acquisition can provide bacterial hosts with adaptive traits and is likely to confer a selective advantage in particular conditions.2 Self-transmissible mobile genetic elements (MGEs) such as prophages and conjugative elements were shown to be an immense resource for genome evolution and bacterial adaptation.1-3
Genomic islands (GIs) also largely participate in bacterial genome diversification. Many GIs were identified thanks to the adaptive traits they encoded and named accordingly, e.g. pathogenicity islands or symbiosis islands. GIs are chromosomal DNA segments typically present in subsets of closely related strains, one of the hallmarks of acquisition by horizontal gene transfer. However, the mechanism of acquisition and dissemination of GIs has remained a conundrum for a long time, often due to the lack of obvious mobility–related genes.4 Recent studies have refined our understanding of the biology and mobility mechanisms of several of mobilizable GIs (MGIs) families including satellite prophages and GIs mobilized by conjugative elements.5-7 Mobility of MGIs involves self-transmissible MGEs that provide them with functions they lack to catalyze their dissemination. Availability of thousands of bacterial genome sequences associated with low-cost, high-throughput modern molecular methods unraveled an even greater diversity of GIs.
We recently reported the discovery of MGIVchHai6, a new mobile resistance island in Vibrio cholerae, that is mobilizable by A/C conjugative plasmids.8 Here, we compare the possible mechanisms of activation and mobilization of MGIVchHai6 with two other A/C-dependent mobilizable GIs as well as a family of GIs mobilized by integrative and conjugative elements (ICEs) of the SXT/R391 family.
Regulation of transfer of A/C plasmids
Plasmids of the incompatibility groups A and C (A/C) are large (> 110 kb) double-stranded molecules that efficiently disseminate by conjugation.9 A/C plasmids drive the spread of multiple antibiotic resistances including last-resort antimicrobial compounds such as carbapenems.10,11
Recent studies demonstrated that control of A/C plasmids mobility is reminiscent of the FlhCD-dependent activation of flagellar motility in Escherichia coli and related motile bacteria.12,13 Nevertheless, the A/C regulatory circuitry is a unique system with specific early molecular actors and plasmid-borne target genes. Two repressors named Acr1 and Acr2 (A/C repressors 1 and 2) repress the constitutive transcription of acr1 from Pacr1.13,14 Upon inducing conditions that remain to be identified, repression of Pacr1 is alleviated, allowing not only the transcription of acr1 but also that of all four downstream genes. Two of these genes, acaC and acaD, were shown to code for subunits of the FlhCD-like master activator of A/C plasmids AcaCD (A/C activator, subunits C and D).13 Thorough investigation revealed that AcaCD targets 18 A/C-borne promoter regions, thereby activating the transcription of genes and operons responsible for conjugative transfer. Surprisingly, genes coding for predicted or demonstrated functions account for a fraction of AcaCD-activated genes as nearly two thirds of these genes code for proteins of unknown function.13,15 Future investigation of this large terra incognita is necessary to fully understand the biology of A/C plasmids.
A/C-dependent mobilization of mobilizable genomic islands
Recent work conducted by our group uncovered the extended role of AcaCD.12 Besides A/C-borne sequences, AcaCD was shown to recognize chromosomal loci that belong to A/C-unrelated MGIs.13
To date, three unrelated families of A/C-dependent MGIs were identified and named after the prototypical elements that were experimentally characterized: MGIVchHai6 of V. cholerae, MGIVmi1 of V. mimicus and Salmonella genomic island 1 (SGI1).8,13,16 Each family encompasses several members sharing a conserved core sequence. Different members of the same family contain distinct insertion of variable DNA coding for adaptive traits or proteins of unknown function.8,15,17-19 While the precise molecular mechanism leading to intercellular mobility of these elements remains to be deciphered, accumulation of evidence suggests the pivotal role of genes under the control of AcaCD.
Based on these observations and the presence of conserved features, we propose two distinct models of mobilization, one for SGI1 and relatives, and the other for MGIVchHai6/MGIVmi1-like elements (Fig. 1).
Figure 1.
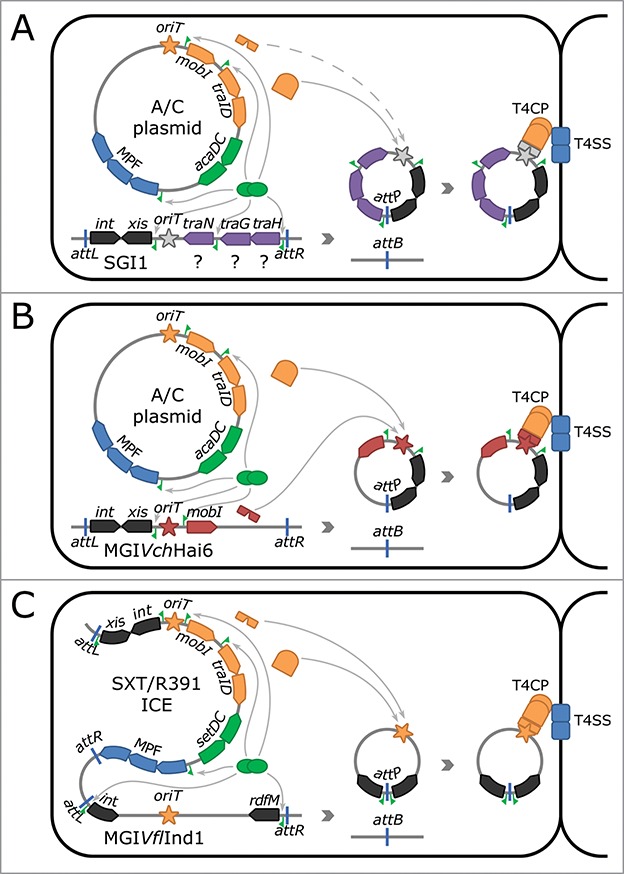
Proposed mobilization mechanisms for three known families of mobilizable genomic islands. (A) Mobilization of SGI1 and related elements by A/C plasmids. (B) Mobilization of MGIVchHai6 and related elements by A/C plasmids. (C) Mobilization of MGIVflInd1 and related elements by SXT/R391 ICEs. Different items are portrayed as follows: arrowed boxes, genes; green pennants, AcaCD/SetCD binding sites; blue strokes, left (attL) and right (attR) junctions as well as chromosomal (attB) and circular form (attP) attachment sites; MPF, genes involved in mating pore formation. oriTSGI1 along with helper plasmid's MobI are depicted in gray and with a dotted line to take into account their highly speculative nature. SGI1 and MGIVchHai6 are integrated into the 3′ end of trmE, whereas SXT/R391 ICEs are integrated into the 5′ end of prfC, and MGIVflInd1 into the 3′ end of yicC.
SGI1: First evidence of A/C-dependent MGI
A/C were firstly shown to specifically mobilize GIs in 2005 with the characterization of SGI1 and by extent the large family of SGI1 elements.13,16,20 SGI1 and its siblings are recognized as major determinants of multidrug resistance in Salmonella enterica and Proteus mirabilis.18 The integron In104 of SGI1 elements confers multidrug resistance.18,19
Five AcaCD binding sites were discovered in SGI1, allowing to posit on the underlying mechanism allowing SGI1 mobilization (Fig. 1A).13,21,22 SGI1 carries int, a constitutively expressed gene coding for the site-specific integrase that catalyzes the integration of SGI1 into the 3′ end of trmE in the chromosome of its host.16,21,23,24 Upon arrival of an A/C plasmid in the cell, associated synthesis of AcaCD triggers the transcription of xis, a gene coding for a recombination directionality factor (RDF).16,21 Xis would displace the integrase-mediated recombination reaction toward excision of SGI1 as a plasmid-like molecule that is the substrate for conjugative transfer (Fig. 1A). To date, no origin of transfer (oriT), the cis-acting locus where DNA transfer is initiated, has been identified on SGI1. Furthermore, A/C- and SGI1-encoded factors that process this putative oriT and enable docking of SGI1 DNA to the mating pore for transfer to the recipient cell remain to be characterized. Once in the recipient, SGI1 is thought to be independent of the helper plasmid in regards to its site-specific integration due to the constitutive expression of int.21
Members of the MGIVchHai6 family are mobilizable by A/C plasmids
MGIVchHai6 is the prototypical member of a new family of MGIs involved in the dissemination of multidrug resistance.8 This 47-kb element was identified in a non-O1/non-O139 V. cholerae clinical isolate recovered from a cholera patient in Haiti in 2010.25 Like SGI1, MGIVchHai6 is integrated into the 3′ end of trmE. It also carries a distinct integron, In36A1, conferring resistance to β-lactams, florfenicol/chloramphenicol, streptomycin/spectinomycin, sulfamethoxazole and trimethoprim (co-trimoxazole), and tetracycline. MGIVchHai6 also likely confers resistance to bacteriophage infection and mercury, as it bears a type I restriction-modification system and Tn6310. MGIVchHai6-like elements are globally distributed in environmental and clinical V. cholerae isolates recovered from 1977 to 2010. All members of this family of MGIs share a ∼8-kb conserved core that likely ensures essential maintenance and transfer functions. Mobility of MGIVchHai6 was shown to be strictly dependent on the presence of an A/C plasmid.
Despite its different size and gene content, MGIVchHai6 shares several features with MGIVmi1, an element integrated into the 3′ end of yicC.8,13,15 In both MGIVchHai6 and MGIVmi1, AcaCD was shown to drive the transcription of the RDF gene xis and of a gene coding for a MobI-like protein (Fig. 1B). MobI is required for transfer of integrative and conjugative elements (ICEs) of the SXT/R391 family and A/C plasmids.26,27 In SXT/R391 ICEs and A/C plasmids, oriT is located in a large intergenic region upstream of mobI.26,27 By analogy, we predict that oriT of MGIVchHai6/MGIVmi1 elements (oriTMGI) is located in the large intergenic region upstream of mobIMGI (Fig. 1B).
A model for MGIVchHai6/MGIVmi1 lifecycle infers that these elements remain quiescent in their integrated chromosomal state. Based on work done on other MGIs, stable integration of MGIVchHai6/MGIVmi1 elements is likely enabled by constitutive expression of int.21,28 Like for SGI1, entry of an A/C plasmid that expresses AcaCD triggers the synthesis of Xis that, in concert with the integrase, mediates the excision of the MGI (Fig. 1B). AcaCD also activates the synthesis of MobIMGI that is thought to recognize and bind to oriTMGI.26,27 MobIMGI would act as an adaptor protein that recruits and assembles the A/C plasmid encoded DNA-processing machinery, called relaxosome, within which the relaxase TraI initiates conjugative transfer through the A/C-encoded mating pore (Fig. 1B). The MGI is then assumed to be able to site-specifically integrate into the genome of the recipient cell regardless of the presence of the A/C plasmid, as expression of the integrase is constitutive.
MGIs come in many flavors
For several decades, studies of members of well-known families of self-transmissible MGEs eclipsed the discovery of new types of mobile elements. New evidence suggests that MGIs could be more abundant conjugative entities than expected, presaging an unforeseen significant ecological role.5,29 So far, their influence has been overlooked likely because of difficulties identifying them and/or identifying their cognate helper element(s).
Early milestones into the discovery of MGIs include the characterization of CTn-dependent non-replicating Bacteroides units (NBUs), phage-mobilizable Staphylococcus aureus pathogenicity islands (SaPIs), and the mobilizable transposon of Streptococcus agalactiae MTnSag1, whose mobility depends on Tn916.5,30-36 The identification of oriTSXT and elucidation of the master activator SetCD of SXT/R391 ICEs also greatly helped our recent investigation on A/C-dependent MGIs.26,37 Indeed, localization of oriTSXT allowed the identification of similar chromosomal loci.38 Further investigations revealed that these chromosomal oriT sequences belonged to integrated MGIs, whose mobilization mechanism is slightly different from the above-described MGIs (Fig. 1C).12,28,37-39 Excision of these elements depends on the SetCD-dependent transcriptional activation of the RDF gene.12,28,37 oriTMGI mimics oriTSXT; hence it is recognized by MobISXT and processed by the ICE-encoded relaxosome prior to transfer through the ICE-encoded mating pore (Fig. 1C). In the recipient cell, the MGI integrates autonomously due to int constitutive expression.12,28
Concluding remarks
The propensity of MGIs to persist into and disseminate between bacterial populations using diverse strategies indicates that they are not defective elements. MGIs have rather adapted to act as parasites of self-transmissible MGEs, while at the same time spreading adaptive traits such as resistance to multiple antimicrobial compounds. An interesting example of this cooperative/antagonistic relationship is provided by SGI1 and variants that rely on IncC plasmids for mobilization.16 Co-transfer of plasmid and GI is rare suggesting that the latter is able to affect plasmid transfer, as also confirmed by the rapid loss of the plasmid when SGI1 is co-present in the same E. coli cell.40
This captivating research area is likely to deepen our understanding of other families of MGEs, including other classes of MGIs. Future investigations must focus on (i) the characterization of master regulators and cognate target sequences of a broad set of self-transmissible MGEs and (ii) the identification of their oriT sequence. Such valuable information will help when performing data mining of genome sequences to identify new DNA elements acquired by horizontal gene transfer. In-depth, step-by-step discoveries will help paving the road to building an atlas of interconnections between MGEs and associated massive flow of genetic material.
Abbreviations
- A/C
plasmids of incompatibility groups A and C
- GI
genomic island
- ICE
integrative conjugative element
- MGE
mobile genetic element
- MGI
mobilizable genomic island
- oriT
origin of transfer
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by a Discovery Grant [2016-04365] from the Natural Sciences and Engineering Council of Canada (NSERC) to VB.
References
- [1].Soucy SM, Huang J, Gogarten JP. Horizontal gene transfer: building the web of life. Nat Rev Genet 2015; 16:472-82; PMID:26184597; http://dx.doi.org/ 10.1038/nrg3962 [DOI] [PubMed] [Google Scholar]
- [2].Koonin EV. Horizontal gene transfer: essentiality and evolvability in prokaryotes, and roles in evolutionary transitions. F1000Research 2016; 5; PMID:27508073; http://dx.doi.org/ 10.12688/f1000research.8737.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Frost LS, Leplae R, Summers AO, Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 2005; 3:722-32; PMID:16138100; http://dx.doi.org/ 10.1038/nrmicro1235 [DOI] [PubMed] [Google Scholar]
- [4].Juhas M, van der Meer JR, Gaillard M, Harding RM, Hood DW, Crook DW. Genomic islands: tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol Rev 2009; 33:376-93; PMID:19178566; http://dx.doi.org/ 10.1111/j.1574-6976.2008.00136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bellanger X, Payot S, Leblond-Bourget N, Guédon G. Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiol Rev 2014; 38:720-60; PMID:24372381; http://dx.doi.org/ 10.1111/1574-6976.12058 [DOI] [PubMed] [Google Scholar]
- [6].Lee CA, Thomas J, Grossman AD. The Bacillus subtilis conjugative transposon ICEBs1 mobilizes plasmids lacking dedicated mobilization functions. J Bacteriol 2012; 194:3165-72; PMID:22505685; http://dx.doi.org/ 10.1128/JB.00301-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Novick RP, Ram G. The floating (pathogenicity) island: a genomic dessert. Trends Genet TIG 2016; 32:114-26; PMID:26744223; http://dx.doi.org/ 10.1016/j.tig.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Carraro N, Rivard N, Ceccarelli D, Colwell RR, Burrus V. IncA/C conjugative plasmids mobilize a new family of multidrug resistance islands in clinical Vibrio cholerae non-O1/non-O139 isolates from Haiti. mBio 2016; 7(4):e00509-16; PMID:27435459; http://dx.doi.org/ 10.1128/mBio.00509-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Harmer CJ, Hall RM. The A to Z of A/C plasmids. Plasmid 2015; 80:63-82; PMID:25910948; http://dx.doi.org/ 10.1016/j.plasmid.2015.04.003 [DOI] [PubMed] [Google Scholar]
- [10].Wailan AM, Sartor AL, Zowawi HM, Perry JD, Paterson DL, Sidjabat HE. Genetic contexts of blaNDM-1 in patients carrying multiple NDM-producing strains. Antimicrob Agents Chemother 2015; 59:7405-10; PMID:26392493; http://dx.doi.org/ 10.1128/AAC.01319-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harmer CJ, Hall RM. pRMH760, a precursor of A/C₂ plasmids carrying blaCMY and blaNDM genes. Microb Drug Resist Larchmt N 2014; 20:416-23; http://dx.doi.org/ 10.1089/mdr.2014.0012 [DOI] [PubMed] [Google Scholar]
- [12].Poulin-Laprade D, Carraro N, Burrus V. The extended regulatory networks of SXT/R391 integrative and conjugative elements and IncA/C conjugative plasmids. Front Microbiol 2015; 6:837; PMID:26347724; http://dx.doi.org/ 10.3389/fmicb.2015.00837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carraro N, Matteau D, Luo P, Rodrigue S, Burrus V. The master activator of IncA/C conjugative plasmids stimulates genomic islands and multidrug resistance dissemination. PLoS Genet 2014; 10:e1004714; PMID:25340549; http://dx.doi.org/ 10.1371/journal.pgen.1004714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lang KS, Johnson TJ. Characterization of Acr2, an H-NS-like protein encoded on A/C2-type plasmids. Plasmid 2016; 87-88:17-27; PMID:27492737; http://dx.doi.org/ 10.1016/j.plasmid.2016.07.004 [DOI] [PubMed] [Google Scholar]
- [15].Carraro N, Matteau D, Burrus V, Rodrigue S. Unraveling the regulatory network of IncA/C plasmid mobilization: when genomic islands hijack conjugative elements. Mob Genet Elem 2015; 5:1-5; http://dx.doi.org/ 10.1080/2159256X.2015.1006109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Doublet B, Boyd D, Mulvey MR, Cloeckaert A. The Salmonella genomic island 1 is an integrative mobilizable element. Mol Microbiol 2005; 55:1911-24; PMID:15752209; http://dx.doi.org/ 10.1111/j.1365-2958.2005.04520.x [DOI] [PubMed] [Google Scholar]
- [17].Levings RS, Lightfoot D, Partridge SR, Hall RM, Djordjevic SP. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar Typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. J Bacteriol 2005; 187:4401-9; PMID:15968049; http://dx.doi.org/ 10.1128/JB.187.13.4401-4409.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hall RM. Salmonella genomic islands and antibiotic resistance in Salmonella enterica. Future Microbiol 2010; 5:1525-38; PMID:21073312; http://dx.doi.org/ 10.2217/fmb.10.122 [DOI] [PubMed] [Google Scholar]
- [19].Boyd DA, Shi X, Hu Q, Ng LK, Doublet B, Cloeckaert A, Mulvey MR. Salmonella genomic island 1 (SGI1), variant SGI1-I, and new variant SGI1-O in Proteus mirabilis clinical and food isolates from China. Antimicrob Agents Chemother 2008; 52:340-4; PMID:18025121; http://dx.doi.org/ 10.1128/AAC.00902-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Douard G, Praud K, Cloeckaert A, Doublet B. The Salmonella genomic island 1 is specifically mobilized in trans by the IncA/C multidrug resistance plasmid family. PloS One 2010; 5:e15302; PMID:21187963; http://dx.doi.org/ 10.1371/journal.pone.0015302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kiss J, Papp PP, Szabó M, Farkas T, Murányi G, Szakállas E, Olasz F. The master regulator of IncA/C plasmids is recognized by the Salmonella genomic island SGI1 as a signal for excision and conjugal transfer. Nucleic Acids Res 2015; 43:8735-45; PMID:26209134; http://dx.doi.org/ 10.1093/nar/gkv758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murányi G, Szabó M, Olasz F, Kiss J. Determination and analysis of the putative AcaCD-responsive promoters of Salmonella genomic island 1. PloS One 2016; 11:e0164561; PMID:27727307; http://dx.doi.org/ 10.1371/journal.pone.0164561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mulvey MR, Boyd DA, Olson AB, Doublet B, Cloeckaert A. The genetics of Salmonella genomic island 1. Microbes Infect Inst Pasteur 2006; 8:1915-22; http://dx.doi.org/ 10.1016/j.micinf.2005.12.028 [DOI] [PubMed] [Google Scholar]
- [24].Kiss J, Nagy B, Olasz F. Stability, entrapment and variant formation of Salmonella genomic island 1. PloS One 2012; 7:e32497; PMID:22384263; http://dx.doi.org/ 10.1371/journal.pone.0032497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hasan NA, Choi SY, Eppinger M, Clark PW, Chen A, Alam M, Haley BJ, Taviani E, Hine E, Su Q, et al.. Genomic diversity of 2010 Haitian cholera outbreak strains. Proc Natl Acad Sci U S A 2012; 109:E2010-2017; PMID:22711841; http://dx.doi.org/ 10.1073/pnas.1207359109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ceccarelli D, Daccord A, René M, Burrus V. Identification of the origin of transfer (oriT) and a new gene required for mobilization of the SXT/R391 family of integrating conjugative elements. J Bacteriol 2008; 190:5328-38; PMID:18539733; http://dx.doi.org/ 10.1128/JB.00150-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Carraro N, Sauvé M, Matteau D, Lauzon G, Rodrigue S, Burrus V. Development of pVCR94ΔX from Vibrio cholerae, a prototype for studying multidrug resistant IncA/C conjugative plasmids. Front Microbiol 2014; 5:44; PMID:24567731; http://dx.doi.org/ 10.3389/fmicb.2014.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Daccord A, Mursell M, Poulin-Laprade D, Burrus V. Dynamics of the SetCD-regulated integration and excision of genomic islands mobilized by integrating conjugative elements of the SXT/R391 family. J Bacteriol 2012; 194:5794-802; PMID:22923590; http://dx.doi.org/ 10.1128/JB.01093-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Guglielmini J, Quintais L, Garcillan-Barcia MP, de la Cruz F, Rocha EPC. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet 2011; 7:e1002222; PMID:21876676; http://dx.doi.org/ 10.1371/journal.pgen.1002222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shoemaker NB, Wang GR, Salyers AA. NBU1, a mobilizable site-specific integrated element from Bacteroides spp., can integrate nonspecifically in Escherichia coli. J Bacteriol 1996; 178:3601-7; PMID:8655560; http://dx.doi.org/ 10.1128/jb.178.12.3601-3607.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kreiswirth BN, Projan SJ, Schlievert PM, Novick RP. Toxic shock syndrome toxin 1 is encoded by a variable genetic element. Rev Infect Dis 1989; 11(Suppl 1):S83-88; discussion S88-89; PMID:2564693; http://dx.doi.org/ 10.1093/clinids/11.Supplement_1.S83 [DOI] [PubMed] [Google Scholar]
- [32].Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol 1998; 29:527-43; PMID:9720870; http://dx.doi.org/ 10.1046/j.1365-2958.1998.00947.x [DOI] [PubMed] [Google Scholar]
- [33].Ruzin A, Lindsay J, Novick RP. Molecular genetics of SaPI1–a mobile pathogenicity island in Staphylococcus aureus. Mol Microbiol 2001; 41:365-77; PMID:11489124; http://dx.doi.org/ 10.1046/j.1365-2958.2001.02488.x [DOI] [PubMed] [Google Scholar]
- [34].Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol 2010; 8:541-51; PMID:20634809; http://dx.doi.org/ 10.1038/nrmicro2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martínez-Rubio R, Quiles-Puchalt N, Martí M, Humphrey S, Ram G, Smyth D, Chen J, Novick RP, Penadés JR. Phage-inducible islands in the Gram-positive cocci. ISME J 2016; PMID:27959343; http://dx.doi.org/ 10.1038/ismej.2016.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Achard A, Leclercq R. Characterization of a small mobilizable transposon, MTnSag1, in Streptococcus agalactiae. J Bacteriol 2007; 189:4328-31; PMID:17416666; http://dx.doi.org/ 10.1128/JB.00213-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Poulin-Laprade D, Matteau D, Jacques P-É, Rodrigue S, Burrus V. Transfer activation of SXT/R391 integrative and conjugative elements: unraveling the SetCD regulon. Nucleic Acids Res 2015; 43(4):2045-56:gkv071; PMID:25662215; http://dx.doi.org/ 10.1093/nar/gkv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Daccord A, Ceccarelli D, Burrus V. Integrating conjugative elements of the SXT/R391 family trigger the excision and drive the mobilization of a new class of Vibrio genomic islands. Mol Microbiol 2010; 78:576-88; PMID:20807202; http://dx.doi.org/ 10.1111/j.1365-2958.2010.07364.x [DOI] [PubMed] [Google Scholar]
- [39].Daccord A, Ceccarelli D, Rodrigue S, Burrus V. Comparative analysis of mobilizable genomic islands. J Bacteriol 2013; 195:606-14; PMID:23204461; http://dx.doi.org/ 10.1128/JB.01985-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harmer CJ, Hamidian M, Ambrose SJ, Hall RM. Destabilization of IncA and IncC plasmids by SGI1 and SGI2 type Salmonella genomic islands. Plasmid 2016; 87-88:51-57; PMID:27620651; http://dx.doi.org/ 10.1016/j.plasmid.2016.09.003 [DOI] [PubMed] [Google Scholar]


