ABSTRACT
The prevalence of diabetes rapidly increased during the last decades in association with important changes in lifestyle. Diabetes and hyperglycemia are well-known for inducing deleterious effects on physiologic processes, increasing for instance cardiovascular diseases, nephropathy, retinopathy and foot ulceration. Interestingly, diabetes also impairs brain morphology and functions such as (1) decreased neurogenesis (proliferation, differentiation and cell survival), (2) decreased brain volumes, (3) increased blood-brain barrier leakage, (4) increased cognitive impairments, as well as (5) increased stroke incidence and worse neurologic outcomes following stroke. Importantly, diabetes is positively associated with a higher risk to develop Alzheimer disease. In this context, we aim at reviewing the impact of diabetes on neural stem cell proliferation, newborn cell differentiation and survival in a homeostatic context or following stroke. We also report the effects of hyper- and hypoglycemia on the blood-brain barrier physiology through modifications of tight junctions and transporters. Finally, we discuss the implication of diabetes on cognition and behavior.
KEYWORDS: blood-brain barrier, brain remodeling, cognitive impairment, diabetes, hyperglycemia, neurogenesis, stroke
Introduction
Hyperglycemia is defined as a high blood glucose concentration (> 1.26 g/L in fasting conditions) reflecting a failure in energy homeostasis and/or in glucose sensing. It can result from chronic disorder of insulin secretion and/or resistance (diabetes) or from acute stress. In the context of diabetes, chronic hyperglycemia leads to severe complications such as cardiovascular diseases, diabetic nephropathy, foot ulceration, retinopathy, and nerve damage.1 More and more evidences document the impact of metabolic disorders such as diabetes and obesity on the central nervous system (CNS).2-6 Diabetes is notably linked to changes in the blood-brain barrier (BBB) physiology of cerebral microvessels (barrier and transport functions).7,8 Furthermore, diabetic patients display lower brain volumes,9 and they increase their vulnerability to cognitive deficits and/or behavioral alterations.4,10
This review aims at further documenting the impact of diabetes/hyperglycemia on the CNS. After introducing the main experimental models of type 1 and type 2 diabetes (T1D and T2D), we will describe the impact of diabetes on neurogenesis, stroke and brain remodeling, as well as its impact on BBB physiology, on cognitive functions and behavior in mammals. Finally, we will discuss the use of zebrafish as an emerging model for studying the impact of diabetes on neurogenesis and brain remodeling.
Part 1: Insights of rodents and mammals
Models of type 1 and type 2 diabetes in rodents
The main models of type 1 diabetes (T1D) in the literature are non obese diabetic (NOD) mice and streptozotocin (STZ)-induced diabetic rodent models. NOD mice spontaneously develop T1D due to immune cell infiltration in the pancreatic islets, leading to the destruction of the insulin-synthesizing β cells.11 Despite divergences in the immune response of NOD mice and T1D subjects, NOD mice remain a representative model of human T1D.11 Another model for inducing T1D in animals is the injection of drugs such as alloxan or streptozotocin, 2 toxic glucose analogs. These compounds accumulate in pancreatic β-cells through the glucose transporter GLUT2 and lead to their destruction.12 It consequently impairs insulin synthesis and secretion and results in increased blood glucose levels.
Several models have been also developed for studying T2D in rodents. For example, db/db mice and zucker diabetic fatty (ZDF) rats are models of T2D and obesity in which the leptin receptor is deficient due to a point mutation. These rodents display high levels of fasted blood glucose. The Goto-Kakizaki (GK) rats are a well-characterized model of non-obese Wistar rats that spontaneous develop T2D.13,14 High fat diets (HFD) also allow the development of T2D in mice and rats.15-17
Diabetes impairs adult neurogenesis in rodents
Adult neurogenesis is a physiologic process involving the proliferation of adult neural progenitors, the genesis of newborn neurons, their differentiation, their migration, and their functional integration into neural networks. Such a process is spatially and temporally controlled by a wide variety of intrinsic and extrinsic factors including transcriptional regulators and hormones.18,19 In the brain of mammals, including humans, adult neurogenesis is mainly detected in 2 regions of the brain: the subventricular zone (SVZ) of the lateral ventricle and the subgranular region of the dentate gyrus (DG) of the hippocampus.20,21 Hippocampal adult neurogenesis appears to be important for learning and memory processes.
Diabetes impairs brain cell proliferation in neurogenic regions
To investigate the impact of diabetes on brain cell proliferation, several studies have used BrdU (5-bromo-2′-deoxyuridine) injection/detection as well as immunohistochemistry using antibodies raised against well-characterized proliferative markers such as PCNA (Proliferative Cell Nuclear Antigen) and Ki-67. In NOD mice, brain cell proliferation was reported to be significantly decreased in the DG compared with their control littermates.22 Similarly, using STZ-induced T1D models of mice and rats, accumulating data show that diabetes results in a decreased cell proliferation in the DG by performing BrdU and Ki-67 stainings.10,23,24 Interestingly, only 6 days after STZ injection, diabetic mice already displayed a lower number of proliferative cells in the DG.25
In a similar way, T2D models mainly exhibit a decrease in brain cell proliferation in the DG, as shown in db/db mice and in HFD mice and rats.17,26 Interestingly, HFD impairs neurogenesis in young rats (when intake started at weaning) but not when it started in adults as revealed by performing DCX-immunostaining, a marker of newly generated neurons.27 It suggests that the hippocampus would be more sensitive to HFD during a critical period of development. In striking contrast, other studies described an increase in hippocampal cell proliferation in both db/db and HFD mice as well as in GK rats,10,28 while another work shows the absence of effects of the diabetic status on cell proliferation of the DG.29 To our knowledge, fewer studies investigate the impact of diabetes on brain cell proliferation in the SVZ. Six week-old GK rats and their respective controls had a similar pattern of neural progenitor proliferation in the SVZ, while 18 week-old GK rats displayed a strong increase in dividing neural progenitors compared with controls.30 Proliferation of the SVZ striatal wall of the lateral ventricle was also reported to be not impacted in HFD rats (Rivera et al., 2011), while a drastic decrease in cell proliferation was observed after only 20 days of treatment STZ-injected mice reported.31 Such discrepancies could be attributed to differences in the age of the animals, the duration and the gravity of the diabetic status, and also the species used.
Taken together, these data tend to show that T1D and T2D mainly induce a significant decrease in cell proliferation of the subgranular zone of the DG (Fig. 1).
Figure 1.
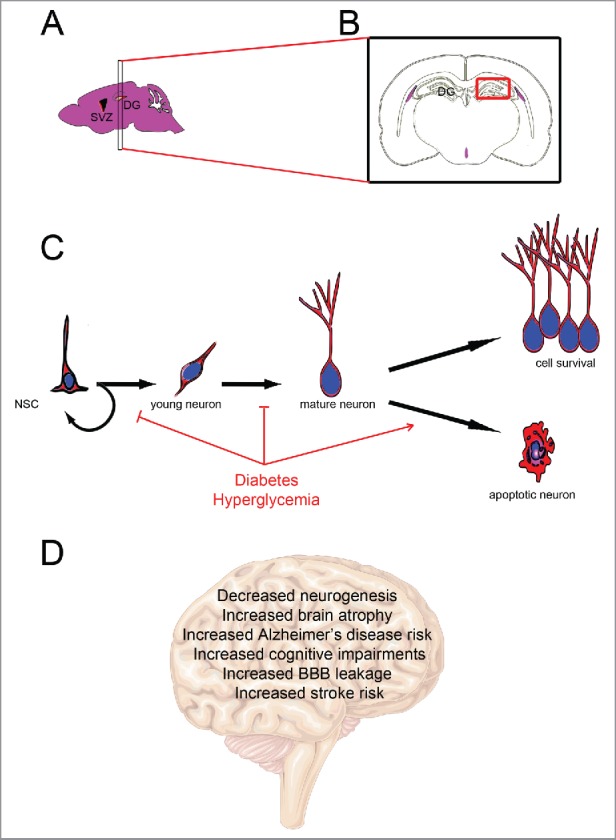
Diabetes impairs adult neurogenesis and brain functions. (A) Sagittal mouse brain section showing the main neurogenic regions: the subventricular zone of the lateral ventricle (SVZ) and the subgranular region of the dentate gyrus of the hippocampus (DG). (B) Coronal section through the dentate gyrus of the hippocampus corresponding to the most studied neurogenic region in diabetes. (C) Neurogenic processes involve neural stem cell proliferation (including self-renewing) and the generation of young neurons that differentiate into mature neurons and integrate preexisting neural networks. Diabetes/hyperglycemia has been shown to inhibit neural stem cell proliferation and neuronal differentiation, and to promote apoptosis. (D) Effects of diabetes on brain functions and physiology.
Diabetes impairs brain cell differentiation in neurogenic regions
Although most studies show that diabetes impairs brain cell proliferation in neurogenic niches, the picture appears more contrasted by investigating the effects of diabetes on neural differentiation. Different studies using BrdU experiments and co-staining with mature and immature neuronal markers (i.e. doublecortin -DCX-, Tuj-1/β-III tubulin) have documented the negative impact of diabetes on neuronal differentiation. For instance, the number of DCX-positive cells in the granule cell layer did not show statistically significant differences between experimental groups.32 However, in STZ-treated mice and rats, a significant decrease in newly generated neurons was observed.24,33,34 T2D models of mice (HFD) and rats (ZDF) also display a reduced neuronal differentiation.26,35 However, few studies reported no change in neuronal differentiation under type 1 and type 2 diabetic status;22,28,36 and in striking contrast, a 2-fold increase in neuronal differentiation was reported in GK rats at 4 months of age compared with controls, assuming a compensatory mechanism induced by neuronal damage.37
In addition, STZ models of mice and rats as well as HFD rats and db/db mice, display a consistent decreased in dendritic density and branching.10 For instance, the dendritic length of pyramidal cells from the prefrontal cortex, the occipital cortex, and the hippocampus of T1D rats was decreased (from 20% to 45%) as well as their density (from 36% to 58%) compared with controls.38 Such an inhibition of synaptogenesis was also reinforced in HFD model in rats and db/db mouse.10,36
Taken together, these data highlight the fact that diabetes impairs neuronal differentiation (Fig. 1), including decreased hippocampal dendritic density and branching leading to an overall decrease in hippocampal volume.39 Such data probably argue in favor of a link between diabetes and cognitive impairment,10 given that the hippocampus is involved in memory processes.
Diabetes impairs brain cell survival
Most studies documented that T1D and T2D result in a decreased survival of newborn cells,10 except in GK rats for which no significant difference was observed in cell survival 3 weeks after BrdU injection, while proliferation was impacted.37 STZ models of rat have shown that diabetes increases neuronal death through apoptotic mechanisms in the hippocampus.40 For instance, the number of TUNEL-positive neurons was increased,41-43 and the expression of pro-apoptotic protein such as Caspase-3 and Bax was upregulated.44-46 Taken together, these data highlight that diabetes impairs neuronal survival by increasing Caspase-3 expression and activity, and apoptotic gene regulators40 (Fig. 1).
Hyperglycemia, stroke and injury-induced neurogenesis in rodents and humans
Hyperglycemia and stroke
Hyperglycemia is an independent factor of poor outcome after acute ischemic stroke (AIS). There is a strong association between admission blood glucose levels and intracerebral hemorrhage following thrombolytic therapy (recombinant tissue plasminogen activator, rtPA) after stroke.47 Fig. 2 shows the increase of hemorrhagic transformation following middle cerebral artery occlusion (MCAO) in mouse. Recently, hyperglycemia was shown to be independently associated with worse outcome at 3 months after mechanical therapy (thrombectomy) in AIS patients.48 The mechanisms by which hyperglycemia affects ischemic tissues include glutamate accumulation, intracellular acidosis, BBB disruption accompanied by an increased matrix metalloprotease (MMP) activity, brain edema formation, and inhibited plasma fibrinolysis.49-52 There is only few data showing deleterious effects of hyperglycemia in very early reperfusion.53 In clinical conditions, the detrimental effect of acute hyperglycemia was shown to be higher after early (< 3 hours) than after delayed or no reperfusion.54 These findings support the notion that acute hyperglycemia was sufficient to increase infarct volume and that this detrimental effect was also dependent on the time of reperfusion.
Figure 2.
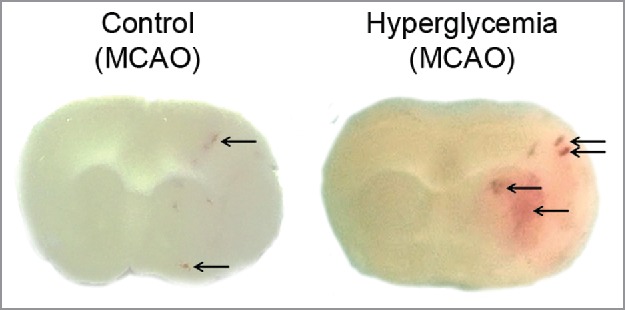
Hyperglycemia promotes hemorrhagic transformation in MCAO models. Representative brain sections of mouse following MCAO (3 hours) under control or hyperglycemic conditions. Arrows highlight hemorrhagic transformation that is obviously increased in hyperglycemia.
In a model of MCAO in db/db mice, increased mortality, infarct volume and cerebral edema were reported relative to db/+ mice. This was associated with a worsened neurologic status.55 Wound healing was reported to be impaired after cerebral ischemia and hypoxia in db/db mice, potentially due to a decreased inflammatory response in diabetic conditions. 56 db/db mice had also increased brain hemorrhage and displayed more severely injured white matter than nondiabetic mice after MCAO. MMP-9 as well as apoptotic markers were upregulated in db/db mice and in primary cortical neuron culture.57 In a different model of hypoxia (8% O2) and ischemia (carotid ligation), gelatinase activity (MMP-2 and 9) was more increased in the infarcted hemisphere of db/db relative to db/+ mice, suggesting a protease-mediated alteration of the BBB.58 Other mechanisms such as increased autophagy may participate in the aggravation of the brain insult under diabetic conditions as shown in a STZ-induced diabetes model in mice.59 In a similar model, microangiopathy associated with diabetes was shown to impact on post-stroke remodeling (vasoreactivity was abolished and the angiogenesis was delayed).60 After ischemic stroke, a decreased vascularization is also noticed in the ischemic cortex and striatum of diabetic rats compared with control.61 In addition, they displayed increased swelling and number of astrocytic processes highlighting that the healing process is impaired. Beyond microvessels diabetic conditions also affect the proliferation and survival of oligodendrocyte progenitor cells that potentially reduce the white matter response to ischemia.62 Whereas epigenetic modifications may occur during diabetes and participate in aggravating ischemia-reperfusion consequences,63 hyperglycemia induced by stress even in nondiabetic condition is sufficient to worsen ischemic stroke outcome.64
Hyperglycemia and injury-induced neurogenesis
Interestingly, neurogenesis plays a crucial role in cerebral recovery occurring after stroke; the formation of new neurons in the SVZ and in the DG being promoted in such a pathological condition.65-67 Severe hyperglycemia leads to a strong decrease in injury-induced proliferation in the SVZ of rats following MCAO.68 The downregulation of CREB phosphorylation and the decrease of BDNF expression appear to be involved in the injury-induced neurogenesis impairment in a hyperglycemic context. In the same line of evidences, diabetes inhibits BDNF expression in the hippocampus and decreases the survival and the differentiation of the newly generated neurons after ischemia in rats.69 Such adverse effects of hyperglycemia in reactive neurogenesis were also highlighted by in vitro ischemia of adult rats neural stem cells in which, hyperglycemia promotes apoptosis and decreases proliferation.70 Hyperglycemia is also known to promote the production of ROS and free radicals and could thus promotes their formation in the ischemic brain, leading to an increased cell death.71
Hyperglycemia and blood-brain barrier in rodents and humans
The BBB plays a key role in maintaining the CNS homeostasis. The BBB separates the peripheral blood circulation from the brain parenchyma. On the one hand, the BBB prevents detrimental substances to enter the brain, and on the other hand, the BBB will facilitate nutrients and oxygen passage in the CNS. The BBB is also involved in the water and electrolyte equilibrium in the brain interstitial fluid.72 The particular structure and the specific properties of the vessels composing the BBB are responsible for the different functions of the BBB. Structurally, the BBB is characterized by the presence of tight junctions, and it has also specific transporters and electric properties. Endothelial dysfunction is a hallmark of diabetes; it has been linked to T2D and to insulin resistance in experimental and clinical studies, and during the onset of cerebral ischemia, the role of endothelial cells composing the BBB is critical. The vasculature appears also as an important component of the neurogenic niches given that endothelial cells regulate neural stem cell activity through diffusible signal that are not clearly identified.73,74
Transporters
Glucose transporters
As recently reviewed, many experimental studies report a downregulation of BBB glucose transporters in hyperglycemic animals, however other works did not reveal significant changes in the BBB glucose transporters expression in DM.75 In hypoglycemia conditions, glucose transporters such as glut1, glut4 or SGLT-1 (Na+/glucose co-transporter) have been reported to be upregulated.75,76
Efflux transporters
Glycemic changes have also been studied on the ABC family transporters, which includes the P-glycoprotein (Pgp) and multidrug resistance-related proteins (MRPs). These transporters play a key role in brain homeostasis and mediate active efflux of many potential toxicants including lipophilic compounds.77 In the hCMEC/D3 BBB cellular model, acute hypoglycemic exposure upregulated BBB endothelial expression of P-glycoprotein, breast cancer resistance protein (BCRP) and MRP1 and 4 proteins, while acute hyperglycemic exposure induced BCRP activity. Repeated hyperglycemic episodes up-regulate P-glycoprotein activity.76 In rat model of diabetes (STZ), contradictory results have been reported in the brain with no change or a decrease of P-glycoprotein expression.78-80 Actually, the modulation of efflux transporters expression in the brain is region dependent, with an increase of P-glycoprotein expression in the hippocampus and in the striatum and a decrease in the cerebral cortex of the diabetic rats when compared with control.81,82 For BCRP, a decrease in the cortex of diabetic rats has been described.83 It is likely that other transporter may be affected in diabetes as expression of transporters such as L-type amino acid transporter 1 (LAT1), have been described to be affected in an inflammatory environment.84
Tight junctions and integrity
Variation of glycemia affects tight junction (VE-cadherin, claudin, zona-occludens (ZO)) and permeability on endothelial cells mimicking the BBB. Hyperglycemia promotes cerebral-barrier dysfunction through activation of PKC-β and consequent stimulations of oxidative stress and tight junction dissolution.85 Hyperglycemia increases VE-cadherin and decreases claudin 5 expression, while hypoglycemia modify ZO-1 localization and also decreases claudin 5 expression and increases the permeability of the BBB.76
Taken together, these data highlight the impact of hypo- and hyperglycemia on the integrity of the BBB and its physiology (Fig. 3). It also suggests that under pathological condition (diabetes), the microenvironment regulating neural stem cell activity could be disturbed, impairing neurogenesis and favoring neurodegeneration. Such links between metabolic disorders (diabetes and obesity) and BBB disruption could favor cognitive impairment and modulation of feeding neuronal networks.
Figure 3.

Hyperglycemia and hypoglycemia impairs BBB physiology and integrity. (A) In normoglycemic context, tight junctions strongly maintain endothelial cells between them avoiding paracellular transport. Efflux pumps, amino acids and glucose transporters work in an appropriate fashion to maintain brain homeostasis. (B) In hyperglycemic context, tight junction expression is decreased allowing paracellular transport leading to BBB leakage. Glucose transporter expression appears to be reduced in hyperglycemia while efflux pump expression is increased. (C) In hypoglycemic context, tight junction expression is decreased allowing paracellular transport leading also to BBB leakage. Glucose transporters and efflux pump expression are increased.
Diabetes, neurotransmission, cognitive impairment and behavior in rodents and humans
The brain is a glucose-dependent organ, which can be damaged by both hypoglycemia and hyperglycemia.86 In the hippocampus, diabetes leads to a reduced hippocampal volume due to a decrease in dendritic branching.10 The membrane potential, the membrane input resistance, and membrane time constant, remain unaffected in diabetes.10 Still in the hippocampus, the basal neurotransmission appears to be uncertain: (1) long-term potentiation (LTP), a cellular mechanism implied in learning and memory, is affected in CA1 neurons of the hippocampus in STZ and HFD treated rodents, (2) LTP appears to be impacted by both the duration and the severity of diabetes.87,88 The impairment of LTP in the different diabetic models results in cognitive impairments observed through different neurologic tests (Y-maze, Morris water maze, novel recognition test). Functional MRI studies showed a dysfunction in glucose metabolism in patient having Alzheimer disease and also in small subcortical structures in patients with Parkinson disease.89,90
Besides showing significantly more cognitive deficits, T1D and T2D patients display equally more behavioral alterations such as depression, related to their condition.4,10 In fact, depression is a risk factor for developing diabetes91 as glycemic control is affected by lifestyle (e.i. self-care, exercise, diet, medication outlet) which is often neglected in the case of depression. Metabolic defects and neuropsychiatric diseases consequently appear to be linked.92 Through ATP generation, glucose is the main source of energy for the brain and a tight glucose regulation is required for a suitable brain homeostasis, as well as neurotransmitter biosynthesis.93 As an illustration, a significant increase in acetylcholinesterase activity was observed in the hippocampus of diabetic rats induced by high fat diet followed by STZ injection. These rats showed reduced glutamate levels in their hippocampus and increased GABA levels in their hippocampus and cortex compared with normal animals. They also display altered performance in various behavioral tests such as Morris water maze, open field and elevated plus maze. A prolonged uncontrolled hyperglycemia and impaired insulin function resulted in cognitive deficits and altered behaviors.94
However, glucose is not only a nutrient, but also an input for neuronal circuits located in the hypothalamus (e.i. VMH, ARC, PVN) and containing pro-opiomelanocortin (POMC), melanin concentrating hormone (MCH), and neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons.95-97 These neuronal populations are involved in energy storage/expenditure and food intake.98,99 Numerous study have proved that (1) mice lacking MCH are hypophagic and lean, (2) direct injection of NPY into the hypothalamus of rats stimulates food intake, (3) mice lacking the POMC-derived peptides become obese and present defective adrenal development, (4) activation of AgRP neurons in mice induced voracious feeding.100-104 Interestingly, it was recently demonstrated that the non-nutritive sweetener sucralose promoted food intake via a NPY-dependent mechanism, supporting a link between synthetic sweetener consumption and metabolic dysregulations.105 Consequently, disruption of such feeding neural networks could result in an increased risk to develop diabetes.
Part 2: Zebrafish as an emerging model for studying the impact of diabetes on neurogenesis and brain remodeling
In few years, zebrafish became a recognized model for studying adult neurogenesis and brain repair mechanisms. In contrast to mammals, the brain of adult zebrafish maintains a lot of neurogenic niches across the whole brain.21 Such a strong neurogenic capacity is due to the persistence of (a) radial glial cells, acting like neural stem cells, and of (b) further committed progenitors (neuroblasts).106 Teleost fish also display a strong capability for brain regeneration.19,107,108 Zebrafish is also a common model for investigating a wide variety of physiologic and physiopathological processes including metabolic syndrome and diabetes.109-113 T2D has been developed by immersing fish in water supplemented with D-glucose,109,111,114 and T1D has been set up by STZ intraperitoneal (ip) injection.109 Acute hyperglycemia can be also mimicked by intraperitoneal injection of D-glucose.112 Consequently, zebrafish appear as interesting models for investigating the role of metabolic disorders such as diabetes on the CNS.
The impact of hyperglycemia on the blood-brain and blood-retinal barriers has been assessed in zebrafish. In our experimental conditions, we recently demonstrated that acute hyperglycemia (2.5 g/kg of body weight) leads to impaired expression of genes involved in the establishment of blood-brain barrier (claudin 5a, zo1a and 1b).112 In addition, Alvarez and colleagues also showed that the blood-retinal barrier is compromised in glucose-treated (110mM; 30 days) fish.115
Recent data also implies hyperglycemia in the disruption of neurogenic processes as well as in memory process in zebrafish. Chronic hyperglycemia induced by putting fish in water supplemented with D-glucose (111mM, 14 days) impairs brain cell proliferation along the neurogenic niches.34 In the same line of evidences, mechanical injury of the zebrafish telencephalon under such chronic hyperglycemia conditions leads to impaired injury-induced stem cell proliferation at 7 days post-injury.34 Until now, the impact of acute and chronic hyperglycemia has not yet been assessed for newborn cell migration and cell survival under homeostatic and regenerative conditions. Last but not least, chronic hyperglycemia (111mM; 14 days) has been shown to impair memory in zebrafish through increased acethylcholinesterase activity.116
Taken together, these data show that diabetes impairs blood-brain and -retinal barriers, constitutive and regenerative neurogenesis as well as cognitive functions in zebrafish, similar to what was shown in mammals. It consequently highlights the use of zebrafish as a model for investigating the impact of hyperglycemia on the CNS and for screening therapeutically molecules to restore impaired functions.
Conclusion
Most studies using different models of diabetes tend to show that diabetes impairs neurogenic processes including neural stem cell proliferation, neuronal differentiation and survival. Hyperglycemia is also a risk factor for stroke and a predictor of bad neurologic outcomes, impairing notably the injury-induced neurogenic process. In addition, diabetes is associated with morphological and functional changes of the CNS. In the ADNI cohort, T2D patients displayed a lower brain volume and a disturbed brain glucose metabolism compared with non-diabetic participants.9 Another study involving 614 patients with T2D has shown a significant association between the duration of diabetes and an increase in abnormal gray and white matters.117 Interestingly, diabetes is implied in cognitive impairment. Indeed, T1D patients exhibit mainly modest, but sometimes severe, cognitive impairments. In contrast, T2D patients display more consistently moderate cognitive impairments in verbal memory tasks and complex information processing.40,118-120 An increasing number of data and epidemiological studies also link diabetes and Alzheimer disease;121,122 T2D being associated with an almost 2-fold increased risk of dementia.123,124 By modifying the BBB physiology, diabetes/hyperglycemia could disturb the microenvironment of neural stem cell niches and impairs neural stem cell activity and newborn cell survival. Diabetes consequently disturbs brain functions and homeostasis leading to cognitive impairment and impaired feeding behavior reinforcing its deleterious impact through a vicious circle.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].World Health Organisation W Diabetes 2016; http://wwwwhoint/diabetes/en/ [Google Scholar]
- [2].Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre d'Hellencourt C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflammation 2016; 13:67; PMID:27012931; http://dx.doi.org/ 10.1186/s12974-016-0530-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cukierman-Yaffe T. Diabetes, dysglycemia and cognitive dysfunction. Diabetes Metab Res Rev 2014; 30:341-5; PMID:24339052; http://dx.doi.org/ 10.1002/dmrr.2507 [DOI] [PubMed] [Google Scholar]
- [4].Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev 2008; 29:494-511; PMID:18436709; http://dx.doi.org/ 10.1210/er.2007-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJ, Pocock SJ. BMI and risk of dementia in two million people over two decades:a retrospective cohort study. Lancet Diabetes Endocrinol 2015; 3:431-6; PMID:25866264; http://dx.doi.org/ 10.1016/S2213-8587(15)00033-9 [DOI] [PubMed] [Google Scholar]
- [6].Letra L, Santana I, Seica R. Obesity as a risk factor for Alzheimer's disease:the role of adipocytokines. Metab Brain Dis 2014; 29:563-8; PMID:24553879; http://dx.doi.org/ 10.1007/s11011-014-9501-z [DOI] [PubMed] [Google Scholar]
- [7].Mooradian AD. Central nervous system complications of diabetes mellitus–a perspective from the blood-brain barrier. Brain Res Brain Res Rev 1997; 23:210-8; PMID:9164671; http://dx.doi.org/ 10.1016/S0165-0173(97)00003-9 [DOI] [PubMed] [Google Scholar]
- [8].Horani MH, Mooradian AD. Effect of diabetes on the blood brain barrier. Curr Pharm Des 2003; 9:833-40; PMID:12678883; http://dx.doi.org/ 10.2174/1381612033455314 [DOI] [PubMed] [Google Scholar]
- [9].Li W, Risacher SL, Huang E, Saykin AJ, Alzheimer's Disease Neuroimaging I. Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 2016; 87:595-600; PMID:27385744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 2013; 37:1346-62; PMID:23680701; http://dx.doi.org/ 10.1016/j.neubiorev.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giarratana N, Penna G, Adorini L. Animal models of spontaneous autoimmune disease: type 1 diabetes in the nonobese diabetic mouse. Methods Mol Biol 2007; 380:285-311; PMID:17876100 [DOI] [PubMed] [Google Scholar]
- [12].Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008; 51:216-26; PMID:18087688; http://dx.doi.org/ 10.1007/s00125-007-0886-7 [DOI] [PubMed] [Google Scholar]
- [13].Portha B, Giroix MH, Tourrel-Cuzin C, Le-Stunff H, Movassat J. The GK rat: a prototype for the study of non-overweight type 2 diabetes. Methods Mol Biol 2012; 933:125-59; PMID:22893405 [DOI] [PubMed] [Google Scholar]
- [14].Goto Y, Suzuki K, Ono T, Sasaki M, Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat). Adv Exp Med Biol 1988; 246:29-31; PMID:3074659 [DOI] [PubMed] [Google Scholar]
- [15].Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004; 53 Suppl 3:S215-9; PMID:15561913; http://dx.doi.org/ 10.2337/diabetes.53.suppl_3.S215 [DOI] [PubMed] [Google Scholar]
- [16].Park HR, Park M, Choi J, Park KY, Chung HY, Lee J. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 2010; 482:235-9; PMID:20670674; http://dx.doi.org/ 10.1016/j.neulet.2010.07.046 [DOI] [PubMed] [Google Scholar]
- [17].Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 2006; 13:1385-8; PMID:17116226; http://dx.doi.org/ 10.1111/j.1468-1331.2006.01500.x [DOI] [PubMed] [Google Scholar]
- [18].Hsieh J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev 2012; 26:1010-21; PMID:22588716; http://dx.doi.org/ 10.1101/gad.187336.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pellegrini E, Coumailleau P, Kah O, Diotel N. Aromatase and estrogens: involvement in constitutive and regenerative neurogenesis in adult zebrafish In: Duncan Kelli A. eds Estrogen Effects on Traumatic Brain Injury - Mechanisms of Neuroprotection and Repair: 51-71; 2015. [Google Scholar]
- [20].Braun SM, Jessberger S. Adult neurogenesis: mechanisms and functional significance. Development 2014; 141:1983-6; http://dx.doi.org/ 10.1242/dev.104596 [DOI] [PubMed] [Google Scholar]
- [21].Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol 2006; 80:281-307; PMID:17218052; http://dx.doi.org/ 10.1016/j.pneurobio.2006.11.007 [DOI] [PubMed] [Google Scholar]
- [22].Beauquis J, Saravia F, Coulaud J, Roig P, Dardenne M, Homo-Delarche F, De Nicola A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol 2008; 210:359-67; PMID:18190910; http://dx.doi.org/ 10.1016/j.expneurol.2007.11.009 [DOI] [PubMed] [Google Scholar]
- [23].Jackson-Guilford J, Leander JD, Nisenbaum LK. The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett 2000; 293:91-4; PMID:11027841; http://dx.doi.org/ 10.1016/S0304-3940(00)01502-0 [DOI] [PubMed] [Google Scholar]
- [24].Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand 2008; 117:205-10; PMID:17854417; http://dx.doi.org/ 10.1111/j.1600-0404.2007.00928.x [DOI] [PubMed] [Google Scholar]
- [25].Revsin Y, Rekers NV, Louwe MC, Saravia FE, De Nicola AF, de Kloet ER, Oitzl MS. Glucocorticoid receptor blockade normalizes hippocampal alterations and cognitive impairment in streptozotocin-induced type 1 diabetes mice. Neuropsychopharmacology 2009; 34:747-58; PMID:18784648; http://dx.doi.org/ 10.1038/npp.2008.136 [DOI] [PubMed] [Google Scholar]
- [26].Hwang IK, Kim IY, Kim DW, Yoo KY, Kim YN, Yi SS, Won MH, Lee IS, Yoon YS, Seong JK. Strain-specific differences in cell proliferation and differentiation in the dentate gyrus of C57BL/6N and C3H/HeN mice fed a high fat diet. Brain Res 2008; 1241:1-6; PMID:18761331; http://dx.doi.org/ 10.1016/j.brainres.2008.08.024 [DOI] [PubMed] [Google Scholar]
- [27].Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012; 22:2095-100; PMID:22593080; http://dx.doi.org/ 10.1002/hipo.22032 [DOI] [PubMed] [Google Scholar]
- [28].Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J Neurosci Res 2011; 89:481-9; PMID:21312223; http://dx.doi.org/ 10.1002/jnr.22565 [DOI] [PubMed] [Google Scholar]
- [29].Rivera P, Romero-Zerbo Y, Pavon FJ, Serrano A, Lopez-Avalos MD, Cifuentes M, Grondona JM, Bermúdez-Silva FJ, Fernández-Llebrez P, de Fonseca FR, et al.. Obesity-dependent cannabinoid modulation of proliferation in adult neurogenic regions. Eur J Neurosci 2011; 33:1577-86; PMID:21395869; http://dx.doi.org/ 10.1111/j.1460-9568.2011.07650.x [DOI] [PubMed] [Google Scholar]
- [30].Lang BT, Yan Y, Dempsey RJ, Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res 2009; 1258:25-33; PMID:19138677; http://dx.doi.org/ 10.1016/j.brainres.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saravia F, Revsin Y, Lux-Lantos V, Beauquis J, Homo-Delarche F, De Nicola AF. Oestradiol restores cell proliferation in dentate gyrus and subventricular zone of streptozotocin-diabetic mice. J Neuroendocrinol 2004; 16:704-10; PMID:15271063; http://dx.doi.org/ 10.1111/j.1365-2826.2004.01223.x [DOI] [PubMed] [Google Scholar]
- [32].Alvarez EO, Beauquis J, Revsin Y, Banzan AM, Roig P, De Nicola AF, Saravia F. Cognitive dysfunction and hippocampal changes in experimental type 1 diabetes. Behav Brain Res 2009; 198:224-30; PMID:19041902; http://dx.doi.org/ 10.1016/j.bbr.2008.11.001 [DOI] [PubMed] [Google Scholar]
- [33].Beauquis J, Roig P, Homo-Delarche F, De Nicola A, Saravia F. Reduced hippocampal neurogenesis and number of hilar neurones in streptozotocin-induced diabetic mice: reversion by antidepressant treatment. Eur J Neurosci 2006; 23:1539-46; PMID:16553617; http://dx.doi.org/ 10.1111/j.1460-9568.2006.04691.x [DOI] [PubMed] [Google Scholar]
- [34].Beauquis J, Roig P, De Nicola AF, Saravia F. Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice. PLoS One 2010; 5:e13993; PMID:21085588; http://dx.doi.org/ 10.1371/journal.pone.0013993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, et al.. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res 2009; 34:1039-46; PMID:18982449; http://dx.doi.org/ 10.1007/s11064-008-9870-y [DOI] [PubMed] [Google Scholar]
- [36].Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 2008; 11:309-17; PMID:18278039; http://dx.doi.org/ 10.1038/nn2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, Portha B, De Nicola AF, Saravia F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol 2010; 222:125-34; PMID:20045412; http://dx.doi.org/ 10.1016/j.expneurol.2009.12.022 [DOI] [PubMed] [Google Scholar]
- [38].Martinez-Tellez R, Gomez-Villalobos Mde J, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res 2005; 1048:108-15; PMID:15916754; http://dx.doi.org/ 10.1016/j.brainres.2005.04.048 [DOI] [PubMed] [Google Scholar]
- [39].Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci 2007; 257:250-60; PMID:17401728; http://dx.doi.org/ 10.1007/s00406-007-0728-0 [DOI] [PubMed] [Google Scholar]
- [40].Sadeghi A, Hami J, Razavi S, Esfandiary E, Hejazi Z. The effect of diabetes mellitus on apoptosis in hippocampus: cellular and molecular aspects. Int J Prev Med 2016; 7:57; PMID:27076895; http://dx.doi.org/ 10.4103/2008-7802.178531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jafari Anarkooli I, Sankian M, Ahmadpour S, Varasteh AR, Haghir H. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp Diabetes Res 2008; 2008:638467; PMID:18923682; http://dx.doi.org/ 10.1155/2008/638467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yonguc GN, Dodurga Y, Adiguzel E, Gundogdu G, Kucukatay V, Ozbal S, Yilmaz I, Cankurt U, Yilmaz Y, Akdogan I. Grape seed extract has superior beneficial effects than vitamin E on oxidative stress and apoptosis in the hippocampus of streptozotocin induced diabetic rats. Gene 2015; 555:119-26; PMID:25445279; http://dx.doi.org/ 10.1016/j.gene.2014.10.052 [DOI] [PubMed] [Google Scholar]
- [43].Chen Z, He Y, Song C, Dong Z, Su Z, Xue J. Sericin can reduce hippocampal neuronal apoptosis by activating the Akt signal transduction pathway in a rat model of diabetes mellitus. Neural Regen Res 2012; 7:197-201; PMID:25767499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen Y, Li L, Li Z, Huang X, Zhang L, Chen W. [Effects of naokang erhao decoction on cognitive ability and hippocampal apoptosis-related proteins in diabetic rats]. Zhongguo Zhong Yao Za Zhi 2011; 36:1519-23; PMID:22779191 [PubMed] [Google Scholar]
- [45].Zhang T, Jia W, Sun X. 3-n-Butylphthalide (NBP) reduces apoptosis and enhances vascular endothelial growth factor (VEGF) up-regulation in diabetic rats. Neurol Res 2010; 32:390-6; PMID:20483006; http://dx.doi.org/ 10.1179/016164110X12670144526264 [DOI] [PubMed] [Google Scholar]
- [46].Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 2009; 92:251-9; PMID:19138703; http://dx.doi.org/ 10.1016/j.pbb.2008.12.012 [DOI] [PubMed] [Google Scholar]
- [47].Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis 2007; 24:1-10; PMID:17519538; http://dx.doi.org/ 10.1159/000103110 [DOI] [PubMed] [Google Scholar]
- [48].Kim JT, Jahan R, Saver JL, Investigators S. Impact of glucose on outcomes in patients treated with mechanical thrombectomy: a post Hoc analysis of the solitaire flow restoration with the intention for thrombectomy study. Stroke 2016; 47:120-7; PMID:26658447; http://dx.doi.org/ 10.1161/STROKEAHA.115.010753 [DOI] [PubMed] [Google Scholar]
- [49].Li PA, Shuaib A, Miyashita H, He QP, Siesjo BK, Warner DS. Hyperglycemia enhances extracellular glutamate accumulation in rats subjected to forebrain ischemia. Stroke 2000; 31:183-92; PMID:10625736; http://dx.doi.org/ 10.1161/01.STR.31.1.183 [DOI] [PubMed] [Google Scholar]
- [50].Pulsinelli WA, Waldman S, Rawlinson D, Plum F. Moderate hyperglycemia augments ischemic brain damage: a neuropathologic study in the rat. Neurology 1982; 32:1239-46; PMID:6890157; http://dx.doi.org/ 10.1212/WNL.32.11.1239 [DOI] [PubMed] [Google Scholar]
- [51].Hafez S, Abdelsaid M, El-Shafey S, Johnson MH, Fagan SC, Ergul A. Matrix metalloprotease 3 exacerbates hemorrhagic transformation and worsens functional outcomes in hyperglycemic stroke. Stroke 2016; 47:843-51; PMID:26839355; http://dx.doi.org/ 10.1161/STROKEAHA.116.013283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Won SJ, Tang XN, Suh SW, Yenari MA, Swanson RA. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production. Ann Neurol 2011; 70:583-90; PMID:22002675; http://dx.doi.org/ 10.1002/ana.22538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Martin A, Rojas S, Chamorro A, Falcon C, Bargallo N, Planas AM. Why does acute hyperglycemia worsen the outcome of transient focal cerebral ischemia? Role of corticosteroids, inflammation, and protein O-glycosylation. Stroke 2006; 37:1288-95; PMID:16601221; http://dx.doi.org/ 10.1161/01.STR.0000217389.55009.f8 [DOI] [PubMed] [Google Scholar]
- [54].Alvarez-Sabin J, Molina CA, Ribo M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke 2004; 35:2493-8; PMID:15472110; http://dx.doi.org/ 10.1161/01.STR.0000143728.45516.c6 [DOI] [PubMed] [Google Scholar]
- [55].Tureyen K, Bowen K, Liang J, Dempsey RJ, Vemuganti R. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem 2011; 116:499-507; PMID:21133923; http://dx.doi.org/ 10.1111/j.1471-4159.2010.07127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab 2007; 27:710-8; PMID:16926846 [DOI] [PubMed] [Google Scholar]
- [57].Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 2011; 42:445-52; PMID:21193743; http://dx.doi.org/ 10.1161/STROKEAHA.110.596486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem 2011; 119:1029-40; PMID:21923664; http://dx.doi.org/ 10.1111/j.1471-4159.2011.07487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wei N, Yu SP, Gu XH, Chen DD, Whalin MK, Xu GL, Liu XF, Wei L. The involvement of autophagy pathway in exaggerated ischemic brain damage in diabetic mice. CNS Neurosci Ther 2013; 19:753-63; PMID:23731488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Poittevin M, Bonnin P, Pimpie C, Riviere L, Sebrie C, Dohan A, Pocard M, Charriaut-Marlangue C, Kubis N. Diabetic microangiopathy: impact of impaired cerebral vasoreactivity and delayed angiogenesis after permanent middle cerebral artery occlusion on stroke damage and cerebral repair in mice. Diabetes 2015; 64:999-1010; PMID:25288671; http://dx.doi.org/ 10.2337/db14-0759 [DOI] [PubMed] [Google Scholar]
- [61].Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 2013; 44:2875-82; PMID:23920018; http://dx.doi.org/ 10.1161/STROKEAHA.113.001660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yatomi Y, Tanaka R, Shimada Y, Yamashiro K, Liu M, Mitome-Mishima Y, Miyamoto N, Ueno Y, Urabe T, Hattori N. Type 2 diabetes reduces the proliferation and survival of oligodendrocyte progenitor cells in ishchemic white matter lesions. Neuroscience 2015; 289:214-23; PMID:25592431; http://dx.doi.org/ 10.1016/j.neuroscience.2014.12.054 [DOI] [PubMed] [Google Scholar]
- [63].Kalani A, Kamat PK, Tyagi N. Diabetic Stroke Severity: Epigenetic Remodeling and Neuronal, Glial, and Vascular Dysfunction. Diabetes 2015; 64:4260-71; PMID:26470785; http://dx.doi.org/ 10.2337/db15-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huang J, Liu B, Yang C, Chen H, Eunice D, Yuan Z. Acute hyperglycemia worsens ischemic stroke-induced brain damage via high mobility group box-1 in rats. Brain Res 2013; 1535:148-55; PMID:24012767; http://dx.doi.org/ 10.1016/j.brainres.2013.08.057 [DOI] [PubMed] [Google Scholar]
- [65].Bachor TP, Suburo AM. Neural stem cells in the diabetic brain. Stem Cells Int 2012; 2012:820790; PMID:23213341; http://dx.doi.org/ 10.1155/2012/820790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yoneyama M, Shiba T, Hasebe S, Ogita K. Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J Pharmacol Sci 2011; 115:425-32; PMID:21422724; http://dx.doi.org/ 10.1254/jphs.11R02CP [DOI] [PubMed] [Google Scholar]
- [67].Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A 2001; 98:4710-5; PMID:11296300; http://dx.doi.org/ 10.1073/pnas.081011098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tan S, Zhi PK, Luo ZK, Shi J. Severe instead of mild hyperglycemia inhibits neurogenesis in the subventricular zone of adult rats after transient focal cerebral ischemia. Neuroscience 2015; 303:138-48; PMID:26126927; http://dx.doi.org/ 10.1016/j.neuroscience.2015.06.041 [DOI] [PubMed] [Google Scholar]
- [69].Han H, Wu LM, Han MX, Yang WM, Wang YX, Fang ZH. Diabetes impairs spatial learning and memory and hippocampal neurogenesis via BDNF in rats with transient global ischemia. Brain Res Bull 2016; 124:269-77; PMID:27233782; http://dx.doi.org/ 10.1016/j.brainresbull.2016.05.011 [DOI] [PubMed] [Google Scholar]
- [70].Chen J, Guo Y, Cheng W, Chen R, Liu T, Chen Z, Tan S. High glucose induces apoptosis and suppresses proliferation of adult rat neural stem cells following in vitro ischemia. BMC Neurosci 2013; 14:24; PMID:23452440; http://dx.doi.org/ 10.1186/1471-2202-14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Bruno A, Liebeskind D, Hao Q, Raychev R, Investigators US. Diabetes mellitus, acute hyperglycemia, and ischemic stroke. Curr Treat Options Neurol 2010; 12:492-503; PMID:20848328; http://dx.doi.org/ 10.1007/s11940-010-0093-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud PO, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, et al.. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 2016; 36:862-90; PMID:26868179; http://dx.doi.org/ 10.1177/0271678X16630991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Philos Trans R Soc Lond B Biol Sci 2008; 363:123-37; PMID:17322003; http://dx.doi.org/ 10.1098/rstb.2006.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 2004; 304:1338-40; PMID:15060285; http://dx.doi.org/ 10.1126/science.1095505 [DOI] [PubMed] [Google Scholar]
- [75].Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil 2014; 2:125; PMID:25632404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sajja RK, Prasad S, Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS 2014; 11:8; PMID:24708805; http://dx.doi.org/ 10.1186/2045-8118-11-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Abbott NJ. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 2013; 36:437-49; PMID:23609350; http://dx.doi.org/ 10.1007/s10545-013-9608-0 [DOI] [PubMed] [Google Scholar]
- [78].Reichel V, Burghard S, John I, Huber O. P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res 2011; 1370:238-45; PMID:21075088; http://dx.doi.org/ 10.1016/j.brainres.2010.11.012 [DOI] [PubMed] [Google Scholar]
- [79].Liu H, Xu X, Yang Z, Deng Y, Liu X, Xie L. Impaired function and expression of P-glycoprotein in blood-brain barrier of streptozotocin-induced diabetic rats. Brain Res 2006; 1123:245-52; PMID:17074306; http://dx.doi.org/ 10.1016/j.brainres.2006.09.061 [DOI] [PubMed] [Google Scholar]
- [80].Liu L, Liu XD. Alterations in function and expression of ABC transporters at blood-brain barrier under diabetes and the clinical significances. Front Pharmacol 2014; 5:273; PMID:25540622; http://dx.doi.org/ 10.3389/fphar.2014.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Wu KC, Pan HJ, Yin HS, Chen MR, Lu SC, Lin CJ. Change in P-glycoprotein and caveolin protein expression in brain striatum capillaries in New Zealand obese mice with type 2 diabetes. Life Sci 2009; 85:775-81; PMID:19891976; http://dx.doi.org/ 10.1016/j.lfs.2009.10.014 [DOI] [PubMed] [Google Scholar]
- [82].Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, Liu L, Duan R, Liu XD, Wang GJ, et al.. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin 2011; 32:956-66; PMID:21685928; http://dx.doi.org/ 10.1038/aps.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Liu YC, Liu HY, Yang HW, Wen T, Shang Y, Liu XD, Xie L, Wang GJ. Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem Pharmacol 2007; 74:1766-72; PMID:17915193; http://dx.doi.org/ 10.1016/j.bcp.2007.08.021 [DOI] [PubMed] [Google Scholar]
- [84].Wittmann G, Mohacsik P, Balkhi MY, Gereben B, Lechan RM. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS 2015; 12:21; PMID:26337286; http://dx.doi.org/ 10.1186/s12987-015-0016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shao B, Bayraktutan U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes Obes Metab 2013; 15:993-9; PMID:23617822; http://dx.doi.org/ 10.1111/dom.12120 [DOI] [PubMed] [Google Scholar]
- [86].Scheen AJ. Central nervous system: a conductor orchestrating metabolic regulations harmed by both hyperglycaemia and hypoglycaemia. Diabetes Metab 2010; 36 Suppl 3:S31-8; PMID:21211733; http://dx.doi.org/ 10.1016/S1262-3636(10)70464-X [DOI] [PubMed] [Google Scholar]
- [87].Artola A, Kamal A, Ramakers GM, Gardoni F, Di Luca M, Biessels GJ, Cattabeni F, Gispen WH. Synaptic plasticity in the diabetic brain: advanced aging? Prog Brain Res 2002; 138:305-14 [DOI] [PubMed] [Google Scholar]
- [88].Kamal A, Biessels GJ, Ramakers GM, Hendrik Gispen W. The effect of short duration streptozotocin-induced diabetes mellitus on the late phase and threshold of long-term potentiation induction in the rat. Brain Res 2005; 1053:126-30; PMID:16038887; http://dx.doi.org/ 10.1016/j.brainres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- [89].Borghammer P, Hansen SB, Eggers C, Chakravarty M, Vang K, et al. (2012) Glucose metabolism in small subcortical structures in Parkinson's disease. Acta Neurol Scand 125: 303-310. [DOI] [PubMed] [Google Scholar]
- [90].Li W, Risacher SL, Huang E, Saykin AJ, Alzheimer's Disease Neuroimaging I (2016) Type 2 diabetes mellitus is associated with brain atrophy and hypometabolism in the ADNI cohort. Neurology 87: 595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Renn BN, Feliciano L, Segal DL. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev 2011; 31:1239-46; PMID:21963669; http://dx.doi.org/ 10.1016/j.cpr.2011.08.001 [DOI] [PubMed] [Google Scholar]
- [92].Petrak F, Herpertz S. Treatment of depression in diabetes: an update. Curr Opin Psychiatry 2009; 22:211-7; PMID:19553878; http://dx.doi.org/ 10.1097/YCO.0b013e3283207b45 [DOI] [PubMed] [Google Scholar]
- [93].Harris JJ, Jolivet R, Attwell D (2012) Synaptic energy use and supply. Neuron 75: 762-777. [DOI] [PubMed] [Google Scholar]
- [94].Jawale A, Datusalia AK, Bishnoi M, Sharma SS. Reversal of diabetes-induced behavioral and neurochemical deficits by cinnamaldehyde. Phytomedicine 2016; 23:923-30; PMID:27387400; http://dx.doi.org/ 10.1016/j.phymed.2016.04.008 [DOI] [PubMed] [Google Scholar]
- [95].Chalmers JA, Jang JJ, Belsham DD. Glucose sensing mechanisms in hypothalamic cell models: glucose inhibition of AgRP synthesis and secretion. Mol Cell Endocrinol 2014; 382:262-70; PMID:24145125; http://dx.doi.org/ 10.1016/j.mce.2013.10.013 [DOI] [PubMed] [Google Scholar]
- [96].Kong D, Vong L, Parton LE, Ye C, Tong Q, Hu X, Choi B, Brüning JC, Lowell BB. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab 2010; 12:545-52; PMID:21035764; http://dx.doi.org/ 10.1016/j.cmet.2010.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, et al.. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007; 449:228-32; PMID:17728716; http://dx.doi.org/ 10.1038/nature06098 [DOI] [PubMed] [Google Scholar]
- [98].Chen Y, Knight ZA. Making sense of the sensory regulation of hunger neurons. Bioessays 2016; 38:316-24; PMID:26898524; http://dx.doi.org/ 10.1002/bies.201500167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Tabe-Bordbar S, Anastasio TJ. Computational Analysis of the Hypothalamic Control of Food Intake. Front Comput Neurosci 2016; 10:27; PMID:27199725; http://dx.doi.org/ 10.3389/fncom.2016.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Catzeflis C, Pierroz DD, Rohner-Jeanrenaud F, Rivier JE, Sizonenko PC, Aubert ML. Neuropeptide Y administered chronically into the lateral ventricle profoundly inhibits both the gonadotropic and the somatotropic axis in intact adult female rats. Endocrinology 1993; 132:224-34; PMID:8380374 [DOI] [PubMed] [Google Scholar]
- [101].Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 1998; 396:670-4; PMID:9872314; http://dx.doi.org/ 10.1038/25341 [DOI] [PubMed] [Google Scholar]
- [102].Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 2011; 14:351-5; PMID:21209617; http://dx.doi.org/ 10.1038/nn.2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 1999; 5:1066-70; PMID:10470087; http://dx.doi.org/ 10.1038/12506 [DOI] [PubMed] [Google Scholar]
- [104].Kurita H, Xu KY, Maejima Y, Nakata M, Dezaki K, Santoso P, Yang Y, Arai T, Gantulga D, Muroya S, et al.. Arcuate Na+,K+-ATPase senses systemic energy states and regulates feeding behavior through glucose-inhibited neurons. Am J Physiol Endocrinol Metab 2015; 309:E320-33; PMID:26081283; http://dx.doi.org/ 10.1152/ajpendo.00446.2014 [DOI] [PubMed] [Google Scholar]
- [105].Wang QP, Lin YQ, Zhang L, Wilson YA, Oyston LJ, Cotterell J, Qi Y, Khuong TM, Bakhshi N, Planchenault Y, et al.. Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell Metab 2016; 24:75-90; PMID:27411010; http://dx.doi.org/ 10.1016/j.cmet.2016.06.010 [DOI] [PubMed] [Google Scholar]
- [106].März M, Chapouton P, Diotel N, Vaillant C, Hesl B, Takamiya M, Lam CS, Kah O, Bally-Cuif L, Strähle U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010; 58:870-88; PMID:20155821 [DOI] [PubMed] [Google Scholar]
- [107].Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol 2012; 72:429-61; PMID:21595047; http://dx.doi.org/ 10.1002/dneu.20918 [DOI] [PubMed] [Google Scholar]
- [108].Diotel N, Vaillant C, Gabbero C, Mironov S, Fostier A, Gueguen MM, Anglade I, Kah O, Pellegrini E. Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm Behav 2013; 63:193-207; PMID:22521210; http://dx.doi.org/ 10.1016/j.yhbeh.2012.04.003 [DOI] [PubMed] [Google Scholar]
- [109].Sarras MP, Intine RV. Use of zebrafish as a disease model provides a unique window for understanding the molecular basis of diabetic metabolic memory. iConcept Press 2013; 2611-9 [Google Scholar]
- [110].Olsen AS, Sarras MP, JrIntine RV. Limb regeneration is impaired in an adult Zebrafish model of diabetes mellitus. Wound Repair Regen 2010; 18:532-42; PMID:20840523; http://dx.doi.org/ 10.1111/j.1524-475X.2010.00613.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Capiotti KM, Antonioli R Jr, Kist LW, Bogo MR, Bonan CD, Da Silva RS. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp Biochem Physiol B Biochem Mol Biol 2014; 171:58-65; PMID:24704522; http://dx.doi.org/ 10.1016/j.cbpb.2014.03.005 [DOI] [PubMed] [Google Scholar]
- [112].Dorsemans AC, Soule S, Weger M, Bourdon E, Lefebvre d'Hellencourt C, Meilhac O, Diotel N. Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J Comp Neurol 2016; 525:442-58; PMID:27339277; http://dx.doi.org/ 10.1002/cne.24065 [DOI] [PubMed] [Google Scholar]
- [113].Nguyen M, Yang E, Neelkantan N, Mikhaylova A, Arnold R, Poudel MK, Stewart AM, Kalueff AV. Developing ‘integrative’ zebrafish models of behavioral and metabolic disorders. Behav Brain Res 2013; 256:172-87; PMID:23948218; http://dx.doi.org/ 10.1016/j.bbr.2013.08.012 [DOI] [PubMed] [Google Scholar]
- [114].Connaughton VP, Baker C, Fonde L, Gerardi E, Slack C. Alternate Immersion in an External Glucose Solution Differentially Affects Blood Sugar Values in Older Versus Younger Zebrafish Adults. Zebrafish 2016; 13:87-94; PMID:26771444; http://dx.doi.org/ 10.1089/zeb.2015.1155 [DOI] [PubMed] [Google Scholar]
- [115].Alvarez Y, Chen K, Reynolds AL, Waghorne N, O'Connor JJ, Kennedy BN. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis Model Mech 2010; 3:236-45; PMID:20142328; http://dx.doi.org/ 10.1242/dmm.003772 [DOI] [PubMed] [Google Scholar]
- [116].Capiotti KM, De Moraes DA, Menezes FP, Kist LW, Bogo MR, et al. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav Brain Res 2014; 274: 319-325. [DOI] [PubMed] [Google Scholar]
- [117].Bryan RN, Bilello M, Davatzikos C, Lazar RM, Murray A, Horowitz K, Lovato J, Miller ME, Williamson J, Launer LJ. Effect of diabetes on brain structure: the action to control cardiovascular risk in diabetes MR imaging baseline data. Radiology 2014; 272:210-6; PMID:24779562; http://dx.doi.org/ 10.1148/radiol.14131494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet 2012; 379:2291-9; PMID:22683129; http://dx.doi.org/ 10.1016/S0140-6736(12)60360-2 [DOI] [PubMed] [Google Scholar]
- [119].Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta 2015; 444:18-23; PMID:25661087; http://dx.doi.org/ 10.1016/j.cca.2015.01.027 [DOI] [PubMed] [Google Scholar]
- [120].Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005; 28:726-35; PMID:15735218; http://dx.doi.org/ 10.2337/diacare.28.3.726 [DOI] [PubMed] [Google Scholar]
- [121].Li X, Song D, Leng SX. Link between type 2 diabetes and Alzheimer's disease: from epidemiology to mechanism and treatment. Clin Interv Aging 2015; 10:549-60; PMID:25792818; http://dx.doi.org/ 10.2147/CIA.S74042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schindler SM, Klegeris A. Elucidating the link between the modifiable risk factors of Alzheimer's disease and neuroinflammation. Neurodegener Dis Manag 2016; 6:375-84; PMID:27600142; http://dx.doi.org/ 10.2217/nmt-2016-0028 [DOI] [PubMed] [Google Scholar]
- [123].Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol 2011; 7:108-14; PMID:21263438; http://dx.doi.org/ 10.1038/nrendo.2010.228 [DOI] [PubMed] [Google Scholar]
- [124].Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012; 42:484-91; PMID:22372522; http://dx.doi.org/ 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]


