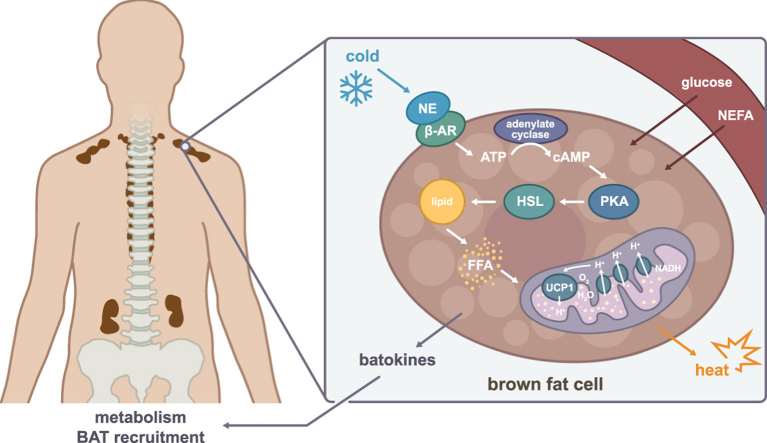
Abstract
Activation of brown adipose tissue (BAT) in adult humans increase glucose and fatty acid clearance as well as resting metabolic rate, whereas a prolonged elevation of BAT activity improves insulin sensitivity. However, substantial reductions in body weight following BAT activation has not yet been shown in humans. This observation raise the possibility for feedback mechanisms in adult humans in terms of a brown fat-brain crosstalk, possibly mediated by batokines, factors produced by and secreted from brown fat. Batokines also seems to be involved in BAT recruitment by stimulating proliferation and differentiation of brown fat progenitors. Increasing human BAT capacity could thus include inducing brown fat biogenesis as well as identifying novel batokines. Another attractive approach would be to induce a brown fat phenotype, the so-called brite or beige fat, within the white fat depots. In adult humans, white fat tissue transformation into beige has been observed in patients with pheochromocytoma, a norepinephrine-producing tumor. Interestingly, human beige fat is predominantly induced in regions that were BAT during early childhood, possibly reflecting that a presence of human beige progenitors is depot specific and originating from BAT. In conclusion, to utilize the anti-obesity potential of human BAT focus should be directed towards identifying novel regulators of brown and beige fat progenitor cells, as well as feedback mechanisms of BAT activation. This would allow for identification of novel anti-obesity targets.
Graphical abstract
1. Introduction
The discovery of substantial amounts of active brown fat being present in adult humans [1], [2], [3], [4], [5] has raised the expectations for the development of new anti-obesity treatments by targeting this tissue. Brown fat consumes energy by activation of non-shivering thermogenesis through sympathetic signalling [6]. In adult humans, brown fat has been verified at a molecular level in the supraclavicular region [7], [8], deep neck [9] and in the perirenal region [10]. From initial PET/CT images, it is clear that active brown fat is also present around the spinal cord in the intercostal regions [1], [2], [3], [4], [5]. This review will focus on human brown fat, however, as brown fat has been studied in rodents for decades, some comparison on key concepts will be provided when considered relevant. In rodents, the association between obesity and reduced brown fat activity is well-established [11] and a sympathetic denervation of intrascapular BAT was demonstrated to mediate an increase in body fat [12]. In humans, however, the efficiency of BAT activation as an anti-obesity strategy could be questioned, as the amount of BAT in relation to whole body mass, is substantially lower than in rodents [13]. The differences in the effects of BAT activation between mice and humans might also include a more complex regulatory network for preserving energy balance and individual body weight set-point present in humans. For example, secreted factors derived from activated brown fat, called batokines [14] should be further explored for their potential role in regulating BAT activity and its effects on whole body metabolism. In this review, we aim to provide an overview of what is known to date about the plasticity of human brown fat abundancy and frequency and its role in adult human metabolism. This overview could serve as a basis for discussing potential strategies for using brown fat in the development of novel treatments against obesity.
2. Sympathetic activation of human brown fat mitochondria
In response to cold, brown fat is activated through sympathetic signalling. Norepinephrine activates β3-adrenergic receptors on the brown adipocytes, initiating lipolysis of the intracellular triglyceride storage. Free fatty acids are released as substrate, triggering mitochondrial respiration, which generates a mitochondrial membrane potential. However, instead of producing ATP, the mitochondrial membrane potential is uncoupled through the brown fat specific protein: Mitochondrial brown fat uncoupling protein 1 (UCP1), resulting in dissipation of the energy as heat [6]. Thus, through this mechanism brown fat consumes energy and has thereby become an attractive target in the battle against obesity, and novel avenues to increase this process are now being intensively investigated. Importantly, mitochondrial uncoupling capacity has been reported to be comparable between mice and humans [15], suggesting that the major species difference might be due to a higher ratio of inactive cells per active brown adipocyte in humans, resulting in a lower abundance of activated mitochondria and ultimately a weaker response than observed in mice. This argue against the proposition that human brown fat is beige [16] or a mixture of classical brown and brite [7]. Rather, this might indicate heterogeneity in the amount and grade of activation of the human brown adipocytes. So why would mice have more activated brown adipocytes? One aspect is that brown fat needs to be induced to differentiate into active brown fat and by living mainly at thermoneutrality, humans fail to keep this channel open. In contrast, experimental mice are in general kept at room temperature, which is below their thermoneutral set-point. Indeed, it has been clearly demonstrated that metabolic response is determined by ambient temperature [17]. Related to this point are the differences in body size between humans and mice. As mice have a relatively large surface in relation to body volume, they lose much more heat than humans, who rather have developed mechanisms to waste heat, e.g. by sweat.
In addition to norepinephrine-induced lipolysis, other factors are important for the heat-producing machinery of brown fat. Following NE-induced lipolysis, an increased substrate availability results in an acceleration of the electron transport chain in the mitochondria which, as a by-product, generate the superoxide radical (•O−). Interestingly, an interaction between superoxides and UCP proteins have been demonstrated [18]. In line with this observation, the levels of mitochondrial reactive oxygen species (ROS) is increased in activated BAT [19]. At the same time, ROS has been shown to be beneficial for providing thermogenesis as it activates UCP1 by sulfenylation at Cyc253 [19]. Another recent study demonstrated a mechanism in brown fat for maintaining a healthy population of mitochondria despite a high level of reactive oxygen stress-induction. This maintenance of mitochondrial integrity was provided by AMPK activation upon cold-induced thermogenesis. It was demonstrated that AMPK induces mitophagy which is crucial for maintaining mitochondrial integrity and thereby also the function of murine brown fat [20]. The study also demonstrated that AMPK is activated in supraclavicular-derived human brown fat cell strains upon norepinephrine stimulation [20], suggesting that this mechanism is conserved between species. An additional pathway identified as important for brown fat activation, is adenosine signalling through the AdenosineA2A receptor. This pathway protects mice from diet-induced obesity and plays an important role in sympathetic BAT activation in both murine and human brown adipocytes [21]. Thus, these conserved pathways might be valuable to consider for improvement of brown fat efficiency in humans.
3. Activation of BAT improves insulin sensitivity
Initial reports on active BAT in adult humans demonstrated glucose uptake in activated BAT depots using Pet/CT-Scan [1], [2], [3], [4], [22]. Since then, sequential cold-stimulations over time have confirmed initial findings and have provided more insight into the metabolic role of brown fat in healthy humans [23], [24], [25]. A recent study demonstrated that the main substrate used by activated brown fat is fatty acids derived from intracellular triglycerides [26]. However, a simultaneous uptake of systemic non-esterified fatty acids (NEFA) [27] and glucose [23] has been described, probably occurring to compensate for the energy loss and for building up new triglycerides [26]. These properties make activation of brown fat to an attractive strategy for counteracting metabolic disease. Increased BAT activation over time, i.e. cold acclimation, has been shown to increase glucose uptake in BAT [23], [28]. Interestingly, the amount of activated BAT increased during winter compared to summer [4] and increased following sleeping in a cold (19 °C) room with light clothing for a month [24], demonstrating an ability of human BAT to adapt to the environment, as already well-established in mice [6]. This adaptation is an important observation strongly suggesting that brown fat has a role in temperature regulation also in adult humans. Moreover, several cold acclimation protocols have, along with increased BAT activation, also demonstrated an improved insulin sensitivity [23], [24], [29]. The molecular mechanisms for this increased insulin sensitivity is not known but might be related to an increased amount of BAT following cold acclimation. The role of human brown fat in regulating glucose levels was further emphasized by another study, suggesting that human BAT activity have a role in buffering glucose fluctuations [30], and raising the idea that human BAT activity is under circadian control as has been clearly demonstrated with murine BAT activity, which is regulated by Rev-Erbα [31]. In fact, even people suffering from obesity [32] and type 2 diabetes [29] experienced an increased insulin sensitivity following a sequential cold stimulation protocol, while BAT volume was associated with adipose tissue insulin sensitivity in a cohort of over-weight and obese subjects [33]. Hence, a role in regulating glucose metabolism of BAT in adult humans is established. Moreover, a pharmacological study using the β3-agonist, mirabegron demonstrated an increased resting metabolic rate with 203±40 kcal/day, mediated by the increased activation of human BAT [34]. In contrast, none of the cold acclimation studies performed in humans have to date demonstrated any loss of total body weight. Although body fat mass has been demonstrated to decrease [35], the total body weight is maintained, also in obese individuals. This is somewhat surprising considering the established negative correlation in the initial studies between BAT activity and obesity [2], [4]. This consistent lack of weight loss in response to increased BAT activation, despite an increased metabolic rate, might be due to the extent and the limited duration of the interventions. However, it cannot be excluded that a compensatory energy intake is induced to preserve the individual body weight set-point. Whether this potential cross-talk exist and how it is regulated remains to be explored.
4. The potential role of batokines in BAT recruitment and metabolism
Cypess and colleagues characterized several levels of brown fat in the neck area and described a core of brown fat which gradually turned into a less pronounced brown fat phenotype in the superficial regions [9]. The precise mechanisms by which the presence or activation grade of human brown fat is determined are currently not well described. Secreted factors from BAT, i.e. batokines might be involved in regulating this process in an autocrine or paracrine manner. Batokines secreted from brown or beige fat represent a so far unexplored field in humans, but has recently caught increased interest and are better described in rodent models which has been nicely been reviewed [14]. For example, members of the bone-morphogenetic proteins have been described to be involved in the differentiation of adipocytes, where BMP7 regulates brown adipogenesis while BMP4 has been described to target beige adipogenesis [14]. Another interesting batokine is VEGFA which targets endothelial cells to induce vascularization of BAT [36]. Knockout of VEGFA resulted in a “whitening” of murine brown fat depots [37], emphasizing the importance of vascularization for maintaining a brown fat phenotype. Interestingly, pro-angiogenic factors was also demonstrated to induce proliferation of human beige adipocyte progenitors in association with expanding capillary networks [38]. It is well-established that white fat communicates with other organs by secreting adipokines, e.g. leptin [39] and adiponectin [40], which contributes to the regulation of whole body energetics. The fat-induced secretion of leptin from white adipocytes, rasie the idea that corresponding brown fat derived adipokines could be involved in regulating appetite and whole body metabolism. Indeed, a beige fat derived Slit-2C fragment was described in vivo in mice to enhance non-shivering thermogenesis, resulting in increased energy expenditure and improved glucose homeostasis h [41]. Hence, identifying and determine the function of potential human batokines might thus lead to the discovery of novel drug targets against obesity.
5. Browning of human visceral fat
Cold acclimation protocols in humans have not revealed any inducible brown fat depots in addition to the constitutively present depots, i.e. the neck, supraclavicular, spinal cord and perirenal depots. This is in sharp contrast to mice where cold-induction of a brown fat phenotype called beige [42] is observed in the subcutaneous inguinal white fat depot [42]. The phenomenon was initially demonstrated in vitro under stimulation of PPARγ in epidydimal adipocytes which were named brite [43]. The brite/beige fat also express UCP1, is induced during cold acclimation and has an increased energy consumption while providing non-shivering thermogenesis [42], [43]. Interestingly, a compensatory browning can occur in the white fat of mice, following ablation of the classical brown fat, supporting the idea that beige and brown fat progenitors are of differential origin but can provide similar functional phenotypes [44]. Although cold challenges have not proven enough to mediate browning of white fat depots of humans, cases of chronically elevated sympathetic activation, has been reported to produce brown fat morphology and function in human white fat (Fig. 1). For example, browning of white fat depots in adult humans have been observed in patients suffering from the catecholamine producing adrenal gland tumor, called pheochromocytoma. Interestingly, the pheochromocytoma-induced brown fat phenotype is not equally distributed among the white fat depots, but is selectively occurring in the visceral fat depots, including the perirenal region and even the omental fat depot [45], [46]. In a case study, a pheochromocytoma patient was PET/CT-scanned at diagnosis, following α-blockade and following removal of the tumor [47]. This subject had dramatic levels of glucose uptake during PET/CT-scanning in the abdominal visceral fat, strongly suggesting brown fat activity. This glucose uptake was reduced upon α-blockade and disappeared following removal of the norepinephrine producing tumor. Interestingly, it has been demonstrated in a larger sample set that whereas a pronounced browning occurs in the visceral fat, pheochromocytoma induced browning does not occur in the subcutaneous depots [45]. Hence, a subcutaneous depot susceptible for browning in adult humans corresponding to the murine subcutaneous inguinal depot, has not been identified. Rather, the visceral fat, traditionally regarded as the unhealthy fat type associating with insulin resistance [48], seems to be more prone to adapt a brown fat phenotype in humans. This could be related to the proximity to the adrenal gland tumor, but would likely also relate to the higher vascularity of visceral fat, as this has been demonstrated as an important factor in determining brown versus white fat cell destiny [37]. In addition to the visceral fat depots, BAT activity in the brown fat regions including supraclavicular, deep neck and intercostal depots, is heavily increasing, suggesting brown fat biogenesis or increase in the amount of active brown fat. Although the browning source is originating from the adrenal gland, these data could be considered as an indicatory map of the human fat regions that are susceptible for sympathetically induced differentiation into mature brown fat. Notably, a brown fat morphology defined from autopsy biopsies, has been found in children in several visceral fat regions, including the mesenteric and omental depot, whereas subcutaneous fat was reported to almost completely lack brown fat morphology [49]. Although the only active brown fat depot in the visceral region detected in healthy adult humans is represented by the perirenal fat, these observations raise the possibility that brown or beige fat progenitor cells are still present in additional visceral depots [46], [47], [49].
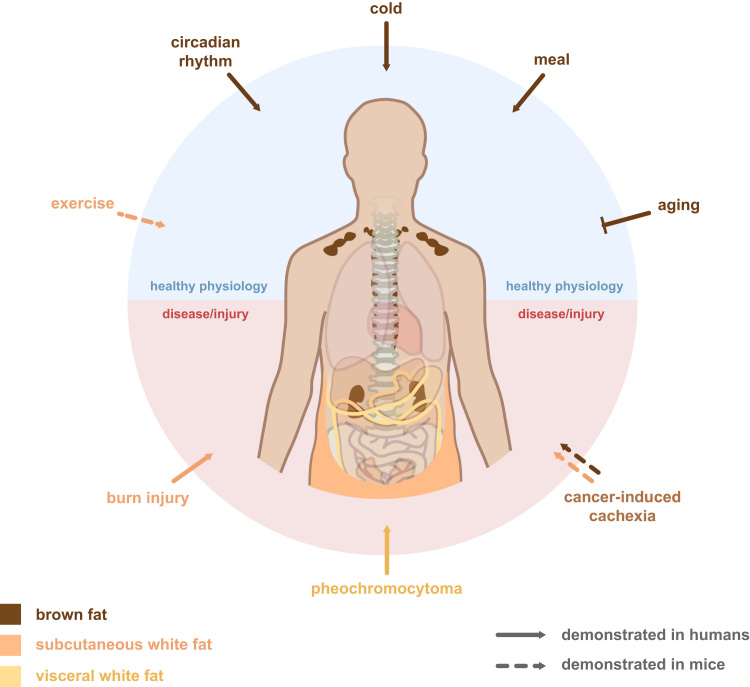
Fig. 1.
Regulation of human brown fat and induction of white fat browning. Several physiological conditions have been demonstrated to influence brown fat activity in humans. When present in human adults, cold activates the brown fat depots which mainly are present at the cervical, supraclavicular and paravertebral areas and sometimes also in the perirenal region. Brown fat is also induced in response to a meal and is activated in a circadian manner in mice and seems to have a biorhythm with an opposing pattern to plasma glucose in humans. Aging is negatively associated with the appearance of active brown fat. In terms of browning of white fat depots, physical exercise has been shown in mice to induce browning of subcutaneous inguinal fat; however, this has not been reproducible in subcutaneous fat humans. In contrast, pheochromocytoma, a catecholamine-producing tumor in the adrenal gland, has a well-described browning effect on the visceral fat depots, including the perirenal, omental and mesenteric fat, while no effect has been observed on subcutaneous depots. Burn injury, on the other hand, demonstrated a gradually increasing brown fat phenotype in the subcutaneous fat following severe burn injury in a mixed cohort of children and adults. The severe burn injuries were associated with chronically elevated levels of norepinephrine. Another disease related browning effect has been observed in cancer cachexic mice were accelerated lipolysis as well as browning was shown to occur in the white fat depots. A tumor-derived factor, PTHrP, was shown to induce this phenotype and was shown to be higher in tumor patients with more severe cachexia. Whether the mechanism of cancer cachexia induced browning occur in humans and which depot is targeted, remains to be investigated.
6. Browning of human subcutaneous fat
In one recent study, browning was observed in the subcutaneous depot in response to severe burn injury [50]. However, human subcutaneous tissue has not been shown to demonstrate brown fat even in early childhood [49] and despite elevated systemic levels of norepinephrine, the mechanism for burn-injury induced browning, which is a complex condition, including immune system mobilization and tissue repair, might therefore be completely separate from the pheochromocytoma-induced browning. In either case, an increase in activated brown fat mass, i.e. browning, can occur in humans, induced through sympathetic activation and norepinephrine release. Whether this browning is induced directly by norepinephrine or mediated by secondary factors previously described in murine or cell based studies [51] remains to be investigated. Another condition where browning have been reported to occur in mice is physical exercise [52], [53]. In humans, however, browning genes have not been shown to change in adipose tissue biopsies [54]. One problem with studying humans is the difficulties of accessing adipose tissue samples in the deeper fat regions. For example, investigating the visceral fat depots for browning following physical exercise would be of great interest, but has to date not been performed, due to the high-risk properties involved in such clinical procedure. Thus, the easy-accessible depot through biopsy sampling is the subcutaneous fat depot- a depot in humans where browning might not occur in other conditions than severe burn injury [50]. Finally, browning has been shown to occur in mice in relation to cancer cachexia [55], [56]. Cancer cachexia is characterized by massive weight loss and is a severe complication in patients suffering from a subgroup of cancers including lung cancer. Browning of white fat as well as increased activation of brown fat has been suggested to be part of the energy wasting mechanism. Interestingly, although not yet demonstrated in humans, it was found that a certain factor, PTH was secreted from the cancer tumor and induced browning. In humans, this factor associated with severity of cachexia, indicating also a role of the PTH in human lung cancer induced cachexia. Whether exercise and cancer cachexia induce browning of white subcutaneous fat in humans thus remains to be further investigated.
7. The appearance of brown fat throughout the aging continuum of humans
Human BAT seems to gradually decline with age as only 14% of humans over 40 years old have been estimated to have cold activated BAT [4], [57], [58]. Thus, it could be discussed whether middle-aged and older people might have a too small amount of BAT to benefit from activation. On the other hand, current methods for in vivo BAT quantifications are limited to detection of activated BAT, and the actual abundance and frequency of the tissue might be underestimated. Autopsy studies of infants and children under the age of 10 have demonstrated presence of multilocular brown fat morphology in multiple fat regions [49]. One early hypothesis which deserves to be mentioned is that the thermogenic regulation is immature and in excess in children, whereas it stabilizes in adults [49]. Revisiting this thought, this would suggest a physiological capacity of adapting to the environmental conditions of the growing child. Thus, a life-long lack of thermogenic demands in humans could possibly explain the age-associated decline in proportional amount of brown fat [4], [57], [58]. In postmortem samples collected from adult humans, a multilocular brown fat morphology was restricted to deeper fat regions close to the vascular structures, consistent with more recent findings were tissue gene expression also was validated [7], [8], [9]. The BAT location in adults support the idea that thermoregulation is provided to the thoracic structures and cervical spinal regions as a “thermogenic jacket” [49]. The age-related abundance observed in the early studies of postmortem samples are consistent with recent reports on cold-induced BAT in humans detected by using PET/CT-Scan technology [4], [35], [49]. Collectively, data indicates that the amount of active brown fat as well as multilocular morphology, declines with age in terms of amount and frequency and is at an adult stage, i.e. age over 40 years old, restricted to the area around neck vessels, intercostal vessels, para-aortic region and the perirenal depot. Visceral fat developed multilocular UCP1-expressing fat structures in pheochromocytoma patients, while the subcutaneous fat was not affected [45], [46]. This express the possibility that immature brown fat progenitor cells still exist in adults in regions where BAT was active during early childhood. A presence of quiescent progenitor cells distributed predominantly in the human visceral fat could explain the massive browning occurring in this depot rather in the subcutaneous region.
8. Human brown fat progenitor cells
It has been described that preadipocytes derived from the stromal vascular fraction of human supraclavicular brown fat establish a brown fat phenotype when differentiated in vitro, despite using the same differentiation protocol as used for subcutaneous white preadipocytes [7]. These results suggest an intrinsic difference between preadipocytes derived from human brown fat depots compared to white fat depots. It was further demonstrated that these cells expressed markers for classical brown fat, but were also positive for some markers previously described in mice as beige fat markers [16]. This observation emphasizes the heterogeneous status of human BAT which is frequently reported in tissue [7]. Subsequent efforts to characterize human brown adipocytes have been made. A study from the Tseng laboratory generated 128 brown and 152 white fat clones from human neck biopsies of 4 subjects [59]. The authors succeeded in identifying a cell surface marker specifically present in the brown fat progenitor cells, CD29, and predicting UCP1 response in the mature adipocytes. They further reported great variability in terms of brown fat cell identity, even between clones derived from the same starting cell strain. Thus, it was confirmed that human brown fat progenitor cells have a unique expression profile compared to white fat progenitor cells and that human brown fat cell cultures are heterogeneous –even within the same cell strain, isolated from the same biopsy. This observation should be considered when interpreting the results of another recent study. Here only two clones derived from supraclavicular human brown fat were compared to mice brown and beige fat, at a transcriptome level [60]. Interestingly, based on a subset of genes, the human clones seemed to resemble murine beige adipocytes more than murine brown adipocytes. Importantly, the amount of human clonal adipocyte cultures (n=2) cannot be considered sufficient to exclude that adipocytes with closer proximity to the murine classical brown also existed in the cell pool, albeit not selected during the cloning process. Furthermore, both studies mentioned above utilize immortalized cell cultures. The immortalization per se could influence cell metabolism and cell fate [61] and there could be a selection occurring for the cell type which more easily can survive the immortalization process. Whether the heterogeneity of human brown fat consist of a mixture of classical brown and beige adipocytes or whether it consists of brown preadipocytes at different stages on its way to final determination currently remains elusive. To completely entangle and classify human fat, lineage tracing should be performed, i.e. tracking back the cell population to a single cell origin. In rodents, brown fat progenitors have been described to have a common Myf5+ progenitor cell with skeletal muscle [62], [63], [64]. In humans, traditional lineage tracing is not feasible. However, the new generation of single cell transcriptomics is rapidly evolving and could be applied on isolated fat progenitor cells from different fat depots. This would allow for grouping into homogenous groups for clarifying differences between these groups in terms of stages of pre-programmed brown fat gene signatures.
9. Perspectives and concluding remarks
Using BAT for mediating weight loss in humans might demand other strategies than sequential BAT activation through cold exposure. This could include identifying pathways for programming and inducing differentiation of brown fat progenitor cells to increase brown fat biogenesis. To fully understand the transcriptional programme of human browning, a transcriptional map at single cell level across depots will be useful. A research area with great potential is represented by secreted brown fat adipokines, i.e. batokines, which might influence brown fat biogenesis, but might also regulate appetite or energy expenditure. Finally, efforts should focus on identification and neutralisation of BAT induced energy-saving negative feedback circuits. In conclusion, unravelling the molecular means of increasing BAT activity in adult humans have a great potential in the search for novel strategies to combat obesity and its related diseases.
Acknowledgements
The Novo Nordisk Foundation Center for Basic Metabolic Research (http://www.metabol.ku.dk) is supported by an unconditional grant from the Novo Nordisk Foundation to University of Copenhagen. The Centre for Physical Activity Research (CFAS) is supported by a grant from TrygFonden. CIM/CFAS is a member of DD2 - the Danish Center for Strategic Research in Type 2 Diabetes (the Danish Council for Strategic Research, grant no. 09-067009 and 09-075724).
References
- 1.Virtanen K.A., Lidell M.E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N.-J., Enerbäck S., Nuutila P., Virtanen N.P., Kirsi A., Martin Lidell E., Janne Orava, Mikael Heglind, Rickard Westergren, Tarja Niemi, Markku Taittonen, Jukka Laine, Nina-Johanna Savisto, Sven Enerbäck. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 2.Van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Kemerink G.J., Bouvy N.D., Schrauwen P., Teule G.J.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 3.Zingaretti M.C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., Nedergaard J., Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 4.Saito M., Okamatsu-ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., Kawai Y., Tsujisaki M. High incidence of metabolically active brown adipose effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 6.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 7.Jespersen N.Z., Larsen T.J., Peijs L., Daugaard S., Homøe P., Loft A., De Jong J., Mathur N., Cannon B., Nedergaard J., Pedersen B.K., Møller K., Scheele C. A classical brown adipose tissue mrna signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17 doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Lidell M.E., Betz M.J., Leinhard O.D., Heglind M., Elander L., Slawik M., Mussack T., Nilsson D., Romu T., Nuutila P., Virtanen K.A., Beuschlein F., Persson A., Borga M., Enerbäck S. Evidence for two types of brown adipose tissue in humans. Nat. Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 9.Cypess A.M., White A.P., Vernochet C., Schulz T.J., Xue R., Sass C.A., Huang T.L., Roberts-Toler C., Weiner L.S., Sze C., Chacko A.T., Deschamps L.N., Herder L.M., Truchan N., Glasgow A.L., Holman A.R., Gavrila A., Hasselgren P.-O., Mori M.A., Molla M., Tseng Y.-H. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betz M.J., Slawik M., Lidell M.E., Osswald A., Heglind M., Nilsson D., Lichtenauer U.D., Mauracher B., Mussack T., Beuschlein F., Enerbäck S. Presence of brown adipocytes in retroperitoneal fat from patients with benign adrenal tumors: relationship with outdoor temperature. J. Clin. Endocrinol. Metab. 2013 doi: 10.1210/jc.2012-3535. [DOI] [PubMed] [Google Scholar]
- 11.Trayhurn P. Origins and early development of the concept that brown adipose tissue thermogenesis is linked to energy balance and obesity. Biochimie. 2016;134:62–70. doi: 10.1016/j.biochi.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Dullo A., Miller D. Energy balance following sympathetic denervation of brown adipose tissue. J. Physiol. Pharmacol. 1983;62:235–240. doi: 10.1139/y84-035. [DOI] [PubMed] [Google Scholar]
- 13.Townsend K.L., Tseng Y.-H. Of mice and men: novel insights regarding constitutive and recruitable brown adipocytes. Int. J. Obes. Suppl. 2015;5:S15–S20. doi: 10.1038/ijosup.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villarroya F., Cereijo R., Villarroya J., Giralt M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2016;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 15.Porter C., Herndon D.N., Chondronikola M., Borsheim E., Toliver-Kinsky T., Sidossis L.S., Chao T., Annamalai P., Bhattarai N., Saraf M.K., Capek K.D., Reidy P.T., Daquinag A.C., Kolonin M.G., Rasmussen B.B. Clinical and translational report human and mouse brown adipose tissue mitochondria have comparable UCP1 function correspondence clinical and translational report human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab. 2016;24:246–255. doi: 10.1016/j.cmet.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J., Boström P., Sparks L.M., Ye L., Choi J.H., Giang A.-H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G., Huang K., Tu H., van Marken Lichtenbelt W.D., Hoeks J., Enerbäck S., Schrauwen P., Spiegelman B.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. Short article UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 18.K.S. Echtay, D. Roussel, J. St-pierre, M.B. Jekabsons, S. Cadenas, J.A. Stuart, J.A. Harper, S.J. Roebuck, A. Morrison, S. Pickering, J.C. Clapham, M.D. Brand, Superoxide activates mitochondrial uncoupling proteins, 415, 2002. [DOI] [PubMed]
- 19.Chouchani E.T., Kazak L., Jedrychowski M.P., Lu G.Z., Erickson B.K., Szpyt J., Pierce K.A., Laznik-Bogoslavski D., Vetrivelan R., Clish C.B., Robinson A.J., Gygi S.P., Spiegelman B.M. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mottillo E.P., Desjardins E.M., Crane J.D., Smith B.K., Green A.E., Ducommun S., Henriksen T.I., Rebalka I.A., Razi A., Sakamoto K., Scheele C., Kemp B.E., Hawke T.J., Ortega J., Granneman J.G., Steinberg G.R. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through brown and beige adipose tissue function. Cell Metab. 2016;24:118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gnad T., Scheibler S., von Kügelgen I., Scheele C., Kilić A., Glöde A., Hoffmann L.S., Reverte-Salisa L., Horn P., Mutlu S., El-Tayeb A., Kranz M., Deuther-Conrad W., Brust P., Lidell M.E., Betz M.J., Enerbäck S., Schrader J., Yegutkin G.G., Müller C.E., Pfeifer A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature. 2014;516:395–399. doi: 10.1038/nature13816. [DOI] [PubMed] [Google Scholar]
- 22.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B., Kuo F.C., Palmer E.L., Tseng Y.-H., Doria A., Kolodny G.M., Kahn C.R. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chondronikola M., Volpi E., Borsheim E., Porter C., Annamalai P., Enerback S., Lidell M.E., Saraf M.K., Labbe S.M., Hurren N.M., Yfanti C., Chao T., Andersen C.R., Cesani F., Hawkins H., Sidossis L.S. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee P., Smith S., Linderman J., Courville A.B., Brychta R.J., Dieckmann W., Werner C.D., Chen K.Y., Celi F.S. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouellet V., Labbé S.M., Blondin D.P., Phoenix S., Guérin B., Haman F., Turcotte E.E., Richard D., Carpentier A.C. Brown adipose tissue oxidative metabolism contributles to energy expenditure during cold exposure in humans. J. Clin. Invest. 2012;122:545. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blondin D.P., Tingelstad H.C., Noll C., Frisch F., Phoenix S., Guérin B., Turcotte É.E., Richard D., Haman F., Carpentier A.C. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat. Commun. 2017;8:14146. doi: 10.1038/ncomms14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blondin D.P., Frisch F., Phoenix S., Guérin B., Turcotte É.E., Haman F., Richard D., Carpentier A.C. Inhibition of intracellular triglyceride lipolysis suppresses cold-induced brown adipose tissue metabolism and increases shivering in humans. Cell Metab. 2017;25:438–447. doi: 10.1016/j.cmet.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 28.van der Lans A.A.J.J., Hoeks J., Brans B., Vijgen G.H.E.J., Visser M.G.W.M.G.W., Vosselman M.J., Hansen J., Jorgensen J.A., Wu J., Mottaghy F.M., Schrauwen P., van Marken Lichtenbelt W.D., Jörgensen J.A., Wu J., Mottaghy F.M., Schrauwen P., van Marken Lichtenbelt W.D. Cold acclimation recruit human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanssen M.J.W., Hoeks J., Brans B., van der Lans A.A.J.J., Schaart G., van den Driessche J.J., Jörgensen J.A., Boekschoten M.V., Hesselink M.K.C., Havekes B., Kersten S., Mottaghy F.M., van Marken Lichtenbelt W.D., Schrauwen P. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015;21:6–10. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 30.Lee P., Bova R., Schofield L., Bryant W., Dieckmann W., Slattery A., Govendir M.A., Emmett L., Greenfield J.R. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 2016;23:602–609. doi: 10.1016/j.cmet.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Gerhart-Hines Z., Feng D., Emmett M.J., Everett L.J., Loro E., Briggs E.R., Bugge A., Hou C., Ferrara C., Seale P., Pryma D.A., Khurana T.S., Lazar M.A. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503:410–413. doi: 10.1038/nature12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanssen M.J.W., Lans A.A.J.J. Van Der, Brans B., Hoeks J., Jardon K.M.C., Schaart G., Mottaghy F.M., Schrauwen P., Van Marken Lichtenbelt W.D. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2016 doi: 10.2337/db15-1372. [DOI] [PubMed] [Google Scholar]
- 33.Chondronikola M., Volpi E., Børsheim E., Porter C., Saraf M.K., Annamalai P., Yfanti C., Chao T., Wong D., Shinoda K., Labbe S.M., Hurren N.M., Cesani F., Kajimura S., Sidossis L.S. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metab. 2016;23:1200–1206. doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cypess A.M., Weiner L.S., Roberts-Toler C., Elía E.F., Kessler S.H., Kahn P.A., English J., Chatman K., Trauger S.A., Doria A., Kolodny G.M. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xue Y., Petrovic N., Cao R., Larsson O., Lim S., Chen S., Feldmann H.M., Liang Z., Zhu Z., Nedergaard J., Cannon B., Cao Y. Article in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu I., Aprahamian T., Kikuchi R., Shimizu A., Papanicolaou K.N., MacLauchlan S., Maruyama S., Walsh K. Vascular rarefaction mediates whitening of brown fat in obesity. J. Clin. Invest. 2014;124:2099–2112. doi: 10.1172/JCI71643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Min S.Y., Kady J., Nam M., Rojas-Rodriguez R., Berkenwald A., Kim J.H., Noh H.-L., Kim J.K., Cooper M.P., Fitzgibbons T., Brehm M.A., Corvera S. Human “brite/beige” adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 2016;22:312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coppari R., Ichinose M., Lee C.E., Pullen A.E., Kenny C.D., McGovern R.A., Tang V., Liu S.M., Ludwig T., Chua S.C., Lowell B.B., Elmquist J.K. The hypothalamic arcuate nucleus: a key site for mediating leptin's effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., Ezaki O., Akanuma Y., Gavrilova O., Vinson C., Reitman M.L., Kagechika H., Shudo K., Yoda M., Nakano Y., Tobe K., Nagai R., Kimura S., Tomita M., Froguel P., Kadowaki T., Xu A., Wang Y., Keshaw H., Xu L.Y., Lam K.S.L., Cooper G.J.S. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 41.Svensson Katrin J., Long Jonathan Z., Jedrychowski Mark P., Cohen Paul, Lo James C., Serag Sara, Kir Serkan, Kajimura Shingo, Gygi Steven P., Spiegelman Bruce M. A secreted Slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metabol. 2016;8:454–466. doi: 10.1016/j.cmet.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seale P., Conroe H.M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma ( PPARG) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz T., Huang P., Huang T., Xue R., McDougall L., Townsend K., Cypess A., Mishina Y., Gussoni E., Tseng Y. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;21:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vergnes L., Davies G., Lin J., Yeh M., Livhits M., Harari A., Symonds M., Sacks H., Reue K. Adipocyte browning and higher mitochondrial function in periadrenal but not SC fat in pheochromocytoma. J. Clin. Endocrinol. Metab. 2016;101:4440–4448. doi: 10.1210/jc.2016-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frontini A., Vitali A., Perugini J., Murano I., Romiti C., Ricquier D., Guerrieri M., Cinti S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim. Biophys. Acta. 1831;2013:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Søndergaard E., Gormsen L.C., Christensen M.H., Pedersen S.B., Christiansen P., Nielsen S., Poulsen P.L., Jessen N. Chronic adrenergic stimulation induces brown adipose tissue differentiation in visceral adipose tissue. Diabet. Med. 2015;32:e4–e8. doi: 10.1111/dme.12595. [DOI] [PubMed] [Google Scholar]
- 48.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.J.M. Heaton, The distribution of brown adipose tissue in the human, 1972, 35–39. [PMC free article] [PubMed]
- 50.Sidossis L.S., Porter C., Saraf M.K., Børsheim E., Radhakrishnan R.S., Chao T., Ali A., Chondronikola M., Mlcak R., Finnerty C.C., Hawkins H.K., Toliver-Kinsky T., Herndon D.N. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harms M., Seale P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 52.Stanford K.I., Middelbeek R.J.W., Goodyear L.J. Exercise effects on white adipose tissue: beiging and metabolic adaptations. Diabetes. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C., Rasbach K.A., Boström E.A., Choi J.H., Long J.Z., Zingaretti M.C., Vind B.F., Tu H., Cinti S., Gygi S.P., Spiegelman B.M. A PGC1a dependent myokine that derives browning of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheele C. Adipose adaptation to exercise training –increased metabolic rate but no signs of browning. Acta Physiol. 2014 doi: 10.1111/apha.12280. [DOI] [PubMed] [Google Scholar]
- 55.Petruzzelli M., Schweiger M., Schreiber R., Campos-Olivas R., Tsoli M., Allen J., Swarbrick M., Rose-John S., Rincon M., Robertson G., Zechner R., Wagner E.F. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 56.Kir S., White J.P., Kleiner S., Kazak L., Cohen P., Baracos V.E., Spiegelman B.M. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;1:1–19. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y., Miyagawa M., Tsujisaki M., Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19:1755–1760. doi: 10.1038/oby.2011.125. [DOI] [PubMed] [Google Scholar]
- 58.Yoneshiro T., Ogawa T., Okamoto N., Matsushita M., Aita S., Kameya T., Kawai Y., Iwanaga T., Saito M. Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int. J. Obes. 2013;37:993–998. doi: 10.1038/ijo.2012.161. [DOI] [PubMed] [Google Scholar]
- 59.Xue R., Lynes M.D.M.S., Dreyfuss J.M., Shamsi F., Schulz T.J., Zhang H., Huang T.L., Townsend K.L., Li Y., Takahashi H., Weiner L.S., White A.P., Lynes M.D.M.S., Rubin L.L., Goodyear L.J., Cypess A.M., Tseng Y.-H. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med. 2015;21:760–768. doi: 10.1038/nm.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinoda K., Luijten I.H.N., Hasegawa Y., Hong H., Sonne S.B., Kim M., Xue R., Chondronikola M., Cypess A.M., Tseng Y.-H., Nedergaard J., Sidossis L.S., Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen J.B., Jørgensen C., Petersen K., Hallenborg P., De Matteis R., Bøye H.A., Petrovic N., Enerba S., Nedergaard J., Cinti S., Klattenhoff C., De Fuente E., Rigotti A., Gonza A. Proc. Natl. Acad. Sci. USA. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmons J.A., Wennmalm K., Larsson O., Walden T.B., Lassmann T., Petrovic N., Hamilton D.L., Gimeno R.E., Wahlestedt C., Baar K., Nedergaard J., Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl. Acad. Sci. USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H.M., Erdjument-Bromage H., Tempst P., Rudnicki M.A., Beier D.R., Spiegelman B.M. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schulz T.J., Huang T.L., Tran T.T., Zhang H., Townsend K.L., Shadrach J.L., Cerletti M., McDougall L.E., Giorgadze N., Tchkonia T., Schrier D., Falb D., Kirkland J.L., Wagers A.J., Tseng Y.-H. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]