Abstract
We report a case of chromoblastomycosis in lesions on the chest and foot. Itraconazole was chosen as the initial treatment for this patient, who was followed up for 8 months before becoming noncompliant. The pathogenic fungal species was identified as Rhinocladiella similis by ITS region sequencing. In vitro analyses indicate that the fungus was sensitive to posaconazole and itraconazole. This report presents R. similis as a new agent of chromoblastomycosis and raises the hypothesis that this species could be more resistant to some antifungals than R. aquaspersa.
Keywords: Rhinocladiella similis, Chromoblastomycosis, In vitro antifungal susceptibility, Molecular identification, NTD
1. Introduction
Chromoblastomycosis is a tropical and subtropical skin disease caused by environmental accidental inoculation of pathogenic fungi. For instance, this disease often affects farmers, and it is believed that the fungus penetrates the skin through injuries caused by contaminated environmental debris [1], [2]. Chromoblastomycosis is a chronic granulomatous infection characterized by muriform cells, tissue proliferation, and microabscess, which may cause several complications to the health of affected individuals [1], including the inability to work if not treated in the early stages. This fungal skin infection is characterized as one of the Neglected Tropical Diseases (NTD) [2], a cover term for a group of ignored infectious diseases affecting the poorest regions in the world [3].
Rhinocladiella is a genus of melanized fungi that can cause chromoblastomycosis. Taxonomically, the Rhinocladiella genus belongs to the Chaetothyriales order (Ascomycota). This order contains several clinically relevant species of the genera Exophialia, Cladophialophora, Fonsecaea, and Phialophora, which are possible etiologic agents of chromoblastomycosis and/or phaeohyphomycosis [1], [2], [4], [5]. Rhinocladiella similis was isolated for the first time in a human ulcerated foot lesion characterized as chromoblastomycosis, whose report was published in a congress abstract book M.A. Resende, R.B. Caligiorne, C.R. Aguilar and M.M. Gontijo, Abstr. 14th Congr. Int. Soc. Human Anim. Mycol., p. 274, 2000 [4]. Later, R. similis was isolated from environmental samples [6], such as hemodialysis water [7], tap water and groundwater [8]. We, therefore, herein report the first case published in a scientific journal and the second case published in the worldwide literature of R. similis causing chromoblastomycosis.
2. Case
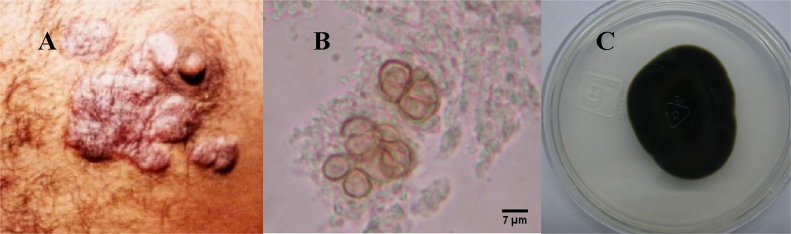
In 2002, a 47-year-old, Mexican rural worker male patient presented asymptomatic scaly lesions on the dorsum of the left foot and on the chest. According to the patient, these manifestations had started 4 years before the physician office visit. The first lesion appearances were characterized as small plaques on the foot, and then later lesions arose on the chest. The patient also declared having no history of traumatic injury in these regions. Upon examination, several erythematous verrucous plaques were seen on both chest and foot (Fig. 1A).
Fig. 1.
(A) Chest lesion caused by Rhinocladiella similis. (B) Sclerotic cells from a lesion sample observed by optic microscopy (400×). (C) Colonial morphology from R. similis in PDA after incubation at 35 °C for 14 days.
In the same day of his first presence in hospital, direct and culture microscopic examinations were performed. Direct microscopic examination of plaques with KOH (20%) revealed muriform cells (5–7 µm in diameter; Fig. 1B) characterizing a chromoblastomycosis and the oral treatment with itraconazole (200 mg/day) was started immediately. Cultures were performed in Sabouraud dextrose agar (Difco, USA) supplemented with chloramphenicol (0.5 mg/mL) and incubated at 30 °C for 14 days. Both macroscopic (Fig. 1C) and microscopic analyses supported the identification of Rhinocladiella sp. colonies.
The patient was followed up during 8 months, and lesion improvement was observed despite his intermittent noncompliance. After this period, the patient quit the treatment. Thirty two months after his first visit, the patient reappeared presenting active lesions in the same places, but he did not want to restart the treatment and did not return to further consultation.
For fourteen years the isolate was maintained in bank collection of fungal in Mexico laboratory. In Brazilian laboratory, the identification at level species was performed with its grown for 14 days in Sabouraud broth at 30 °C, without shaking. Total genomic DNA was extracted and purified from cultures using the Power Soil DNA Isolation Kit (Mobio, USA). Sequencing of the ITS1-5.8S rDNA-ITS2 region was performed using the universal primers ITS1 and ITS4. The amplification conditions were: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 10 min [5]. The PCR product was purified using ExoSAP-IT (Affymetrix, USA) and sequenced in the ABI-PRISM 3100 Genetic Analyzer (Applied Biosystems), according to the manufacturer's instructions. The sequence was assembled and compared with sequences of type strains reported in GenBank using the Basic Local Alignment Search Tool (BLAST) algorithm. The etiologic agent was confirmed as Rhinocladiella similis, since it presented sequence identity at 99% and coverage at 98% with the type strain of this species (CBS 111763). This strain was added to GenBank as number KY657562.
Due to the low effectiveness of itraconazole observed in patient, the absence of an antifungal test in the literature against this new agent of chromoblastomycosis, and the existence of an in vitro antifungal activity protocol, made available in 2008, the antifungal assay was evaluated with the isolate. Therefore, the susceptibility assay was performed according to the protocol M38-A2 of the Clinical and Laboratory Standards Institute (CLSI), utilizing the microdilution technique [9]. For inoculum preparation, Rhinocladiella similis isolates were grown on potato dextrose agar at 30 °C for 14 days. The colonies were covered with approximately 3 mL 0.85% sterile saline solution, and a suspension was prepared by scraping across all colonies with a rigid sterile plastic loop. The suspension was filtered through a filter paper to separate hyphae and conidia. Conidial-only presence in suspensions was verified by microscopy, and the conidia quantitation was performed with a Neubauer chamber [10]. The final concentration in the wells was 2.5×104 CFU/mL [9], [10], [11].
The following antifungals were evaluated: amphotericin B, itraconazole, ketoconazole, voriconazole, posaconazole and terbinafine (all from Sigma-Aldrich, USA) in the final concentration range of 0.03–16 μg/mL [9]. The incubation temperature was 35 °C for up to five days [11]. The minimal inhibitory concentrations (MICs) were determined when there was 100% of visual inhibition by comparing antifungal concentration to the growth in the drug-free wells (growth control) [9]. This assay was performed in triplicate.
After evaluating the MIC, the minimum fungicidal concentration (MFC) was determined. A 50 µL aliquot from the wells in which no growth was observed was transferred to a plate (1 mL 96-well type plate) containing 800 µL/well of Sabouraud-dextrose broth. The plate was incubated for 15 days at 30 °C and growth was visually observed. MFC was defined as the minimum concentration in which no fungal growth occurred [12]. This assay was performed in duplicate.
The MIC obtained of antifungals were (µg/mL): posaconazole and terbinafine (0.5); itraconazole (1.0); ketoconazole and voriconazole (2.0); amphotericin B (8.0). The MFC of posaconazole was 2.0 µg/mL and for all the others, the MFC were ≥16.0 µg/mL.
3. Discussion
With the advent of molecular biology, it has been noticed that clade Exophialia spinifera contains several morphologically similar species, being Rhinocladiella similis one of them [4], [13]. For many years, fungal identification was based on micro and macroscopic characteristics, which led to a series of misidentifications, leading to under- or overestimation of the number of infection cases caused by different species [4], [5]. Currently, in research, the identification based on sequencing of the Internal Transcribed Spacer (ITS) region of ribosomal DNA has been considered a reliable source of identification for this clade [13], but it has not been frequently used in clinical practices.
This is the first report published in a scientific journal and the second case published in the worldwide literature of Rhinocladiella similis as chromoblastomycosis agent. The first case was isolated before 2000 from the foot of a 72-year-old Caucasian male in Minas Gerais, Brazil, and the report was presented at a scientific meeting (M.A. Resende, R.B. Caligiorne, C.R. Aguilar and M.M. Gontijo, Abstr. 14th Congr. Int. Soc. Human Anim. Mycol., p. 274, 2000). This isolate was identified as R. similis in 2003 and it became the type strain for comparison in Genbank (CBS 111763) [4]. The following species of the genus Rhinocladiella have also been reported to relate to chromoblastomycosis: R. aquaspersa, a classical chromoblastomycosis agent [1], [2]; R. tropicalis, which was isolated from 4 Brazilians patients [1]; and R. phaeophora, whose only report derives from a case in Thailand [14].
In the case presented herein, lesions were observed both on the foot, a common site of chromoblastomycosis lesion, and in the chest, which is less common. Typically, disease dissemination occurs slowly, with continuous propagation around the infection source; in contrast, noncontiguous or remote-site lesions may result from autoinoculation caused by itching [2]. In this case, the primary lesion was on the foot, whereas autoinoculation may have occurred on the chest, given the distance to those lesions without lymphatic progression.
Using the cutoff proposed by CLSI [9], the isolate was considered sensitive to posaconazole and itraconazole. The isolate response was characterized as intermediate to voriconazole and resistant to amphotericin B. For terbinafine and ketoconazole, there is no reference of cutoff in the protocol.
The MICs of posaconazole and voriconazole against R. similis were similar to those of both antifungals against R. aquaspersa [10], [15], and the MICs of ketoconazole, itraconazole, terbinafine and amphotericin B were similar to those against two other strains of the genus [11]. However, the R. similis isolate presented MICs 3-fold higher for these last three antifungals, in comparison to R. aquaspersa, indicating that R. similis may be less susceptible than R. aquaspersa to those antifungals. This is an important observation because itraconazole is the standard therapy for chromoblastomycosis [2]. Therefore, according to these results, treatment with itraconazole may result ineffective or of decreased effectiveness when the infection is caused by R. similis. Taking this into account, identification at the species level of Rhinocladiella spp. and/or antifungal susceptibility assay should be performed in clinical practices to avoid a treatment with low effectiveness.
To our knowledge, the MFC test for Rhinocladiella genus causing chromoblastomycosis had not been reported until now. For this isolate, MFCs of the antifungals (except for posaconazole) were high, indicating lower fungicidal activity against R. similis, which can make the treatment difficult if the patient is immunocompromised [12].
In this case, itraconazole was chosen as the initial treatment for the patient in 2002 because it was and still is the standard medication against CBM agents [2] and evaluating the in vitro antifungal test performed in 2016, the isolate was considered sensitive to itraconazole, since it was in the concentration range. Terbinafine and posaconazole, antifungals that have low MIC (both) and MFC (posaconazole), are expensive drugs [2] for the patient's economic condition, therefore they would not be used as treatment.
The duration of treatment with itraconazole is variable, but a range from 8 to 10 months has been reported in many cases [2]. The patient's follow-up occurred during the first 8 months of treatment, and lesion regression was observed. Unfortunately, after this period the patient quit the treatment, returning 2 years later with active lesions which were probably due to the intermittent and incomplete therapy. Thus, it is impossible to inform if the treatment with itraconazole would be effective against R. similis if the patient had properly followed treatment.
This report present R. similis as a new agent of chromoblastomycosis and raise the hypothesis that this species could be more resistant to some antifungals, such as itraconazole, than R. aquaspersa. In addition, this article stress the importance of species-level identification and antifungal susceptibility testing in clinical practice.
Conflict of interest
None.
Acknowledgements
The authors thank CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the scholarships and Comissão de Pesquisa de Ciências Básicas da Saúde from Universidade Federal do Rio Grande do Sul for the financial support (23903).
Contributor Information
Gloria M. González, Email: gloria62@hotmail.com.
Maria Lucia Scroferneker, Email: scrofern@ufrgs.br.
References
- 1.Gomes R.R., Vicente V.A., Azevedo C.M.P.S., Salgado C.G., da Silva M.B., Queiroz-Telles F. Molecular epidemiology of agents of human chromoblastomycosis in Brazil with the description of two novel species. PLoS Negl. Trop. Dis. 2016;10:1–20. doi: 10.1371/journal.pntd.0005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Queiroz-Telles F., de Hoog S., Santos D.W.C.L., Salgado C.G., Vicente V.A., Bonifaz A. Chromoblastomycosis. Clin. Microbiol. Rev. 2017;30:233–276. doi: 10.1128/CMR.00032-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Utzinger J., Becker S.L., Knopp S., Blum J., Neumayr A.L., Keiser J. Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med. Wkly. 2012;142:1–24. doi: 10.4414/smw.2012.13727. [DOI] [PubMed] [Google Scholar]
- 4.de Hoog G.S., Vicente V., Caligiorne R.B., Kantarcioglu S., Tintelnot K., Gerrits van den Ende A.H.G. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. J. Clin. Microbiol. 2003;2003(41):4767–4778. doi: 10.1128/JCM.41.10.4767-4778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daboit T.C., Duquia R.P., Magagnin C.M., Mendes S.D.C., Castrillón M.R., Steglich R. A case of Exophiala spinifera infection in Southern Brazil: molecular identification and antifungal susceptibility. Med. Mycol. Case Rep. 2012;1:72–75. doi: 10.1016/j.mmcr.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madrid H., Hernández-Restrepo M., Gené J., Cano J., Guarro J., Silva V. New and interesting chaetothyrialean fungi from Spain. Mycol. Prog. 2016;15:1179–1201. [Google Scholar]
- 7.Figel I.C., Marangoni P.R.D., Tralamazza S.M., Vicente V.A., do P., Dalzoto R., do Nascimento M.M.F. Black yeasts-like fungi Isolated from dialysis water in hemodialysis units. Mycopathologia. 2013;175:413–420. doi: 10.1007/s11046-013-9633-4. [DOI] [PubMed] [Google Scholar]
- 8.Babic M.N., Zalar P., Zenko B., Dzeroski S., Gunde-Cimerman N. Yeasts and yeast-like fungi in tap water and groundwater, and their transmission to household appliances. Fungal Ecol. 2016;20:30–39. [Google Scholar]
- 9.Clinical and Laboratory Standards Institute (CLSI), Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed., Wayne, PA: Clinical and Laboratory Standards Institute (CLSI), Approved Standard M38-A2, 2008.
- 10.González G.M., Rojas O.C., González J.G., Kang Y., de Hoog G.S. Chromoblastomycosis caused by Rhinocladiella aquaspersa. Med. Mycol. Case Rep. 2013;2:148–151. doi: 10.1016/j.mmcr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daboit T.C., Magagnin C.M., Heidrich D., Antochevis L.C., Vigolo S., Meirelles L.C. In vitro susceptibility of chromoblastomycosis agents to five antifungal drugs to the combination of terbinafine and amphotericin B. Mycoses. 2014;57:116–120. doi: 10.1111/myc.12111. [DOI] [PubMed] [Google Scholar]
- 12.Magagnin C.M., Stopiglia C.D., Vieira F.J., Heidrich D., Machado M., Vetoratto G. Antifungal susceptibility of dermatophytes isolated from patients with chronic renal failure. An. Bras. Dermatol. 2011;86:694–701. doi: 10.1590/s0365-05962011000400011. [DOI] [PubMed] [Google Scholar]
- 13.Zeng J.S., de Hoog G.S. Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med. Mycol. 2008;46:193–208. doi: 10.1080/13693780701799217. [DOI] [PubMed] [Google Scholar]
- 14.Kampirapap K., Reangchainam S., Ornpaew P., Tresukosol P. Chromoblastomycosis masquerading as dermatophytosis, with the description of a new opportunistic species. Southeast Asian J. Trop. Med. Public Health. 2015;46:105–109. [PubMed] [Google Scholar]
- 15.Badali H., Bonifaz A., Barrón-Tapia T., Vázquez-González D., Estrada-Aguilar L., Oliveira N.M. Rhinocladiella aquaspersa, proven agent of verrucous skin infection and a novel type of chromoblastomycosis. Med. Mycol. 2010;48:696–703. doi: 10.3109/13693780903471073. [DOI] [PubMed] [Google Scholar]