Abstract
Adipocytes hydrolyze triglycerides and secrete free fatty acids and glycerol into the circulation. The molecular mechanism involved in glycerol transport from adipocytes has not been elucidated. Here, we investigated glycerol and glucose metabolism in mice lacking aquaporin 7 (Aqp7), a member of the aquaglyceroporins expressed in adipose tissue, and demonstrated that Aqp7 functions as a glycerol gateway molecule in vivo. Aqp7-knockout (KO) mice had lower plasma glycerol levels compared with WT mice but had normal plasma free fatty acid levels. The increase in plasma glycerol level in response to β3-adrenergic agonist was severely impaired in KO mice. Epinephrine-stimulated glycerol secretion was also impaired in Aqp7 knockdown adipocytes. During prolonged fasting, plasma glycerol was elevated and the plasma glucose level was maintained in WT mice. In contrast, KO mice showed a disrupted increase of plasma glycerol and rapid reduction of plasma glucose during prolonged fasting. Our findings indicate that the lack of effective glycerol transport from adipocytes by glycerol gateway molecule causes defective adaptation to prolonged fasting.
Keywords: adipocyte, gluconeogenesis, lipolysis, knockout mice
Aquaporins (AQPs) are integral membrane proteins that facilitate water movement across the cell membrane (1-3). The discovery of AQPs allowed the recognition of the significance of water channels in maintaining water homeostasis (4, 5). The fact that the lack of AQP2 causes nephrogenic diabetes insipidus in rodents (6, 7) and humans (8) has emphasized the significance of water channel in physiology and homeostasis. Some of AQPs are known to permeabilize glycerol as well as water (9, 10) and are subcategorized as aquaglyceroporins. We previously cloned a cDNA belonging to aquaglyceroporin from a human adipose tissue cDNA library, designated as AQP adipose (AQPap) (11-15). Subsequently, other groups demonstrated that AQPap was a human homologue of Aqp7.
Triglycerides in adipocytes are hydrolyzed to fatty acids and glycerol, and both are released into the circulation (16-20). The molecular mechanism involved in the transport of glycerol from adipocytes remains unclear. Previously, we reported that exercise-induced increase of plasma glycerol was impaired in a subject carrying a loss of function mutation of AQP7 (14). We postulated that AQP7 might function as a glycerol channel molecule in adipose tissue. Here, we generated mice lacking Aqp7 and demonstrated that these knockout (KO) mice showed impaired glycerol release and severe fasting-induced hypoglycemia. These results indicate that AQP7 functions as a glycerol channel in vivo.
Materials and Methods
Animals. The targeting vector for Aqp7 KO was constructed by using the pGT vector containing the neomycin resistance gene. The Aqp7 targeting vector was designed to replace 8.0 kb of genomic locus with the Neo cassette. This 8.0-kb targeting region was located in exons 1, 2, and 3 containing the translation initiation sites. The targeting construct was electroporated into mouse embryonic stem (ES) cells (129/SvJ). ES cell clones, having undergone homologous recombination with the targeting vector, were verified by Southern blotting. Targeted ES cells were microinjected into C57BL/6 mice. Chimeric mice were bred to C57BL/6N mice, and chimeric males gave offspring that carried the disrupted mouse Aqp7 allele through the germ line. We analyzed mice backcrossed to C57BL/6N for five generations. All experiments were performed in 7- to 10-week-old male mice. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Graduate School of Medicine of Osaka University.
Northern Blot Analysis. Total RNA was isolated from mice tissues and cultured cells by using RNA STAT-60 (Tel-Test, Friends-wood, TX). Ten micrograms of total RNA was electrophoresed on 1% agarose/formaldehyde gel and transferred to nylon membranes (Hybond-N+, Amersham Pharmacia Life Science). The membrane was hybridized with the indicated cDNA probe labeled with [α-32P]dCTP. The hybridized membrane was exposed to x-ray film.
Analysis of Metabolic Parameters. Plasma glucose, insulin, and free fatty acid (FFA) levels were measured by the Glucose CII-Test (Wako Pure Chemical, Osaka), the Glazyme Insulin EIA kit (Sanyo, Tokyo), and the Nescauto NEFA kit (Azwell, Osaka), respectively. Glycerol concentrations in plasma, medium, and tissue were determined by using a fluorometric/colorimetric enzyme method. Plasma glucagon and cortisol levels were measured by ELISA with the Glucagon EIA kit (Yanaihara, Shizuoka, Japan) and the Cortisol Enzyme Immunoassay kit (Assay Designs, Ann Arbor, MI), respectively.
Assessment of Metabolic Regulation. Glucose or insulin tolerance tests were conducted as described in ref. 21. Briefly, for glucose tolerance test, mice were fasted for 6 h and a basal blood sample was taken, followed by i.p. injection of D(+)-glucose (2 mg per g of body weight). Blood samples were collected from tail vein at 15, 30, 60, and 120 min after injection. For insulin tolerance test, baseline glucose levels were measured in fed mice, then the mice were injected i.p. with human regular insulin (0.75 milli-units per g of body weight). Blood samples were collected from the tail vein at 15, 30, 60, and 90 min after injection. For the in vivo lipolysis assay, fed mice were i.p. injected with BRL26830A (a selective β3-adrenergic agonist) at 5 μg per g of body weight. Plasma glycerol and FFA were measured by tail bleeding at 0, 10, and 20 min after the injection. For the glycerol administration tests, 6-h fasted mice were i.p. injected with glycerol at 1 mg per g of body weight. Plasma glucose levels were then measured at indicated time points after glycerol injection.
Evaluation of Insulin Signaling. Twelve-hour-overnight-fasted mice were injected with either human regular insulin (1 unit per g of body weight) or saline through the inferior vena cava, and the gastrocnemius muscle, epididymal fat, and liver were excised at 2, 3, and 4 min after injection, respectively. Tissue samples were homogenized and centrifuged at 15,000 × g for 30 min in ice-cold homogenization buffer (50 mM Hepes, pH 7.4/1% Triton X-100/10% glycerol/10 mM sodium pyrophosphate/0.1 M sodium fluoride/10 mM EDTA/5 mM sodium orthovanadate/10 μg/ml aprotinin/5 μg/ml leupeptin/1.5 mg/ml benzamidine/2 mM PMSF). Supernatants were collected, and protein concentration was measured with the BCA protein assay kit (Pierce), using BSA as the standard. A 30-μg protein sample was boiled for 5 min, cooled on ice, and subjected to 12.5% SDS/PAGE, followed by transblotting to a nitrocellulose membrane. Western blot analysis was carried out by using antibodies to Akt and Phospho-Akt (Cell Signaling Technology, Beverly, MA).
Determination of Hormone-Sensitive Lipase (HSL) Activity. The HSL activity assay was performed as described in refs. 22 and 23. In brief, epididymal fat tissues were homogenized in ice-cold buffer (250 mM sucrose/10 mM Tris·HCl, pH 7.5/1 mM EDTA/10 μg/ml aprotinin/10 μg/ml leupeptin/1 μg/ml pepstatin A) and centrifuged at 100,000 × g for 45 min. The supernatant was used for enzyme assay. The protein samples were incubated at 37°C for 30 min in a final volume of 200 μl of a reaction mixture containing 105 μM tri[3H]oleoylglycerol (99.4 μCi/μmol; 1 Ci = 37 GBq), 23.7 μM lecithin, 12.5 μM sodium taurocholate, 1 M NaCl, and 85 mM potassium phosphate (pH 7.0).
Cell Culture. The mouse 3T3-L1 cell line was obtained from Health Science Research Resources Bank (Osaka). 3T3-L1 preadipocytes were grown to confluence and induced to differentiate into adipocytes as described in ref. 12.
Design and Transfection of siRNA. Two pairs of siRNA were chemically synthesized by Qiagen, annealed, and transfected into 3T3-L1 adipocytes by using Lipofectamine 2000 (Invitrogen) as described in ref. 24. The sequences of the sense siRNAs were as follows: Aqp7 siRNA, 5′-UGCCGCAGUGACUUUCACCdTdT-3′; control siRNA, 5′-UUCUCCGAACGUGUCACGUdTdT-3′. Twenty-four hours after transfection, cells were subjected to total RNA extraction and lipolysis assay, as described below.
In Vitro Lipolysis Assay. 3T3-L1 adipocytes cultured in 12-well plates were treated with 0.5% fatty acid-free BSA containing 1 μM epinephrine. The medium was collected exactly at 0, 15, and 30 min after stimulation.
Statistical Analysis. Results were expressed as the mean ± SEM of n separate experiments. Differences between groups were examined for statistical significance by using Student's t test or ANOVA with Fisher's protected least significant difference test.
Results
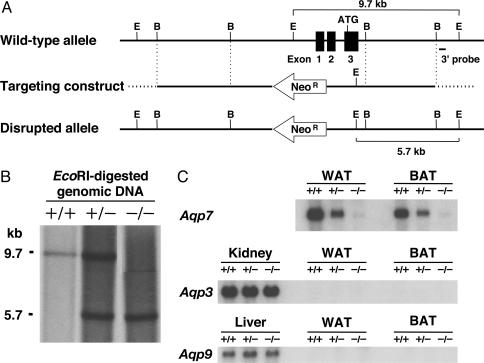
To disrupt the Aqp7 gene, we replaced exons 1-3, which contain the 5′ UTR and the translation initiation site, by the neomycin resistance gene (Fig. 1A). We confirmed disruption of the target allele by Southern blotting, using EcoRI-digested genomic DNA (Fig. 1B), and also confirmed the lack of Aqp7 mRNA in white (WAT) and brown (BAT) adipose tissue of KO mice (Fig. 1C). Furthermore, no Aqp7 mRNA was found in KO mice testis, which is known to express Aqp7 (data not shown). No ectopic expression of other aquaglyceroporins, such as Aqp3 and Aqp9, was detected in adipose tissues of KO mice (Fig. 1C).
Fig. 1.
Targeted disruption of the mouse AQP7 gene. (A) Schematic representation of the gene targeting strategy. Partial restriction map of the mouse AQP7 (Aqp7) locus for the WT allele (Top). The targeting construct for Aqp7 was generated by replacing the region of exons 1-3 containing the translation initiation site ATG with the Neo cassette (NeoR) (Middle). The transcriptional direction of the NeoR gene is indicated by the arrow. The expected disrupted allele was obtained by homologous recombination (Bottom). E and B indicate EcoRI and BamHI restriction enzyme sites, respectively. (B) EcoRI-digested genomic Southern blot analysis. The 9.7-kb band corresponds to the WT allele (+/+), and the 5.7-kb corresponds to band to the disrupted allele (-/-), using the probe denoted in A. (C) Northern blotting of WAT, BAT, kidney, and liver. +/+, WT; +/-, heterozygous; -/-, homozygous mice.
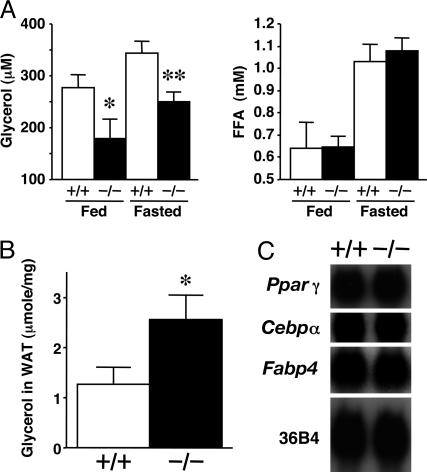
KO mice had significantly lower plasma glycerol concentrations, both in fed and 12-h-fasted conditions, than WT control mice (Fig. 2A). However, there were no differences in plasma FFA concentrations between WT and KO mice under both fed and fasted states. KO mice exhibited a higher content of glycerol in adipose tissue under the fasted state (Fig. 2B). However, they gained body weight at a rate equivalent to that of WT mice until 10 weeks of age (data not shown). No significant difference was observed in the tissue weight and gross appearance of adipose tissue between KO and WT mice. Normal expression levels were detected in KO mice for peroxisome proliferator-activated receptor γ (Pparγ), CCAAT/enhancer-binding protein α (Cebpα), and adipocyte fatty acid binding protein 4 (Fabp4), which are genes for adipocyte differentiation markers (Fig. 2C).
Fig. 2.
Low plasma glycerol concentrations and high adipose glycerol contents in KO mice. (A) Plasma levels of glycerol and FFA under fed and 12-h-fasted conditions (n = 6 per group). (B) Glycerol content in adipose tissue of 12-h-fasted mice (n = 5-6). (C) Northern blot analysis of mRNAs related to adipogenic marker genes in WAT. +/+, WT; -/-, KO mice. In A and B, data are mean ± SEM. *, P < 0.05; **, P < 0.01, compared with the values of WT mice under the same conditions.
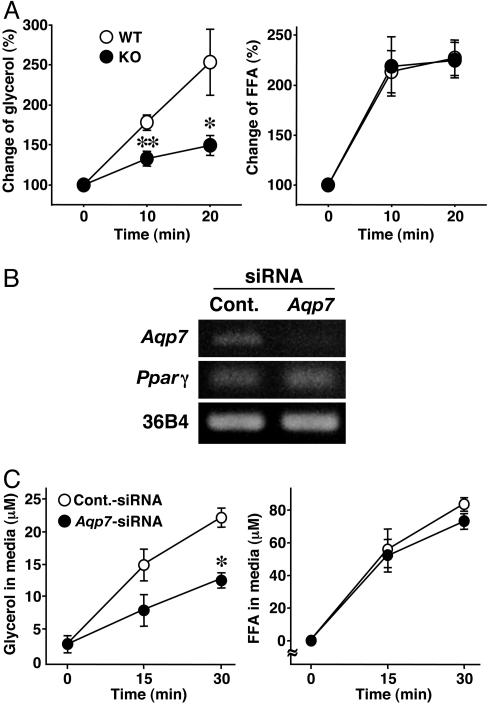
In the fasting state, adipocytes hydrolyze triglycerides and rapidly liberate glycerol and FFAs into the circulation. Next, we investigated the effects of BRL26830A, a β3-adrenergic agonist, which enhances lipolysis in adipose tissue, in WT and KO mice. Administration of BRL26830A increased plasma glycerol concentrations in WT mice. However, such increases were impaired in KO mice (Fig. 3A). The increase of plasma FFA in KO mice in response to β3-adrenergic agonist was similar to that in WT (Fig. 3A). To confirm that the lack of Aqp7 retards the release of glycerol from adipocytes, we knocked down Aqp7 in 3T3-L1 adipocytes by using siRNA. Introduction of siRNA markedly decreased Aqp7 mRNA expression level but had no effect on the mRNA level of Pparγ, which is a master regulator of adipocyte differentiation (Fig. 3B). Epinephrine-stimulated release of glycerol was significantly suppressed in Aqp7 siRNA-transfected adipocytes (Fig. 3C). However, epinephrine-stimulated release of FFAs was similar (Fig. 3C).
Fig. 3.
Impaired glycerol release from Aqp7-KO and -knockdown adipocytes. (A) Lipolysis measurement in vivo. Fed mice were i.p. administered BRL26830A (n = 6-7). Plasma glycerol and FFA levels were normalized to those at 0 min (100%). (B) Knockdown of Aqp7 in 3T3-L1 adipocytes by introducing siRNA. Total RNAs were extracted after a 24-h transfection of siRNA and subjected to RT-PCR. Cont., control. (C) Lipolysis assay in vitro. 3T3-L1 adipocytes transfected with the indicated siRNA were treated with epinephrine (n = 4 per group). +/+, WT; -/-, KO mice. Pparγ, peroxisome proliferator-activated receptor γ. In A and C, data are mean ± SEM. *, P < 0.05; **, P < 0.01, compared with WT mice or control-siRNA.
The activity of hormone-sensitive lipase, which is a key regulator of lipolysis (22), was similar in WT and KO mice in the 12-h fasting state (WT, 539.5 ± 40.3 pmol·min-1 per mg of protein; KO, 565.5 ± 14.1 pmol·min-1 per mg of protein). These results indicate that Aqp7 is crucial for the effective release of glycerol from adipocytes.
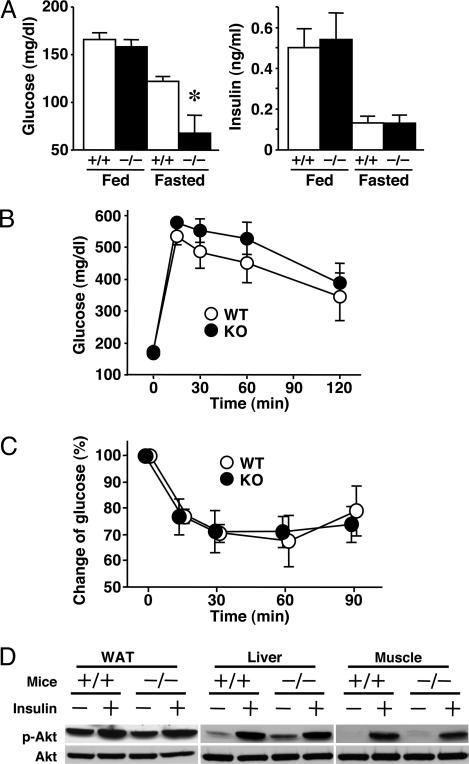
Plasma glucose concentrations were significantly lower after 12-h fasting in KO mice but were similar in the fed condition (Fig. 4A). Plasma insulin levels of KO mice were suppressed in the fasting state, similar to WT mice (Fig. 4A). We further examined glucose metabolism and insulin sensitivity in these mice. Changes in plasma glucose concentrations in glucose-tolerance test (Fig. 4B) and insulin-mediated suppression of plasma glucose (Fig. 4C) were similar in the two groups. Insulin-stimulated phosphorylation of Akt in WAT, liver, and muscle was also similar (Fig. 4D). These results suggest that insulin signaling is normal in KO mice.
Fig. 4.
Plasma glucose levels and insulin sensitivity in KO mice. (A) Plasma levels of glucose and insulin under fed and 12-h-fasted conditions (n = 6 per group). (B) Glucose curves under the glucose-tolerance test (n = 6-8). (C) Glucose curves under the insulin-tolerance test (n = 6-7). Plasma glucose levels were normalized to those at 0 min (100%). (D) Insulin-stimulated phosphorylation of Akt. Equal amounts of protein from each pooled fraction (n = 5 per group) were immunoblotted with anti-phospho-Akt (Ser-473) and anti-Akt antibodies. +/+, WT mice; -/-, KO mice. In A-C, data are mean ± SEM. *, P < 0.05, compared with the values of WT mice under the same conditions.
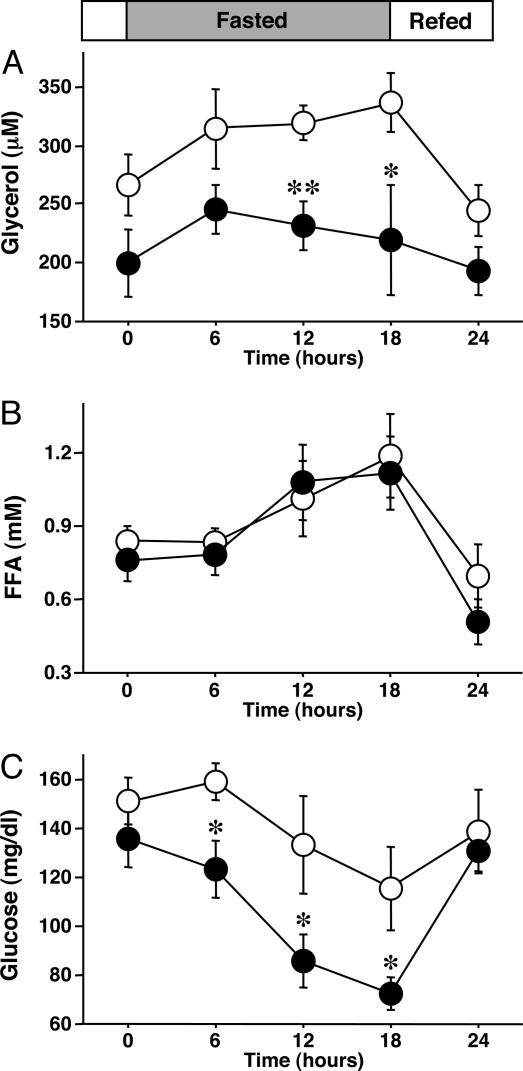
Next, we examined the metabolic changes in WT and KO mice during long periods of starvation; mice were kept fasted for 18 h after the 12-h feeding (Fig. 5). The reduction of body weight was similar in the WT and KO mice (data not shown). Plasma glycerol concentrations increased during starvation in WT mice but not in KO mice (Fig. 5A). FFA levels were elevated in both groups (Fig. 5B). The WT mice could maintain plasma glucose during a 12-h fast. However, plasma glucose levels decreased in KO mice after only a 6-h fast (Fig. 5C).
Fig. 5.
Fasting-induced hypoglycemia in KO mice. Mice were fasted for 18 h after a 12-h feeding (n = 6 per group). Shown is the effect of long fasting on plasma glycerol (A), FFA (B), and glucose (C). Data are mean ± SEM. *, P < 0.05; **, P < 0.01, compared with WT mice.
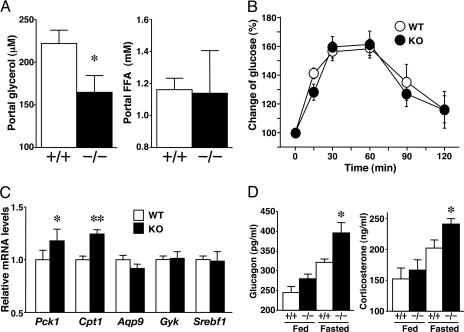
Glycerol and FFA released from mesenteric fat tissue flow into the portal vein during starvation, and glycerol is used as a substrate for gluconeogenesis in the liver (25, 26). KO mice showed a lower portal concentration of glycerol in the 12-h-fasting state compared with that in WT (Fig. 6A), as observed in peripheral blood. There was no difference in portal FFA levels between the two groups of mice. Therefore, a low concentration of portal glycerol apparently underlies the fasting hypoglycemia in KO mice. We next subjected these mice to the glycerol administration test (Fig. 6B). The extent of elevation of plasma glucose induced by i.p. administration of exogenous glycerol was similar in WT and KO mice. These results of experiments using exogenous glycerol as a substrate indicate no impairment of the hepatic gluconeogenesis in KO mice.
Fig. 6.
Liver gluconeogenesis in KO mice. (A) Plasma glycerol and FFA levels in the portal vein of 12-h-fasted mice (n = 6 per group). (B) Rate of glucose clearance after exogenous glycerol administration (n = 5 per group). Plasma glucose levels were normalized to those at 0 min (100%). (C) mRNA levels in liver of 12-h-fasted mice (n = 6 per group). (D) Plasma glucagon and corticosterone concentrations under fed and 12-h-fasted conditions (n = 6 per group). +/+, WT; -/-, KO. Pck1, phosphoenolpyruvate carboxykinase 1; Gyk, glycerol kinase; Srebf1, sterol regulatory element-binding factor 1. In all panels, data are mean ± SEM. *, P < 0.05; **, P < 0.01, compared with WT mice.
We also examined the expression of genes associated with glucose and lipid metabolism in the liver and muscle under 12-h-fasting conditions. The mRNA levels of phosphoenolpyruvate carboxykinase 1 (Pck1) and carnitine palmitoyltransferase 1 (Cpt1) were slightly elevated in the liver of KO mice compared with WT mice (Fig. 6C). We also examined the counterregulatory hormones against hypoglycemia. KO mice showed higher levels of plasma glucagon and corticosterone than WT mice in the 12-h-fasted state (Fig. 6D). Pck1 and Cpt1 mRNA levels might be elevated by increased counterregulatory hormones in KO mice (27-31). There were no apparent changes in liver Aqp9, Gyk, and Srebf1, which are usually regulated by insulin, in KO and WT mice. Peroxisome proliferator-activated receptor α (Pparα), Cd36, fatty acid transport protein 1 (Fatp1), and glucose-transporter protein 4 (Glut4) mRNA levels in the skeletal muscle of KO mice were similar to those of WT mice (data not shown).
In summary, we demonstrated in the present study that aquaglyceroporin acts as a glycerol channel in vivo. Adipocytes require a glycerol channel molecule for the efficient release of glycerol. Adaptation to fasting was impaired in mice lacking glycerol channel molecule in adipose tissue and caused fasting hypoglycemia.
Discussion
The major finding of the present study was that aquaglyceroporin acts as a glycerol channel in the adipose tissue in vivo. Triglyceride in adipocytes is hydrolyzed to glycerol and FFA, and both are released into the blood stream. Several membrane proteins participate in efficient efflux of FFA from adipocytes. These include fatty acid binding protein (FABP), fatty acid translocase (FAT, CD36), and fatty acid transporter protein (FATP). However, the molecule(s) responsible for the effective transport of glycerol has not been identified. We postulated that AQP7 acts as the adipose glycerol channel because AQP7 is glycerol-permeable and is expressed abundantly in adipose tissue (11, 12). In this regard, epinephrine-stimulated glycerol release from 3T3-L1 adipocytes and plasma glycerol concentrations in rodents increased in parallel with the induction of Aqp7 mRNA in the cells and adipose tissue, respectively. Based on genetic screening, we found a subject carrying loss of function mutation in the AQP7 gene with a substitution of Gly-264 to Val, which is located in the center of GXXGXXG motif in the sixth transmembrane domain and crucial for functional confirmation (14). Expression study in Xenopus oocytes revealed AQP7 with G264V mutation lost both water and glycerol transport. The subject was clinically normal, but the increase of plasma glycerol in response to exercise was impaired (14). Although the observation supported the role of AQP7 in glycerol metabolism, we experienced only one subject with this mutation in a homozygous form. In the present study, we generated Aqp7 null mice and clearly demonstrated the presence of low plasma glycerol concentration in KO mice, and we also showed that a β3-adrenergic agonist-induced increase in plasma glycerol was decreased by ≈2-fold in KO mice. Further analysis showed an ≈2-fold reduction in the velocity of glycerol secretion in Aqp7 knockdown adipocytes. Similar results were obtained in cultured adipocytes from the KO mice (data not shown). These results emphasize the importance of AQP7 in adipocytes for the efficient release of glycerol. It should be noted, however, that glycerol secretion was not completely abolished in Aqp7 null adipocytes, suggesting that other mechanisms, such as simple diffusion and/or other glycerol transporter, must be also involved in glycerol secretion.
Another important finding of this study was that Aqp7 null mice showed impaired adaptation to fasting and exhibited profound hypoglycemia under such condition. KO mice exhibited a low concentration of portal glycerol but a normal increase in plasma glucose concentrations in response to exogenous i.p. administered glycerol, suggesting that the lack of glycerol as a substrate for gluconeogenesis is responsible for the hypoglycemia in this model. In the fasted state, plasma insulin levels were suppressed in KO as well as WT mice, and plasma glucagon and corticosterone, which are the counterregulatory hormones against hypoglycemia, were increased in KO mice. Furthermore, the expression of Pck1 and Cpt1 induced by glucagon and corticosterone was up-regulated in KO mice. These results emphasize the significance of the glycerol gateway molecule in the metabolic regulation in vivo.
Sweet and coworkers (32) showed that GlpF is an aquaglyceroporin prototype in Escherichia coli (32). Aquaglyceroporins facilitate glycerol transport according to glycerol gradient. Using atomic resolution analysis, Weissenborn and colleagues (33) revealed that GlpF possess a wider pore in the hourglass-like structure compared with the water-selective AQPs.
In mammals, AQP3, AQP7, and AQP9 belong to the aquaglyceroporin family. AQP3 is expressed in the basal layer of keratinocytes and is thought to transport glycerol to the epidermis in response to dermal-to-epidermal gradient. Previous studies on Aqp3 null mice showed reduced glycerol content in the epidermis and impaired skin elasticity, barrier recovery, and wound healing, but normal serum glycerol concentrations (34-36). On the other hand, AQP9 is expressed in the liver and coordinately regulated with AQP7 in response to fasting/feeding (15). Aqp7 deletion did not affect the expression of Aqp9 in this study. Generation and analysis of Aqp9 KO mice should provide further information on the significance of the glycerol gateway molecule in the homeostasis of glucose metabolism in vivo. Recently, Liu et al. (37) reported that AQP7 facilitates the uptake of arsenite, a carcinogenic metalloid. Exposure of Aqp7 KO mice to arsenite may further enhance our understanding of the carcinogenic action of this metalloid. In the present study, we focused on the function of adipose AQP7 on the glycerol metabolism. However, kidney and testis also express AQP7. The divergent functions of Aqp7 in kidney and testis should be examined precisely in future by using the KO mice.
In summary, we demonstrated AQP7 acts as a glycerol channel in vivo by disrupting mouse Aqp7 gene. Fasting-induced severe hypoglycemia observed in Aqp7 null mice indicates that glycerol derived from adipocytes is a significant substrate for gluconeogenesis.
Acknowledgments
We thank S. Tarui, Y. Tochino, and the members of the Funahashi Adiposcience Laboratory for useful discussion; H. Okazaki and S. Ishibashi for providing the method of the hormone-sensitive lipase activity assay; and S. Tanaka and K. Yamamoto for excellent technical assistance. This work was supported in part by Grants-in-Aid for Scientific Research (B) 14370327 (to T.F.) and 15390287 (to T.N.), Grants-in-Aid for Scientific Research on Priority Areas 13137206 (to T.F.) and 15081208 (to S.K.), and the Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (to N.M.) and The Cell Science Research Foundation (to N.M.).
Author contributions: N.M., T.F., K.K., H.K., T.N., S.K., and Y.M. designed research; N.M., T.H., A.N., and K.K. performed research; N.M. analyzed data; and N.M., T.F., and I.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AQP, aquaporin; BAT, brown adipose tissue; FFA, free fatty acid; WAT, white adipose tissue.
References
- 1.Denker, B. M., Smith, B. L., Kuhajda, F. P. & Agre, P. (1988) J. Biol. Chem. 263, 15634-15642. [PubMed] [Google Scholar]
- 2.Verkman, A. S. (2002) J. Anat. 200, 617-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agre, P. & Kozono, D. (2003) FEBS Lett. 555, 72-78. [DOI] [PubMed] [Google Scholar]
- 4.Preston, G. M., Carroll, T. P., Guggino, W. B. & Agre, P. (1992) Science 256, 385-387. [DOI] [PubMed] [Google Scholar]
- 5.Giles, J. (2003) Nature 425, 651. [DOI] [PubMed] [Google Scholar]
- 6.Fushimi, K., Uchida, S., Hara, Y., Marumo, F. & Sasaki, S. (1993) Nature 361, 549-552. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen, S., DiGiovanni, S. R., Christensen, E. I., Knepper, M. A. & Harris, H. W. (1993) Proc. Natl. Acad. Sci. USA 90, 11663-11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deen, P. M., Verdijk, M. A., Knoers, N. V., Wieringa, B., Monnens, L. A., van Os, C. H. & van Oost, B. A. (1994) Science 264, 92-95. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi, K., Sasaki, S., Fushimi, K., Uchida, S., Kuwahara, M., Saito, H., Furukawa, T., Nakajima, K., Yamaguchi, Y., Gojobori, T., et al. (1994) Proc. Natl. Acad. Sci. USA 91, 6269-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agre, P., King, L. S., Yasui, M., Guggino, W. B., Ottersen, O. P., Fujiyoshi, Y., Engel, A. & Nielsen, S. (2002) J. Physiol. (London) 542, 3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuriyama, H., Kawamoto, S., Ishida, N., Ohno, I., Mita, S., Matsuzawa, Y. & Okubo, K. (1997) Biochem. Biophys. Res. Commun. 241, 53-58. [DOI] [PubMed] [Google Scholar]
- 12.Kishida, K., Kuriyama, H., Funahashi, T., Shimomura, I., Kihara, S., Ouchi, N., Nishida, M., Nishizawa, H., Matsuda, M., Takahashi, M., et al. (2000) J. Biol. Chem. 275, 20896-20902. [DOI] [PubMed] [Google Scholar]
- 13.Kishida, K., Shimomura, I., Kondo, H., Kuriyama, H., Makino, Y., Nishizawa, H., Maeda, N., Matsuda, M., Ouchi, N., Kihara, S., et al. (2001) J. Biol. Chem. 276, 36251-36260. [DOI] [PubMed] [Google Scholar]
- 14.Kondo, H., Shimomura, I., Kishida, K., Kuriyama, H., Makino, Y., Nishizawa, H., Matsuda, M., Maeda, N., Nagaretani, H., Kihara, S., et al. (2002) Eur. J. Biochem. 269, 1814-1826. [DOI] [PubMed] [Google Scholar]
- 15.Kuriyama, H., Shimomura, I., Kishida, K., Kondo, H., Furuyama, N., Nishizawa, H., Maeda, N., Matsuda, M., Nagaretani, H., Kihara, S., et al. (2002) Diabetes 51, 2915-2921. [DOI] [PubMed] [Google Scholar]
- 16.Spiegelman, B. M. & Flier, J. S. (2001) Cell 104, 531-543. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, J. M. (2000) Nature 404, 632-634. [DOI] [PubMed] [Google Scholar]
- 18.Saltiel, A. R. & Kahn, C. R. (2001) Nature 414, 799-806. [DOI] [PubMed] [Google Scholar]
- 19.Moller, D. E. (2001) Nature 414, 821-827. [DOI] [PubMed] [Google Scholar]
- 20.Kahn, B. B. & Flier, J. S. (2000) J. Clin. Invest. 106, 473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda, N., Shimomura, I., Kishida, K., Nishizawa, H., Matsuda, M., Nagaretani, H., Furuyama, N., Kondo, H., Takahashi, M., Arita, Y., et al. (2002) Nat. Med. 8, 731-737. [DOI] [PubMed] [Google Scholar]
- 22.Osuga, J., Ishibashi, S., Oka, T., Yagyu, H., Tozawa, R., Fujimoto, A., Shionoiri, F., Yahagi, N., Kraemer, F. B., Tsutsumi, O., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki, H., Osuga, J., Tamura, Y., Yahagi, N., Tomita, S., Shionoiri, F., Iizuka, Y., Ohashi, K., Harada, K., Kimura, S., et al. (2002) Diabetes 51, 3368-3375. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin, L., Karelsky, S. & Andino, R. (2002) Nature 418, 430-434. [DOI] [PubMed] [Google Scholar]
- 25.Baba, H., Zhang, X. J. & Wolfe, R.R. (1995) Nutrition 11, 149-153. [PubMed] [Google Scholar]
- 26.Peroni, O., Large, V. & Beylot, M. (1995) Am. J. Physiol. 269, E516-E523. [DOI] [PubMed] [Google Scholar]
- 27.Jiang, G. & Zhang, B. B. (2003) Am. J. Physiol. 284, E671-E678. [DOI] [PubMed] [Google Scholar]
- 28.Puigserver, P., Rhee, J., Donovan, J., Walkey, C. J., Yoon, J. C., Oriente, F., Kitamura, Y., Altomonte, J., Dong, H., Accili, D., et al. (2003) Nature 423, 550-555. [DOI] [PubMed] [Google Scholar]
- 29.Louet, J. F., May, C. L., Pegorier, J. P., Decaux, J. F. & Girard, J. (2001) Biochem. Soc. Trans. 29, 310-316. [DOI] [PubMed] [Google Scholar]
- 30.Previs, S. F. & Brunengraber, H. (1998) Curr. Opin. Clin. Nutr. Metab. Care 1, 461-465. [DOI] [PubMed] [Google Scholar]
- 31.Louet, J. F., Hayhurst, G., Gonzalez, F. J., Girard, J. & Decaux, J. F. (2000) J. Biol. Chem. 277, 37991-38000. [DOI] [PubMed] [Google Scholar]
- 32.Sweet, G., Gandor, C., Voegele, R., Wittekindt, N., Beuerle, J., Truniger, V., Lin, E. C. & Boos, W. (1990) J. Bacteriol. 172, 424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weissenborn, D. L., Wittekindt, N. & Larson, T. J. (1992) J. Biol. Chem. 267, 6122-6131. [PubMed] [Google Scholar]
- 34.Ma, T., Hara, M., Sougrat, R., Verbavatz, J. M. & Verkman, A. S. (2002) J. Biol. Chem. 277, 17147-17153. [DOI] [PubMed] [Google Scholar]
- 35.Hara, M., Ma, T. & Verkman, A. S. (2002) J. Biol. Chem. 277, 46616-46621. [DOI] [PubMed] [Google Scholar]
- 36.Hara, M. & Verkman, A. S. (2003) Proc. Natl. Acad. Sci. USA 100, 7360-7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, Z., Shen, J., Carbrey, J. M., Mukhopadhyay, R., Agre, P. & Rosen, B. P. (2002) Proc. Natl. Acad. Sci. USA 99, 6053-6058. [DOI] [PMC free article] [PubMed] [Google Scholar]