Abstract
Collagen quantification has long been relevant to biomedical research and clinical practice to characterize tissues and determine disease states. The hydroxyproline assay, while a broadly employed method of quantifying collagen, uses perchloric acid to dissolve Ehrlich's reagent. Since perchloric acid poses occupational safety hazards and high costs, in this study, a new hydroxyproline assay was developed that replaces perchloric acid with a relatively safer and cheaper alternative, hydrochloric acid (HCl). To validate this biochemical technique, first, using either acid to dissolve Ehrlich's reagent, the assays were completed for native and engineered collagenous tissues. No statistical differences were identified between the assays (p = 0.32). Subsequently, both biochemical techniques were compared to amino acid analysis, considered a proteomics gold standard. Interestingly, utilizing HCl in lieu of perchloric acid yielded greater concordance with amino acid analysis (ρc = 0.980) than did the traditional assay (ρc = 0.947); that is, the HCl-based assay more closely estimates hydroxyproline content, and, consequently, true collagen content. Thus, using Ehrlich's reagent containing HCl in the hydroxyproline assay represents an advance in both mitigating laboratory safety hazards and improving biochemical collagen quantification.
Keywords: : hydroxyproline, 4-dimethyl(amino)benzaldehyde, perchloric acid, quantitative biochemistry
Introduction
Collagen, the most abundant protein in mammals, has been heavily studied for over a century.1,2 It primarily gives rise to the mechanical integrity of connective tissues, such as those of the musculoskeletal system, including bone, cartilage, and tendon.3–5 Additionally, other biological structures (e.g., skin, eye, lung, gut, and vasculature) incorporate copious collagen.6 For any of these systems, a change in collagen density could alter tissue biomechanics leading to impaired biological function.7 The importance of structure-function relationships is paramount, and the specific role of collagen has been extensively characterized. At a smaller scale, collagen also affects cellular behavior via cell-matrix mechanotransduction. Collagen bound to cell-surface proteins (e.g., integrins) can either promote proper cell phenotype, as in chondrocytes, or contribute to cell dysregulation, as in tumor progression.8,9 Degradation or loss of collagen due to age or trauma remains clinically relevant since these processes can adversely affect physiologic function of the aforementioned organ systems. Excessive collagen production associated with chronic or severe tissue damage can also deteriorate normal tissue function.10 Thus, in both healthy and diseased states, collagen is a biologically important protein; for research and clinical purposes, methods to accurately quantify collagen in a target tissue or as a metabolite in urine, for example, have been developed since the mid-1900s.11
It was recognized that collagen could be quantified indirectly through hydroxyproline content since this amino acid is present almost exclusively in collagen. The gold standard for measuring hydroxyproline is chromatographic amino acid analysis, but this technique is not practical for all experiments due to its low-throughput nature and relatively high cost. As such, Neuman and Logan11 devised a simple colorimetric assay that generates a chromophore from hydroxyproline via reaction with p-dimethylaminobenzaldehyde (DMAB, a.k.a. Ehrlich's reagent). Uniquely, their assay required relatively small amounts of protein, which represented a marked advance in hydroxyproline quantification compared to previous methods. Since that initial breakthrough, hydroxyproline quantification, and its use toward quantifying collagen, has been continually improved12–19 and was eventually commercialized. An alternative approach to quantifying collagen, based on Sirius Red dye binding, was later introduced20 and is also available as a commercial assay. More recently, a method was described for quantifying collagen in collagenase-digested tissue based on binding of a fluorescent molecule to peptides containing N-terminyl glycine residues.21 Each of these biochemical methods for measuring collagen exploits a different, unique attribute of collagen's amino acid sequence or structure.
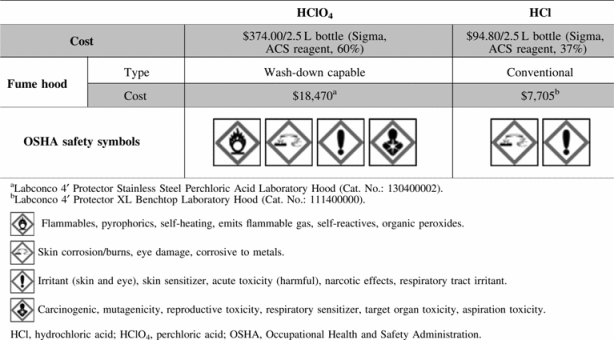
Despite the advent of additional techniques for measuring collagen content, the colorimetric hydroxyproline assay remains a commonly used protocol in biologic research; however, the safety and cost of this assay could be improved. To dissolve DMAB, a solution of perchloric acid (HClO4) and 2-propanol is typically used as the solvent for this assay. HClO4, an unstable and oxidative strong acid, must be used in specially designed fume hoods with wash-down capabilities to prevent accumulation of unstable, explosive perchlorate salts in ventilation systems. Failure to use an appropriate fume hood could create a safety hazard, and not all laboratories contain fume hoods rated for HClO4. The need for specialty fume hoods thus potentially increases assay cost. Additionally, HClO4 is more expensive to purchase than other strong acids, such as hydrochloric acid (HCl). Due to occupational hazards, as defined by the Occupational Health and Safety Administration,22 and relatively high cost, an alternative to HClO4 in the broadly used hydroxyproline assay is desirable (Table 1).
Table 1.
A Cost and Safety Comparison Between Perchloric Acid and Hydrochloric Acid
 |
HCl, which does not need a wash-down capable fume hood, is a less expensive and safer alternative to HClO4. It is also widely used in research laboratories. While HClO4 is often used in the hydroxyproline assay to dissolve DMAB, HCl acts as the strong acid solvent to dissolve DMAB for a recreational drug spot test assay based on similar biochemistry.23 Since the chemistry involved in both cases is similar—chromophore development from heterocyclic organic compounds—we hypothesized that HCl could replace HClO4 in the hydroxyproline assay while causing no differences in hydroxyproline detection for various tissues. To ensure validity of this modified assay across a range of samples, tissue types tested included articular cartilage, tendon, meniscus, liver, and testis, and self-assembled articular cartilage. The primary purpose of this experiment was to ensure hydroxyproline assays, using both acid formulations, yield comparable results for various tissue explants and tissue-engineered constructs.
Methods
Tissue preparation
Samples of bovine articular cartilage were obtained from the distal femora of juvenile bovine pelvic limbs obtained from a commercial provider of animal tissues (Research 87, Boylston, MA). The femorotibial joints were dissected using sterile technique and osteochondral explants were obtained using an 8-mm dermal biopsy punch. Subchondral bone was removed and the cartilage trimmed to a thickness of 2 mm from the articular surface using a custom jig. Tissue-engineered neocartilage samples were produced according to previously published methods.24 Briefly, bovine chondrocytes were isolated from distal bovine femora, seeded into non-adherent 5 mm agarose wells, and fed daily with chondrogenic media. Neocartilage samples were cultured for 16–28 days. Select samples were treated with chondroitinase-ABC or transforming growth factor β-1 to vary the neocartilage glycosaminoglycan (GAG) and collagen contents. Samples of leporine liver, testis, meniscus, and common calcanean tendon were obtained from an adult New Zealand White rabbit humanely euthanized as part of a separate study that was approved by the University Institutional Animal Care and Use Committee. Approximately 5 mg portions of each hydrated tissue were weighed, subject to lyophilization for 72 h, and then reweighed to determine tissue water content. Each dehydrated sample underwent enzymatic digestion in a papain solution (3.75 U/mL) containing 5 mM N-acetyl-L-cysteine and 5 mM ethylenediaminetetraacetic acid (EDTA) in 50 mM potassium phosphate buffer (pH = 6.9) at 60°C for 18 h. Liver and testis tissues were sonicated at ∼5 W for 1 s to achieve complete dissolution (QSonica, Newton, CT). Samples were stored at 4°C following papain digestion and allowed to come to room temperature before conducting the hydroxyproline assay.
Hydroxyproline assay overview
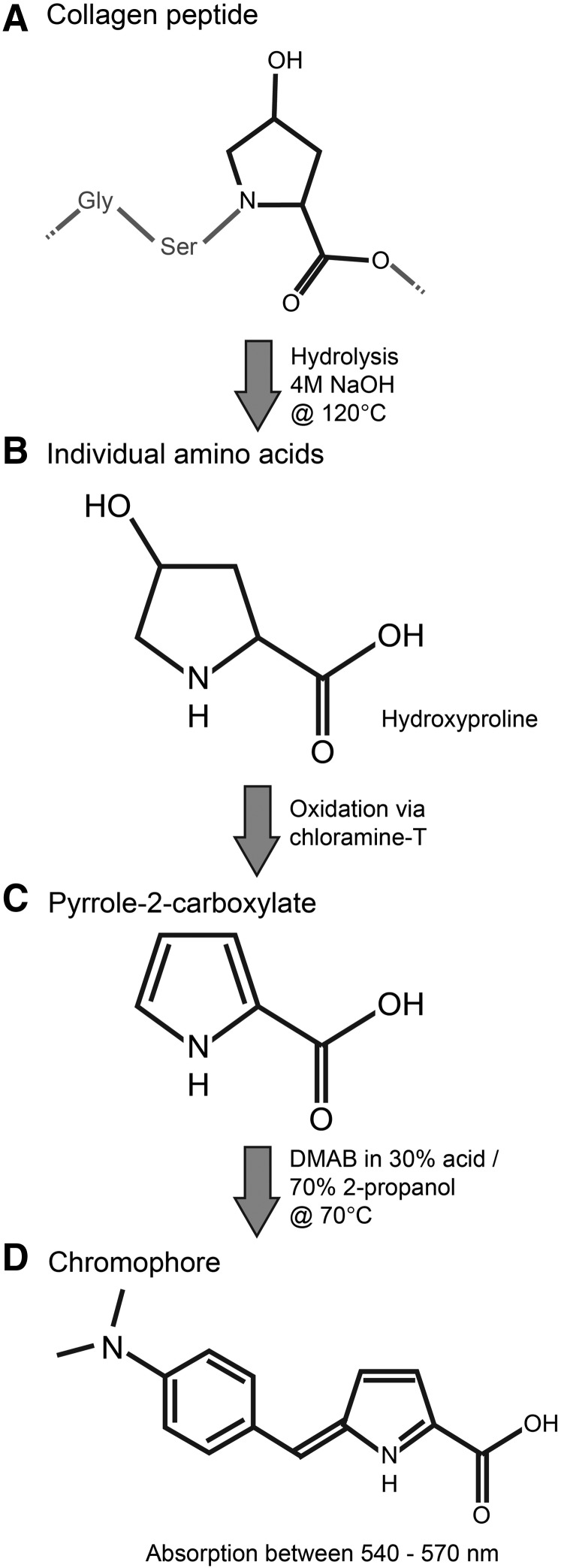
A collagen standard curve was prepared from serial dilutions of a bovine collagen reference standard (Biocolor Ltd., Carrickfergus, UK) diluted with papain solution. Aliquots of 10–30 μL of tissue digest were diluted to a final volume of 100 μL with excess papain solution to achieve a concentration of collagen expected to fall within the range of the standard curve. Specifically, 10 μL of tissue digest were used for tendon and meniscus, 20 μL were used for engineered and native bovine articular cartilage, and 30 μL were used for leporine liver and testis. An overview of the assay chemistry is provided in Figure 1. Soluble peptides and proteins in each standard and tissue sample were hydrolyzed to individual amino acids by adding 100 μL of 4 N sodium hydroxide (NaOH) and incubating at 120°C and 15 psi above atmospheric pressure for 15 min. Samples were allowed to cool to room temperature and then neutralized with 100 μL of 4 N HCl. Hydroxyproline amino acids were converted to pyrolle-2-carboxylate by oxidation via addition of 0.625 mL of 0.05 M chloramine-T in 74% v/v H2O, 26% v/v 2-propanol, 0.629 M NaOH, 0.140 M citric acid (monohydrate), 0.453 M sodium acetate (anhydrous), and 0.112 M acetic acid (glacial), followed by incubation at room temperature for 20 min. Finally, 0.625 mL of 15% w/v DMAB (1 M) in 2-propanol plus concentrated acid (a.k.a. Ehrlich's solution) was added to each sample and vortexed immediately to facilitate mixing. Samples were incubated at 65°C for 20 min and then rapidly cooled by immersion in room temperature water to stop chromophore development.
FIG. 1.
Overview of the hydroxyproline assay. Collagen peptides (A) are hydrolyzed in 4 M NaOH to individual amino acids including hydroxyproline (B). Oxidation of the hydroxylated amino ring of hydroxyproline by chloramine-T yields a pyrrole (C) that reacts with DMAB to produce a chromophore (D) with peak absorption of light with wavelengths between 540 and 570 nm. DMAB, p-dimethylaminobenzaldehyde; NaOH, sodium hydroxide.
Suggested hydroxyproline assay protocol
-
• Digest tissue in papain solution for 18 h at 60°C.
○ Papain solution: 3.875 U/mL papain in 5 mM EDTA tetrasodium hydrate, 5 mM N-acetyl-L-cysteine, and 50 mM potassium phosphate buffer at pH 7.
• Dilute samples with excess papain solution to bring expected concentration of collagen within the assay standard range and to a final volume of 100 μL in an autoclave safe, screw-top tube (e.g., 2 mL cryovial). Leave cap loose.
• Add 100 μL of 4 N NaOH to each sample.
• Autoclave at 120°C and 15 psi above atmospheric pressure (i.e., typical liquid cycle) for 15 min.
• Allow samples to return to room temperature.
• Add 100 μL of 4 N HCl to neutralize the pH.
-
• Add 625 μL Chloramine-T solution.
○ Chloramine-T solution: 0.05 M Chloramine-T in 74% v/v H2O, 26% v/v 2-propanol, 0.629 M NaOH, 0.140 M citric acid (monohydrate), 0.453 M sodium acetate (anhydrous), and 0.112 M acetic acid (glacial).
• Let stand at room temperature for 20 min.
-
• Add 625 μL Ehrlich's solution and vortex immediately to ensure complete mixing.
○ Ehrlich's solution: 1 M DMAB in 30% v/v HCl and 70% v/v 2-propanol.
• Incubate in water bath at 65°C for 20 min.
• Immediately quench reaction by immersing tubes in cool water.
• Plate standards and samples in triplicate in a 96-well clear, flat-bottomed plate (200 μL/well).
• Read plate in a spectrophotometer at an absorbance wavelength between 550 and 565 nm.
Optimization of HCl concentration
To identify the optimum proportion of HCl for the Ehrlich's solution, formulations with 50%, 30%, and 10% v/v concentrated HCl (Fisher Scientific, Waltham, MA) were prepared in a balance of 2-propanol. The different proportions of HCl were compared to 30% HClO4 Ehrlich's solution for quantifying hydroxyproline content via the hydroxyproline assay as described above.
Absorbance measurements
Aliquots of 100 μL of each sample were added in triplicate to a clear 96-well plate. To evaluate potential differences in wavelength of maximal light absorption, absorbance measurements were made at varying wavelengths in 10 nm increments from 300 to 500 nm, in 5 nm increments from 500 to 600 nm, and in 10 nm increments from 600 to 800 nm using a microplate reader (Synergy HT, BioTek Instruments, Winooski, VT). Background absorbance was measured from samples of papain solution without added collagen standard or tissue digest subject to the same reaction steps as described above. Subsequently, absorbance measurements were made within the range of the optimal absorbance wavelengths using a microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
Comparison of Ehrlich's solution using hydrochloric or perchloric acid for collagen quantification
Samples of tissue with differing collagen content and extracellular matrix compositions were used to assess agreement between collagen quantification by the hydroxyproline assay using DMAB dissolved in HClO4 or HCl. Paired samples of each collagen standard and tissue digest were prepared and treated identically as described above up to the final chromophore development step. Chromophore development was then performed with DMAB dissolved in either 30% v/v HClO4 or 30% v/v HCl combined with 70% v/v 2-propanol.
Four separate iterations of the assay were performed using different samples of tissue-engineered neocartilage, native bovine articular cartilage, and native leporine tissues. Samples in the first iteration of the assay consisted of neocartilage and native cartilage that were prepared exactly as described above. In the second iteration, samples with varying collagen content were prepared by performing three serial, 1:1 dilutions of native cartilage digest in papain solution. For the third iteration, samples of neocartilage were chosen with low GAG or high GAG content, and 0, 1.25, 2.50, or 5.00 μg of soluble collagen (Sircol bovine collagen standard; Biocolor Ltd.) per 100 μL were added to each digest solution. Samples in the fourth iteration consisted of leporine tissues prepared as described above. For each iteration of the hydroxyproline assay, absorbance measurements were recorded and the concentration of collagen in each original specimen calculated based on standard curves created for each microplate.
Amino acid analysis
For comparison of colorimetric assay results to a proteomics gold standard, papain digested samples underwent amino acid analysis at the UC Davis Proteomics Core Facility. Selected samples of bovine native cartilage, bovine neocartilage, and leporine tissues were subjected to formic acid/acetonitrile transfer, dried, subjected to liquid phase hydrolysis in 6 N HCl with 1% phenol at 110°C for 24 h, and dried again. Finally, samples were resuspended in S-2-aminoethyl-L-cysteine dilution buffer and loaded into the amino acid analyzer (Hitachi L-8900, Tokyo, Japan) for quantification. Sircol bovine collagen reference standard (Biocolor Ltd.) was also analyzed to relate results to the hydroxyproline assay.
Statistics
Standard curves were fit between measured absorbance values and standards of known collagen concentration by least squares linear regression for all repetitions of the hydroxyproline assay. Coefficients of determination (R2) were calculated for each standard curve. Potential differences in measurements of sample collagen or hydroxyproline content among the different assays were examined by matched pairs two-tailed t-tests with significant differences defined by p < 0.05. Additionally, reproducibility between the different assays was assessed by calculating the Lin's concordance correlation coefficient (ρc), a measure of agreement between paired results that accounts for linearity and proximity to a line with a slope of one and a y-intercept of 0.25,26 Finally, a posteriori power analyses (power = 0.8 or 0.9, p < 0.05) were completed to determine how many samples would be necessary to observe potentially significant differences between groups.
Results
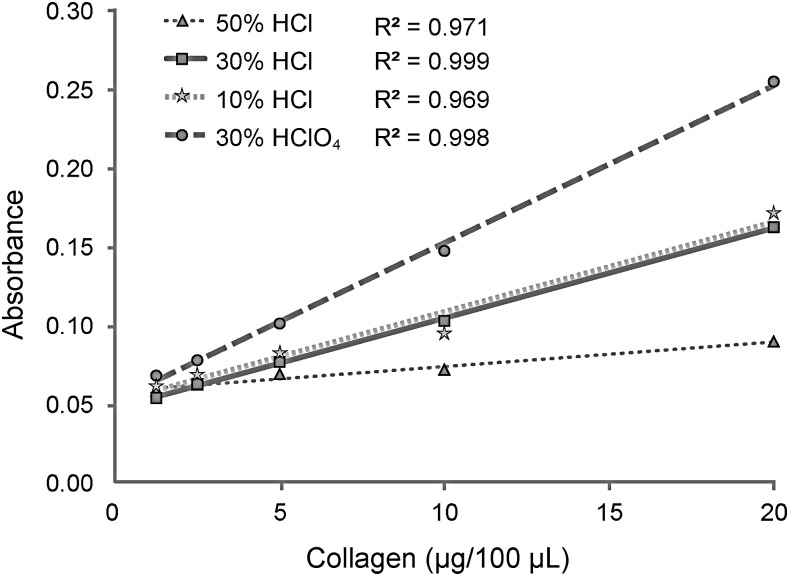
Hydroxyproline assay standard curves for each acid formulation were generated (Fig. 2). A standard curve using a 50% HCl concentration was developed and yielded the lowest absorbance, whereas the 30% HClO4 formulation led to the greatest absorbance. The 30% HClO4 and 30% HCl solutions produced standard curves with R2 values of 0.998 and 0.999, respectively. While the 10% HCl group exhibited similar absorbance to the 30% HCl group, it had a lower R2 value (0.969 vs. 0.999) and demonstrated phase separation during chromophore development. Thus, the 10% HCl group along with 50% HCl were not considered as replacements for 30% HClO4.
FIG. 2.
Spectrophotometric absorbance as a function of collagen concentration. Sircol collagen standard at different concentrations was quantified via hydroxyproline assay using DMAB dissolved in a solution consisting of isopropanol and strong acid (30% HClO4, 10% HCl, 30% HCl, or 50% HCl). The HClO4 group generated a curve with the greatest slope. For the groups using HCl, a 30% strong acid solution most closely mimicked the results of HClO4 and displayed the greatest linearity. These two groups were carried forward into later phases of this project for further comparison. HCl, hydrochloric acid; HClO4, perchloric acid.
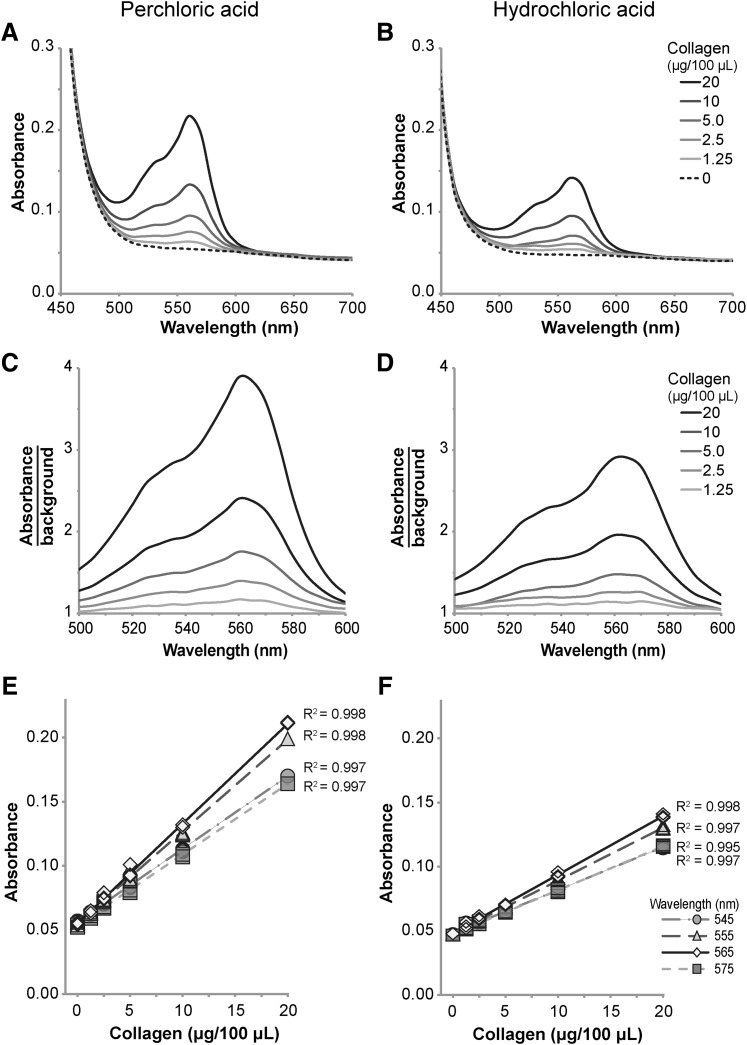
Ehrlich's solutions produced with HClO4 or HCl resulted in similar absorption spectra (Fig. 3). Both formulations of the reagent reacted with collagen to yield the greatest specific absorbance at light wavelengths between 540–575 nm and peak absorbance occurring at 565 nm (Fig. 3A, B). Due to unreacted DMAB, other reagents, and solutions involved in the assay, marked non-specific absorbance occurred for wavelengths <500 nm regardless of solvent and collagen concentration. Ehrlich's solution produced with HClO4 achieved greater absolute absorbance and greater absorbance to background ratio compared to HCl (Fig. 3A–D), but both versions of the reagent exhibited highly linear responses to concentrations of collagen between 1.25 and 20 μg/100 μL for wavelengths of light between 545 and 575 nm (Fig. 3E, F).
FIG. 3.
Absorbance as a function of light wavelength and collagen concentration for paired hydroxyproline assays using Ehrlich's solution made with HClO4 (A, C, E) or HCl (B, D, F). Marked nonspecific absorption occurred at wavelengths <500 nm and negligible absorption occurred at wavelengths >600 nm (A, B). Both solutions produced peak absorption and absorption:background ratios at wavelengths of 560–565 nm (C, D). The maximum absorption and absorption:background was less for Ehrlich's solution made from HCl, but both solutions produced a linear absorption response for concentrations of collagen between 1.25 and 20 μg/100 μL for wavelengths between 545 and 575 nm (E, F).
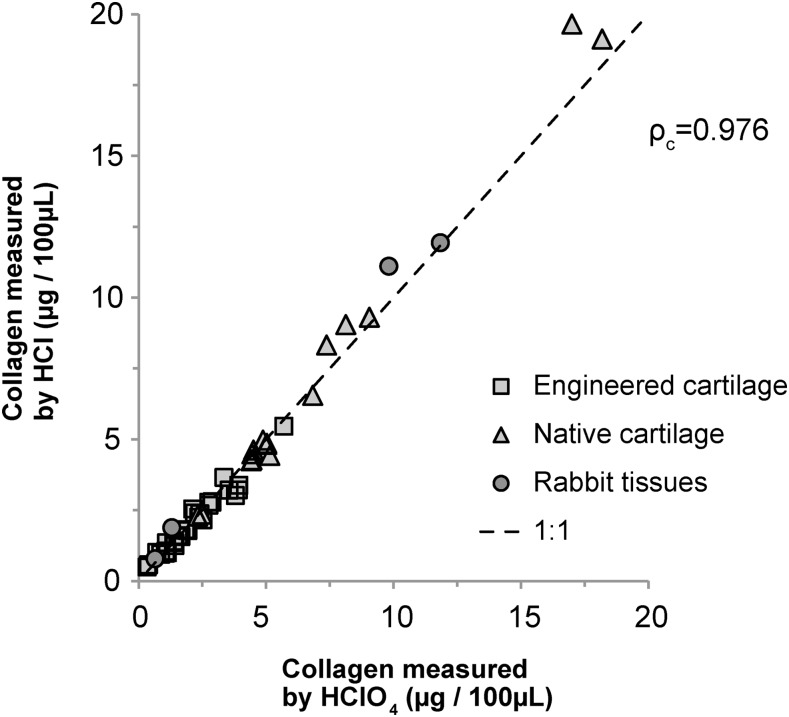
Quantification of collagen using the colorimetric hydroxyproline assay achieved a concordance correlation coefficient of ρc = 0.976 for all paired samples assayed with Ehrlich's solution using either HClO4 or HCl (Fig. 4). Within specific tissue types, ρc was 0.972, 0.986, and 0.991 for engineered cartilage (n = 36), native cartilage (n = 16), and leporine tissues (n = 4), respectively. No significant difference was observed between the two collagen assays applied in samples of bovine native cartilage, tissue-engineered cartilage, or leporine liver, testis, meniscus, or tendon (n = 58, p = 0.321). Based on our power analysis, 89 or 118 measurements would be necessary to achieve power of 0.8 or 0.9, respectively, to identify a statistically significant difference (p < 0.05) if the small, observed differences between the two assays are not due to random variation.
FIG. 4.
Concentrations of collagen measured by the hydroxyproline assay using DMAB dissolved in 30% HClO4 or HCl plus 70% isopropanol. Source tissues, which were differentially treated, included native (n = 16) or engineered (n = 36) bovine cartilage and leporine tissues (n = 4). The leporine tissues included, in ascending order of collagen concentration, liver, testis, meniscus, and tendon. Aliquots of each tissue digest were diluted with a variable amount of excess papain solution to a volume of 100 μL to achieve concentrations of collagen within the limits of the assay standard curve. There was excellent concordance (ρc = 0.976) between collagen concentrations measured using HClO4 or HCl.
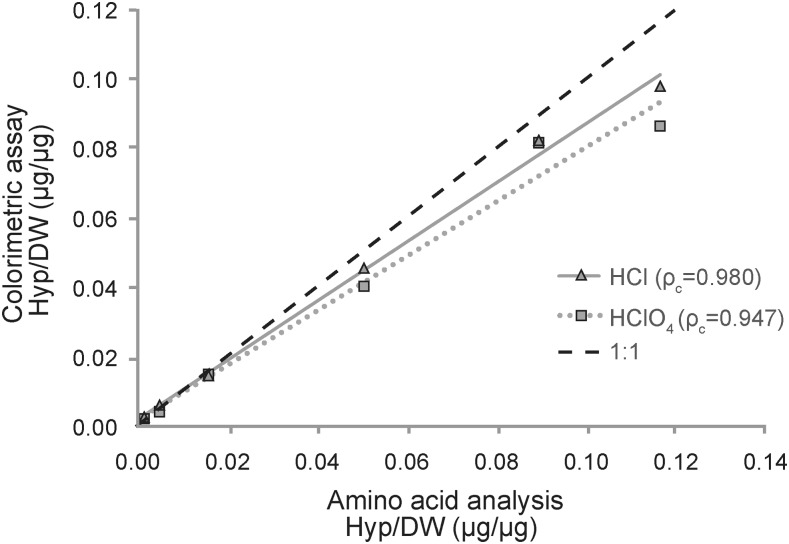
Hydroxyproline biochemical assay results were compared to hydroxyproline amino acid quantification (Fig. 5). The HCl group correlated more strongly with amino acid quantification data than the HClO4 group. Furthermore, the slope of the regression line for the HCl group was closer to unity. Thus, ρc was greater for HCl than for HClO4 (0.980 vs. 0.947). Although both versions of the colorimetric assay underestimated hydroxyproline content compared to chromatographic amino acid analysis, the observed differences were not statistically significant (p = 0.195 for HCl and p = 0.110 for HClO4). To identify a statistically significant difference with power of 0.8 and p < 0.05, 20 measurements would be necessary for HClO4, while 43 measurements would be necessary for HCl.
FIG. 5.
Concentrations of hydroxyproline, from lowest to highest collagen content, in rabbit liver, rabbit testis, engineered bovine articular cartilage, bovine articular cartilage, rabbit meniscus, and rabbit tendon, as measured by colorimetric assay or by amino acid analysis. The dotted line corresponds to the hydroxyproline assay using Ehrlich's solution made with 30% HClO4, while the solid line represents the hydroxyproline assay that replaces 30% HClO4 with 30% HCl. Amino acid analysis quantification shared the greatest concordance with the HCl group (ρc = 0.980), while the HClO4 group did not agree as closely with amino acid quantification (ρc = 0.947). Concordance is illustrated by experimental curve proximity to the 1:1 unity dashed line. Both colorimetric assays underestimated hydroxyproline content as determined by amino acid analysis.
Discussion
Measurement of collagen is an important aspect of biological research toward characterizing the composition of normal tissues, understanding certain pathologic processes, and for development of biomaterials and tissue-engineered constructs. Collagen content of a tissue or biomaterial can be measured by different commonly used approaches; examples include (1) quantification of hydroxyproline; (2) detection of a dye that binds to solubilized collagen molecules; and (3) quantification of antibody binding via an enzyme-linked immunosorbent assay (ELISA). In this study, we report a modification to colorimetric hydroxyproline measurement for collagen quantification that improves on assay safety and cost, and achieves similar or improved accuracy. The modified assay substitutes HCl for HClO4 to make Ehrlich's solution; no difference was observed between measurements of collagen using either formulation of Ehrlich's solution for any of the tissues tested, including liver, testis, cartilage, meniscus, and tendon. Additionally, the hydroxyproline content of samples measured using the colorimetric assay with HCl strongly agrees with results obtained by the gold standard, chromatographic amino acid analysis.
Our proposed colorimetric assay represents a simple improvement for the quantification of hydroxyproline and collagen in biologic tissues by substituting HCl for HClO4 in the formulation of Ehrlich's solution (15% w/v DMAB, 70% 2-propanol, 30% concentrated acid). Colorimetric quantification of collagen is based on oxidation of hydroxyproline residues to form pyrrole-2-carboxylate followed by reaction with DMAB to yield a detectable chromophore.11 Multiple revisions of the original colorimetric hydroxyproline assay have been introduced;18,19 in the case of this study, we have replaced HClO4 with HCl in Ehrlich's solution, which achieved more accurate quantification of hydroxyproline content in tissues as confirmed by amino acid analysis. The HCl-based assay exhibited strong concordance with amino acid analysis (ρc = 0.980) and on average differed from amino acid analysis by 10.3%, whereas HClO4 had weaker concordance (ρc = 0.947) and erred by an average of 14.8%. The limited number of samples included in amino acid analysis prevents evaluating assay accuracy as a function of tissue type, but trends suggest that both colorimetric assays slightly underestimate hydroxyproline content in collagen-rich samples. The ∼33% greater absorbance to background ratio produced by Ehrlich's solution with HClO4 may provide slightly greater sensitivity for detecting very small differences in collagen between tissues. Thus, future refinements of the assay should be aimed at achieving greater absorption to background to further improve the assay sensitivity. Nonetheless, substituting HCl for HClO4 in Ehrlich's solution improves the assay's accuracy.
The results of this study indicate that both acid formulations result in a highly linear absorbance response for concentrations of collagen between 0 and 20 μg/100 μL. The assay as presented here is capable of accurately quantifying hydroxyproline in a wide variety of tissues, from those with minimal collagen (e.g., liver) to those composed of nearly 100% collagen (e.g., tendon). A limitation of our study is that the upper limit of linearity using HCl was not explored. Previous research using HClO4 demonstrated linearity of the absorbance response for dilutions of hydroxyproline up to 20 μg/50 μL, far exceeding any concentrations tested in this study.16 Linearity of the absorbance response should be validated for concentrations of collagen in excess of 20 μg/100 μL before using the HCl-based assay for measuring highly concentrated hydroxyproline or collagen samples. Nonetheless, our modified hydroxyproline assay exhibits a linear absorbance range sufficient for accurately measuring collagen content in most biologic tissues.
Commonly used assays indirectly measure collagen content in biological samples by exploiting its unique amino acid sequence, which can over- or underestimate the true sample collagen content.11,27 Using hydroxyproline to measure collagen in a sample relies on the repeating amino acid sequence and predictable proportion of hydroxyproline in a given type of collagen. All types of collagen exhibit a repeating glycine-X-Y amino acid sequence in which “X” and “Y” most often represent proline and hydroxyproline residues.28 The proportion of hydroxyproline by mass is highly conserved within a given type of collagen and ranges from 11.3% in type I collagen to 15% in type III collagen.29 Importantly, these assays are not specific to detection of hydroxyproline in collagen and the presence of hydroxyproline associated with other proteins could inflate the apparent collagen content. Serum complement, acetylcholinesterase, and elastin are all proteins with relatively high hydroxyproline contents, and their potential presence should be considered when choosing an appropriate assay for collagen quantification.30 Similarly, the hydroxyproline assay is not specific to fibrillar collagen, because hydroxylation of proline to hydroxyproline occurs very soon after translation. Thus, intracellular procollagen and extracellular tropocollagen are also detected by the hydroxyproline assay. Although, the assay reported here demonstrates strong agreement with amino acid analysis for measurement of hydroxyproline, the assay shares the same limitations as previous hydroxyproline-based methods of collagen quantification.
Other methods for quantifying collagen include Sirius red dye binding and ELISA, each associated with their own advantages and disadvantages. For example, Sirius red dye binds to the highly basic residues of solubilized fibrillar collagen amino acid side chains31 and does not bind to most noncollagenous proteins that are high in hydroxyproline content.29,30 The Sirius red binding assay also obviates the need for hydrolysis of collagen to individual amino acids and allows for quantification of different collagen fractions based on their solubility.32,33 Nonetheless, binding of Sirius red to noncollagen proteins has been reported to cause inaccurate results, and modification of the assay may be necessary depending on the tissue source.34 Moreover, a direct comparison of the hydroxyproline and dye-binding based methods for quantifying collagen in a pulmonary fibrosis model found the hydroxyproline-based assay to more accurately reflect total collagen and differences among samples than Sirius red binding.27 Assays based on hydroxyproline or Sirius red binding do not distinguish between different types of collagen. ELISA is typically the best choice for quantifying a specific type of collagen and is capable of discriminating between collagen types that may be present in the same tissue, such as types I and II collagen in fibrocartilage.35 Disadvantages of using ELISA for collagen quantification include cost and potential need for more than one assay when working with tissues from different species. The specific goals of a study, tissue sources, and limitations of each assay should be considered when choosing an appropriate collagen quantification method.
Using HCl in place of HClO4 improves the cost and safety of this assay. Since this assay uses relatively large volumes of reagent in comparison to other biochemical assays, the 75% reduction in cost for the strong acid component of this assay can provide significant cost savings for laboratories.36 In terms of safety, HCl is not associated with the build-up of explosive salts, which is known to occur with HClO4. Therefore, HCl does not necessitate the use of an expensive wash-down capable fume hood. HClO4 also presents the potential issues of flammability, toxicity, and mutagenicity, while HCl can be used without these concerns. Based on improvements in efficacy, cost, and safety, this modified assay represents an improved tool for measuring hydroxyproline and collagen content in biological samples.
In conclusion, the extra hazard, cost, and need for specialized fume hoods can be obviated for the purposes of collagen quantification via the hydroxyproline assay by substituting concentrated HCl for HClO4 to produce Ehrlich's solution. Use of HCl accurately quantifies hydroxyproline and collagen in enzymatically digested collagenous tissues with no change in the wavelength of peak absorption. As when using HClO4, care must be taken to avoid phase separation upon addition of Ehrlich's solution containing HCl. Although Ehrlich's solution containing HClO4 yielded greater absorption than HCl for the same concentration of collagen/hydroxyproline, both versions of the assay were accurate over the range of collagen concentrations and tissues tested in this study, and HCl more closely corresponded to values obtained by amino acid analysis.
Acknowledgments
This work was made possible with the support of the National Institutes of Health (NIH) (Grant Nos. R01AR067821 and R01DE015038). D.D.C. was in part funded by an NIH T32 grant (OD011147). J.M.L. was in part funded by a National Science Foundation Graduate Research Fellowship (Grant No. 1650042).
Disclosure Statement
No competing financial interests exist.
References
- 1.Siegfried M. Reticulin and collagen. J Physiol 28, 319, 1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tebb M.C. Reticulin and collagen. J Physiol 27, 463, 1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair A.K., Gautieri A., and Buehler M.J. Role of intrafibrillar collagen mineralization in defining the compressive properties of nascent bone. Biomacromolecules 15, 2494, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Eleswarapu S.V., Responte D.J., and Athanasiou K.A. Tensile properties, collagen content, and crosslinks in connective tissues of the immature knee joint. PLoS One 6, e26178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigozzi S., Muller R., Stemmer A., and Snedeker J.G. Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-AFM observations at the nanoscale. J Biomech 46, 813, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Miller E.J. The structure of fibril-forming collagens. Ann N Y Acad Sci 460, 1, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Sherman V.R., Yang W., and Meyers M.A. The materials science of collagen. J Mech Behav Biomed Mater 52, 22, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Loeser R.F. Integrins and chondrocyte-matrix interactions in articular cartilage. Matrix Biol 39, 11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang M., Yuan J., Peng C., and Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol 35, 2871, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.King T.E., Jr., Pardo A., and Selman M. Idiopathic pulmonary fibrosis. Lancet 378, 1949, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Neuman R.E., and Logan M.A. The determination of hydroxyproline. J Biol Chem 184, 299, 1950 [PubMed] [Google Scholar]
- 12.Prockop D.J., Lindstedt S., and Udenfriend S. Simple technique for measuring specific activity of labeled hydroxyproline in biological materials. J Biol Chem 236, 1395, 1961 [PubMed] [Google Scholar]
- 13.Kivirikko K.I., Laitinen O., and Prockop D.J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem 19, 249, 1967 [DOI] [PubMed] [Google Scholar]
- 14.Parekh A.C., and Jung D.H. An improved method for determination of total hydroxyproline in urine. Biochem Med 4, 446, 1970 [DOI] [PubMed] [Google Scholar]
- 15.Pødenphant J., Larsen N.-E., and Christiansen C. An easy and reliable method for determination of urinary hydroxyproline. Clin Chim Acta 142, 145, 1984 [DOI] [PubMed] [Google Scholar]
- 16.Reddy G.K., and Enwemeka C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 29, 225, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Colgrave M.L., Allingham P.G., and Jones A. Hydroxyproline quantification for the estimation of collagen in tissue using multiple reaction monitoring mass spectrometry. J Chromatogr A 1212, 150, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Huszar G., Maiocco J., and Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem 105, 424, 1980 [DOI] [PubMed] [Google Scholar]
- 19.Stegemann H., and Stalder K. Determination of hydroxyproline. Clin Chim Acta 18, 267, 1967 [DOI] [PubMed] [Google Scholar]
- 20.Junquiera L.C., Junqueira L.C., and Brentani R.R. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem 94, 96, 1979 [DOI] [PubMed] [Google Scholar]
- 21.Yasmin H., Kabashima T., Rahman M.S., Shibata T., and Kai M. Amplified and selective assay of collagens by enzymatic and fluorescent reactions. Sci Rep 4, 4950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.OSHA QUICK CARD. Hazard Communication Standard Pictogram. Occupational Safety and Health Administration,Washington, DC, 2016 [Google Scholar]
- 23.Maunder M.J.d.F. A field test for hallucinogens: further improvements. J Pharm Pharmacol 26, 637, 1974 [DOI] [PubMed] [Google Scholar]
- 24.Makris E.A., Responte D.J., Paschos N.K., Hu J.C., and Athanasiou K.A. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci USA 111, E4832, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin L.I. A Concordance correlation-coefficient to evaluate reproducibility. Biometrics 45, 255, 1989 [PubMed] [Google Scholar]
- 26.Lin L., Hedayat A.S., Sinha B., and Yang M. Statistical methods in assessing agreement. J Am Statist Assoc 97, 257, 2002 [Google Scholar]
- 27.Kliment C.R., Englert J.M., Crum L.P., and Oury T.D. A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int J Clin Exp Pathol 4, 349, 2011 [PMC free article] [PubMed] [Google Scholar]
- 28.Ramshaw J.A., Shah N.K., and Brodsky B. Gly-X-Y tripeptide frequencies in collagen: a context for host-guest triple-helical peptides. J Struct Biol 122, 86, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Etherington D.J., Pugh D., and Silver I.A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger 40, 1625, 1981 [PubMed] [Google Scholar]
- 30.Walsh B.J., Thornton S.C., Penny R., and Breit S.N. Microplate reader-based quantitation of collagens. Anal Biochem 203, 187, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Junqueira L.C., Bignolas G., and Brentani R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11, 447, 1979 [DOI] [PubMed] [Google Scholar]
- 32.Bornstein P., and Sage H. Structurally distinct collagen types. Annu Rev Biochem 49, 957, 1980 [DOI] [PubMed] [Google Scholar]
- 33.Miller E.J., and Rhodes R.K. Preparation and characterization of the different types of collagen. Methods Enzymol 82, 33, 1982 [DOI] [PubMed] [Google Scholar]
- 34.Lareu R.R., Zeugolis D.I., Abu-Rub M., Pandit A., and Raghunath M. Essential modification of the Sircol Collagen Assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater 6, 3146, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Rennard S.I., Berg R., Martin G.R., Foidart J.M., and Robey P.G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem 104, 205, 1980 [DOI] [PubMed] [Google Scholar]
- 36.Acids. Sigma-Aldrich. 2017. [cited January 8, 2017]. Available from: www.sigmaaldrich.com/chemistry/chemistry-products.html?TablePage=114333318