Abstract
Traumatic brain injury (TBI) in children can cause persisting cognitive and behavioral dysfunction, and inevitably raises concerns about lost potential in these injured youth. Lateral fluid percussion injury (FPI) in weanling rats pathologically affects hippocampal N-methyl-d-aspartate receptor (NMDAR)- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated glutamatergic neurotransmission subacutely within the first post-injury week. FPI to weanling rats has also been shown to impair enriched-environment (EE) induced enhancement of Morris water maze (MWM) learning and memory in adulthood. Recently, improved outcomes can be achieved using agents that enhance NMDAR function. We hypothesized that administering D-cycloserine (DCS), an NMDAR co-agonist, every 12 h (i.p.) would restore subacute glutamatergic neurotransmission and reinstate experience-dependent plasticity. Postnatal day 19 (P19) rats received either a sham or FPI. On post-injury day (PID) 1–3, animals were randomized to saline (Sal) or DCS. Firstly, immunoblotting of hippocampal NMDAR and AMPAR proteins were measured on PID4. Second, PID4 novel object recognition, an NMDAR- and hippocampal- mediated working memory task, was assessed. Third, P19 rats were placed in an EE (17 days), and MWM performance was measured, starting on PID30. On PID4, DCS restored reduced NR2A and increased GluR2 by 54%, and also restored diminished recognition memory in FPI pups. EE significantly improved MWM performance in shams, regardless of treatment. In contrast, FPI-EE-Sal animals only performed to the level of standard housed animals, whereas FPI-EE-DCS animals were comparable with sham-EE counterparts. This study shows that NMDAR agonist use during reduced glutamatergic transmission after developmental TBI can reinstate early molecular and behavioral responses that subsequently manifest in experience-dependent plasticity and rescued potential.
Keywords: : behavioral assessments, learning and memory, neuroplasticity, receptors, TBI
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability in youth.1 Children with TBI develop persisting cognitive and behavioral deficits at least 5 years post-injury, with worse outcomes than adults with similar severity of injury. Previous studies have shown that TBI may be disrupting experience-dependent plasticity in the young brain through acute indiscriminate glutamate release and the pathological activation of excitatory glutamate-mediated neurotransmitter receptors, which eventually affect neural activation and connectivity.2,3
Experience-dependent plasticity is the process through which coordinated patterns of stimulation from the environment help shape brain structure and function. Glutamate-mediated receptors, such as the N-methyl-d-aspartic acid receptor (NMDAR) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), are known mediators of experience-dependent plasticity and are crucial in development.4–7 The NMDAR is a tetramer assembly containing NR1 and NR2 subunits, and is activated by binding of the co-agonists glutamate and glycine. During normal development, NR2B subunit containing NMDARs predominate. Depending on the brain region, NR2A begins to be expressed during the second and third postnatal weeks in rodents and goes on to exceed NR2B expression in adulthood.5,6,8–10 NMDAR activation dramatically increases 15 min after experimental TBI in adult mice followed by diminished NMDAR expression (hours to days) in the cortex and hippocampus.11 A decrease in NMDAR binding has been reported 3 h post-injury in the hippocampus and neocortex,12 as well as reduced protein expression as early as 6 h.13,14 Lateral fluid percussion injury (FPI) in postnatal day 19 (P19) weanling rats resulted in reduced hippocampal NR2A subunit protein levels from post-injury day (PID)1–415 and in failure of these FPI animals to manifest enhanced cognition, behavior, and neuroanatomy after being housed in an enriched environment (EE). When tested between PID30 and PID50, EE rearing in FPI weanling rats did not enhance spatial learning on the Morris water maze task (MWM) compared with shams.16,17 Cortical thickness and dendritic arborization did not significantly increase in FPI animals when measured at approximately PID50.16,18 Moreover, FPI in this age group did not result in overt histological damage.19,20 Therefore, FPI-induced deficits suggest neuronal dysfunction rather than extensive cell death.
AMPARs, much like NMDARs, also play a central role in synaptic plasticity, learning, and memory.7 Changes in the AMPAR tetrameric complex, which is composed of four subunits (GluR1–4), can also mediate postsynaptic cellular excitotoxicity and neuronal damage. For example, the presence of GluR2-containing AMPARs renders the postsynaptic cell impermeable to calcium influx.7 Following FPI in adult rats, GluR2 expression has been shown to be reduced,21,22 suggesting that reduced GluR2 plays a pathological role in increased excitotoxicity after TBI.
Previous work has focused on the blockade of NMDARs as an attempt to halt continuing damage caused by TBI-induced excitotoxicity. Although this approach has been implemented successfully in experimental models of TBI,23–25 NMDAR blockade has failed to show neuroprotection or promote functional recovery. In some cases, it has even led to worsened clinical outcome.26–31 One reason NMDAR antagonists may have failed is that by the time the antagonists are administered, the NMDARs are already downregulated following TBI, missing the critical window of hyperactivity of these receptors. Inhibition of NMDARs during this time would interfere with normal physiological receptor function. Therefore, this idea has led to a shift of focus toward NMDAR agonists rather than antagonists. Recently, the use of NMDA or D-cycloserine (DCS), an NMDAR partial agonist at the glycine site, has shown neuroprotection and has facilitated more rapid recovery after TBI in rodents,11,32,33 with a wide therapeutic window.34
The goal of this study was to investigate whether DCS administration systemically during a critical time window following developmental FPI would restore experience-dependent plasticity, which is disrupted following injury. We approached this study with three experiments. First, we investigated the subacute effects of DCS on protein levels of NMDAR and AMPAR subunits on PID4. Second, on the same PID4, we investigated the effects of FPI and DCS on a novel object recognition (NOR) task, which is an NMDAR hippocampal dependent working memory test.35–38 We posited that the inherent ability to distinguish novelty would be pertinent to experience-dependent plasticity and to gaining benefits from an EE rearing. Lastly, we investigated the effects of FPI and DCS on EE-induced experience-dependent plasticity using the MWM in early adulthood. We expected that the timely administration of DCS after FPI would restore subacute hippocampal NMDAR and AMPAR levels that would be reflected in the early rescue of NOR and in the reinstatement of experience-dependent plasticity later in life.
Methods
Experimental design and subjects
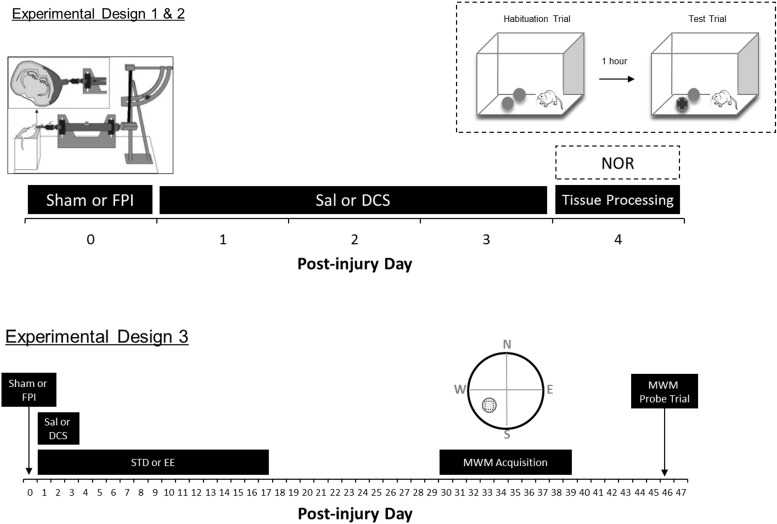
Male Sprague–Dawley rat pups (Charles River, Boston, MA) underwent sham or moderate-severe FPI on P19 (Table 1). In all three experiments, animals were randomized to receive i.p. injections every 12 h with either saline (Sal) or DCS (Sigma Aldrich, Boston, MA) on PID1–3. Animals were maintained in a 12 h light–dark cycle with food and water ad libitum. The University of California Chancellor's Committee for Animal Research approved all animal studies. Figure 1 shows the experimental designs for each experiment.
Table 1.
Injury Characteristics
| n | Groups | Drop < | Apnea (sec) | LOC (sec) | |
|---|---|---|---|---|---|
| Experiment 1 | 8 | Sham-Sal | NA | NA | NA |
| 8 | Sham-highDCS | NA | NA | NA | |
| 8 | FPI-Sal | 16 | 244.5 ± 29.0 | 255.4 ± 21.7 | |
| 8 | FPI-lowDCS | 16 | 230.1 ± 36.7 | 250.6 ± 33.9 | |
| 8 | FPI-highDCS | 16 | 245.3 ± 26.9 | 264.9 ± 29.6 | |
| Experiment 2 | 15 | Sham-Sal | NA | NA | NA |
| 9 | Sham-DCS | NA | NA | NA | |
| 15 | FPI-Sal | 16 | 173.9 ± 19.4 | 194.9 ± 19.7 | |
| 9 | FPI-DCS | 16 | 173.3 ± 23.3 | 214.2 ± 23.4 | |
| Experiment 3 | 9 | Sham-STD-Sal | NA | NA | NA |
| 8 | Sham-STD-DCS | NA | NA | NA | |
| 8 | Sham-EE-Sal | NA | NA | NA | |
| 8 | Sham-EE-DCS | NA | NA | NA | |
| 8 | FPI-STD-Sal | 16 | 201.2 ± 37.9 | 242.7 ± 52.8 | |
| 8 | FPI-STD-DCS | 16 | 161.4 ± 14.5 | 179.0 ± 13.7 | |
| 9 | FPI-EE-Sal | 16 | 275.2 ± 64.4 | 370.2 ± 93.0 | |
| 9 | FPI-EE-DCS | 16 | 190.9 ± 47.5 | 208.3 ± 43.9 |
LOC, loss of consciousness; DCS, D-cycloserine; FPI, fluid percussion injury; EE, enriched environment.
FIG. 1.
Experimental designs. Experiment 1 animals underwent sham or fluid percussion injury (FPI surgery) on postnatal day 19 (P19) (designated as post-injury day [PID]0). After 24 h of recovery, animals were weaned and randomized into either saline (Sal) or D-cycloserine (DCS) treatment (lowDCS = 10 mg/kg, i.p.; or highDCS = 30 mg/kg, i.p., every 12 h) from PID1 to PID3. On PID4, hippocampal brain regions were harvested, dissected, and processed for immunoblotting. Experiment 2 animals followed the same design as Experiment 1, except that performance in the novel object recognition task (NOR) was measured on PID4. In Experiment 3, P19 rats were administered the same sham or FPI surgery on PID0 and were treated with either saline or DCS. Only the 30mg/kg DCS dose was used in Experiments 2 and 3. The upper left inset illustrates a schematic diagram of the FPI device and injury induction. The upper right inset shows the NOR procedure with the presentation of two identical objects during habituation, followed by a 1 h inter-trial interval, and then followed by the presentation of one original object and one novel object. Lower right inset illustrates the Morris water maze (MWM) quadrants and the target zone area (circled square).
Experiment 1 consisted of five groups (n = 8/group): Sham-Sal, Sham-highDCS (30 mg/kg, i.p.), FPI-Sal, FPI-lowDCS (10 mg/kg, i.p.) and FPI-highDCS (30 mg/kg, i.p.). Tissue was harvested from rats on PID4, and brains were dissected for synaptoneurosome immunoblotting of NMDAR (NR1, NR2A, andNR2B) and AMPAR (GluR1 and GluR2) subunits.
Experiment 2 included four groups: Sham-Sal (n = 15), Sham-DCS (n = 9), FPI-Sal (n = 15), and FPI-DCS (n = 9). DCS treated groups were administered 30 mg/kg (i.p.) per dose. On PID4, animals were tested on the NOR task using a 1 h retention interval.
In Experiment 3, rat pups were weaned and differentially housed in standard (STD) or EE conditions for 17 days after 24 h of recovery post-surgery. They were returned to standard vivarium housing until behavioral testing using the MWM task, which began on PID30. Experiment 3 consisted of eight groups: Sham-STD-Sal (n = 9), Sham-STD-DCS (n = 8), FPI-STD-Sal (n = 8), FPI-STD-DCS (n = 8), Sham-EE-Sal (n = 11), Sham-EE-DCS (n = 10), FPI-EE-Sal (n = 9), and FPI-EE-DCS (n = 10). DCS treated groups were also administered 30 mg/kg (i.p.) per dose.
FPI
We followed our standard lateral FPI protocol previously described in detail.19 In brief, following general anesthesia induction using 3% isoflurane (1.0–1.5 mL/min in 100% O2) in a chamber, 1.5–2.5% isoflurane was maintained via a nose cone in spontaneously respiring P19 rat pups. Body temperature was kept constant (37–38°C) by a thermostatically controlled heating pad. Following aseptic surgical preparation, the head was secured in a stereotaxic frame, and a midline skin incision was made to expose the skull. A 3.0 mm diameter craniotomy was made 3.0 mm posterior to bregma and 6.0 mm lateral (left) of midline. The injury cap was secured over the craniotomy with silicone, and dental cement and was later filled with 0.9% saline.
Once the injury cap was secured, anesthesia was discontinued and the animal was attached to the fluid percussion device. When the animal exhibited a hindpaw withdrawal reflex initiated by a toe pinch, a moderate fluid pulse (∼2 atm) was delivered. Apnea time was determined by the resumption of spontaneous respiration and loss of consciousness (LOC) time by the return of the hindpaw withdrawal reflex. Positive pressure ventilation was administered through the mask with 100% O2 if the animal remained apneic for at least 30 sec until spontaneous respiration returns. Upon return of the hindpaw withdrawal reflex, the animal was placed back under anesthesia for the removal of the injury cap and the cleaning and closure of the surgical wound. Intradermal injections of 0.25% bupivacaine and topical antibiotic ointment were administered at the surgical site. The animal was then removed from anesthesia and placed in a heated recovery chamber. Sham animals underwent identical surgical procedures, but without the attachment of the injury cap or fluid pulse induction.
EE
Used in Experiment 3, the EE chamber consisted of various toys, tunnels, and ladders placed in a two level cage measuring 78 × 36 × 48 cm. Between 14 and 16 animals were housed together in the chamber at a time for 17 days and were then returned to standard vivarium conditions until the start of the MWM training on P50. A total of three EE batches were formed to achieve balanced groups. Every day the animals were removed from the EE cage and returned after the toys and objects were changed and rearranged. A light–dark cycle of 12 h was maintained, and food and water were available ad libitum.
Drugs
DCS was obtained from Sigma Aldrich (Boston, MA). DCS was mixed in sterile 0.9% saline with a constant injection volume of 0.25 mL/kg. DCS was stored at −20°C. DCS was thawed 15 min prior to each injection time.
Tissue processing and Western blotting
Experiment 1 animals were euthanized on PID4. The brains were removed and sectioned into four 1 mm thick slabs, and dissected on ice into regions of interest. Hippocampal regions ipsilateral and contralateral to the injury site were isolated and homogenized in buffer (0.137 M NaCl, 2.7 mM KCl, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 10 mM HEPES, 10 mM glucose, pH 7.4) with protease inhibitors (Complete™, Roche, Germany). The homogenate was loaded into a 1 mL syringe attached to a 13 mm diameter Millipore syringe filter holder and forced through one 100 μm nylon filter and then again through two 5 μm nitrocellulose filters. The filtered samples were centrifuged at 1000g for 20 min, followed by 1000g for 10 min. The resulting pellet was re-suspended in 150 μL homogenization buffer with protease inhibitors and stored at −70°C.39
Protein concentrations of synaptoneurosome fractions were determined using the Bio-Rad DC Protein Assay. Protein (10–20 μg) was prepared in Laemmli sample buffer, electrophoresed on 7.5 and 10% pre-cast Tris-HCl gels (Bio-Rad, Hercules, CA) and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Total protein was then quantified using Sypro® Ruby Protein Blot Stain for normalization. Membranes were subsequently blocked in 5% milk in tris-buffered saline with tween20 (TBST) for 1 h at room temperature and probed overnight with 1:1000 anti-NR1, NR2A, NR2B, GluR1, and GluR2 (Millipore, Billerica, MA). Membranes were incubated in horseradish peroxidase (HRP) conjugated secondary antibodies (Santa Cruz Biotech, Santa Cruz, CA) and developed using enhanced chemiluminescence (ECL) reagents (Cell Signaling, Boston, MA). Membranes were imaged with Bio-Rad ChemiDoc XRS system and analyzed using Quantity One® software.
Raw subunit density signal was controlled to total protein from the same lane on the same blot. Protein-controlled subunit values from each individual sample were normalized to the average of the Sham-Sal group. Protein levels are presented as mean percent change from Sham-Sal (mean ± standard error of the mean [SEM]).
NOR task
The NOR task has been previously shown to measure hippocampal-, NMDA-mediated working memory.35–38 The testing chamber was a white, Plexiglas arena (32 × 52 × 30 cm) that approximated the size of the home cage, which was optimal for weanling rats.40 Testing objects were similar in form and color, yet discernibly different. The arena and objects were wiped down with 70% ethanol between subjects. The NOR task consisted of a familiarization phase followed by a testing phase.
Familiarization phase
Animals were allowed to freely explore the testing chamber without objects for 5 min per session. One session per day was administered on PID2 and PID3.
Testing phase
The testing phase took place on PID4 and consisted of a 1) habituation trial, followed by a 2) test trial 1 h later. During the habituation trial, the animal was placed in the testing chamber for 5 min with two identical objects. A digital tracking system (SMART, San Diego Instruments) was used to measure the animal's locomotion and interaction with the objects. Interaction was defined as direct object contact with at least the nose or whiskers. The test trial was administered the same way as the habituation trial, but used one object from the habituation trial and one novel object. Positions of the novel and familiar objects were counterbalanced in the chamber.
MWM training
Acquisition training
Experiment 3 animals began the MWM acquisition training 30 days after surgery. Animals were trained for 10 consecutive days. The MWM was a blue circular tank (1.5 m in diameter, 0.6 m in height) filled with water maintained between 18° and 20°C. Each animal underwent two blocks of training per day (28 min between blocks). Each block consisted of four trials wherein the animal was released from the four cardinal directions (north-N, south-S, east-E, and west-W) in random order. For each trial, the animal was given 45 sec to locate the hidden platform, and was guided to the platform if the time elapsed. A 15 × 15 cm platform submerged 2 cm below water level was placed in the SW quadrant of the tank. The animal remained on the platform for 60 sec in between trials. The time it took the animal to reach the platform (latency) was recorded. The swim paths and velocity were also recorded using a digital tracking system (SMART, San Diego Instruments, San Diego, CA).
The goal was to discover whether EE rearing, injury, or drug treatment were important predictors for reduced latency in MWM acquisition. Additionally, a “trials to criterion” and the slope of learning were also determined to measure learning of the MWM task. The trials to criterion, the mastery of learning the MWM task, was the number of trials achieved to reach the hidden platform in ≤5 sec in one block (four consecutive trials). The learning curve was determined from the slope of the linear regression of the mean latencies between the first block and the block when the animal reached the trials to criterion.
Probe trial
Retention of the hidden platform location was tested 7 days later. The hidden platform was removed and each animal was released at the center of the tank facing N for a single 60 sec trial. Animals that were able to recall the learned location of the platform were expected to spend a greater amount of time swimming in the quadrant that had contained the platform. Swim path and velocity were also quantified. The first 15 sec of the probe trial were used for analysis of recall. It has been shown that the animal has peak activity within the first 15 sec in the MWM probe trial.41
Statistical analysis
All data are expressed as the mean ± SEM. An analysis of covariance (ANCOVA) was performed on the injury characteristic parameters (apnea and LOC) with the PID0 weight as a covariate within and across experiments. Post-hoc comparisons were Bonferroni adjusted. For all analyses, p < 0.05 was considered significant.
Immunoblotting analysis
All statistical analyses were conducted using SPSS version 16. Multivariate analyses of variance (ANOVAs) were implemented, with injury and drug as independent variables and hippocampal Western blot signal and general open field behavior as dependent variables. Post-hoc tests were Bonferroni adjusted comparisons.
NOR performance analysis
Data from the first 3 min were analyzed for percent object interaction with the novel object (time spent with novel object divided by total object interaction time × 100%). Subject speed and total distance traveled in the chamber were also measured. The subject speed was computed only when the animal was in motion. Object recognition was defined as an interaction rate with the familiar object that was significantly greater than chance performance. An animal that did not recognize the object from the habituation trial was expected to split its object interaction time 50:50 between the novel and familiar object at testing. Therefore the percentage of total object interaction time spent with the novel object was tested against chance performance (50%) for each group using one sample t tests. Remembrance occurs when the percent time spent with the novel object is significantly higher (p < 0.05) than chance performance.
MWM test analysis
Latencies to reach the platform were analyzed using a linear mixed-effects model fit by restricted maximum likelihood in R.42 MWM acquisition (trials to criterion and slope of learning) and probe trial parameters (number of target zone entries) were analyzed using multivariate ANOVA with injury (Sham or FPI), housing (STD or EE), and drug (Sal or DCS) as between-subject factors. Post-hoc comparisons were Bonferroni adjusted. For all analyses, p < 0.05 was considered significant.
Results
Injury severity
All animals included were administered either a sham surgery or moderate-severe FPI. Moderate-severe FPI was defined as having duration of unresponsiveness to toe pinch (LOC) of at least 120 sec. Six, nine, and twenty-six animals were excluded from Experiments 1, 2, and 3, respectively, because of complications from surgery or injury or to LOC times of <120 sec. Between and within experimental groups, mean apnea, LOC, and PID0 weight did not significantly differ among FPI groups (Table 1).
Experiment 1: FPI and DCS subacute effects on NMDAR and AMPAR levels
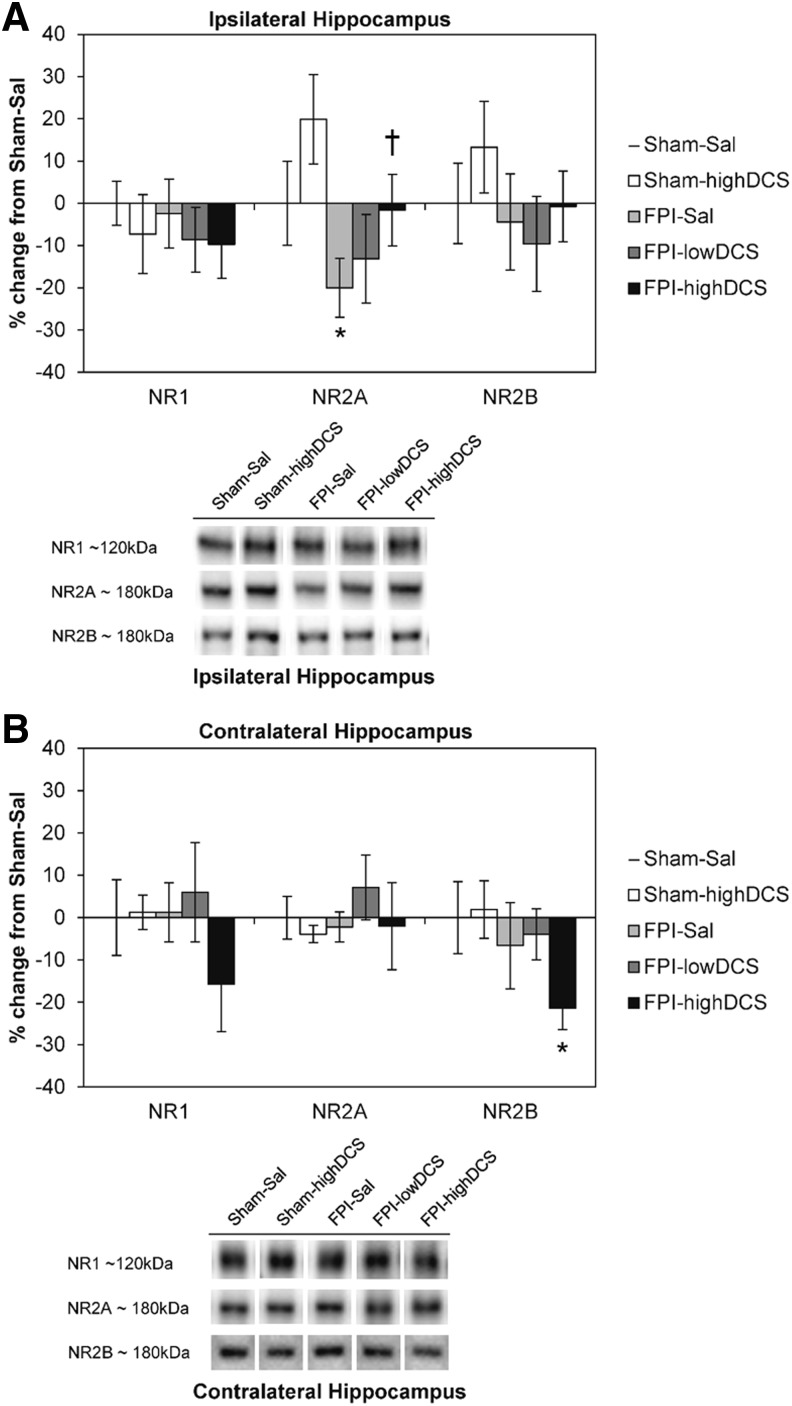
In Experiment 1, two dose levels were used: 10 mg/kg (lowDCS) and 30 mg/kg (highDCS) were used to evaluate DCS effects on PID4 hippocampal NMDAR and AMPAR subunit levels. Across all Sal and DCS samples, there was an overall effect of injury on the NR2A subunit in the ipsilateral hippocampus (F1,37 = 4.740, p = 0.037). No overall effects were observed for NR1 and NR2B. When comparing Sal and the 30 mg/kg DCS groups, there was a main effect of injury (F1,29 = 5.148, p = 0.032) and drug treatment (F1,29 = 4.384, p = 0.046) on ipsilateral NR2A (Fig. 2A). FPI reduced ipsilateral hippocampal NR2A by 20% regardless of drug treatment, and DCS increased hippocampal NR2A levels by 20% in both sham and FPI pups. We found that DCS resulted in a dose-dependent restoration of NR2A levels in the ipsilateral hippocampus. No injury or drug effects were observed for NR1 and NR2B levels. Additionally, there was a trend toward an overall effect of injury on NR2B levels in the contralateral hippocampus (F1,31 = 3.590, p = 0.068) but not for DCS treatment. There were no main effects of injury or drug for contralateral NR1 and NR2A levels (Fig. 2B). Although no main effects were observed for NR1, the initial calculation of a Pearson's r correlation coefficient showed a strong positive correlation between NR1 and NR2B in the contralateral hippocampus (r = 0.559, n = 40, p < 0.001). In addition to a significant reduction of NR2B (−21%) from Sham-Sal levels (independent samples test, p = 0.05), there was a relative decrease in NR1.
FIG. 2.
Post-injury day (PID)4 N-methyl-d-aspartate receptor (NMDAR) subunits. (Mean ± standard error of the mean [SEM]) NMDAR NR1, NR2A, and NR2B subunit levels in the hippocampus, (A) ipsilateral and (B) contralateral to the fluid percussion injury (FPI) site. Representative Western blots are shown below the graphs. In the ipsilateral hippocampus, main effects of FPI and D-cycloserine (DCS) treatment were observed for the NR2A subunit. FPI did not significantly affect NR1 and NR2B levels. The askterisk indicates a significant difference from Sham-Sal (*p < 0.05, Student t test, Bonferroni corrected), and the dagger denotes a significant difference from FPI-Sal (†p < 0.05). In the contralateral hippocampus, FPI and DCS had no significant effects on NR1 and NR2A subunits. Independent samples t test showed a significant decrease in FPI-highDCS (*p = 0.05) compared to Sham-Sal.
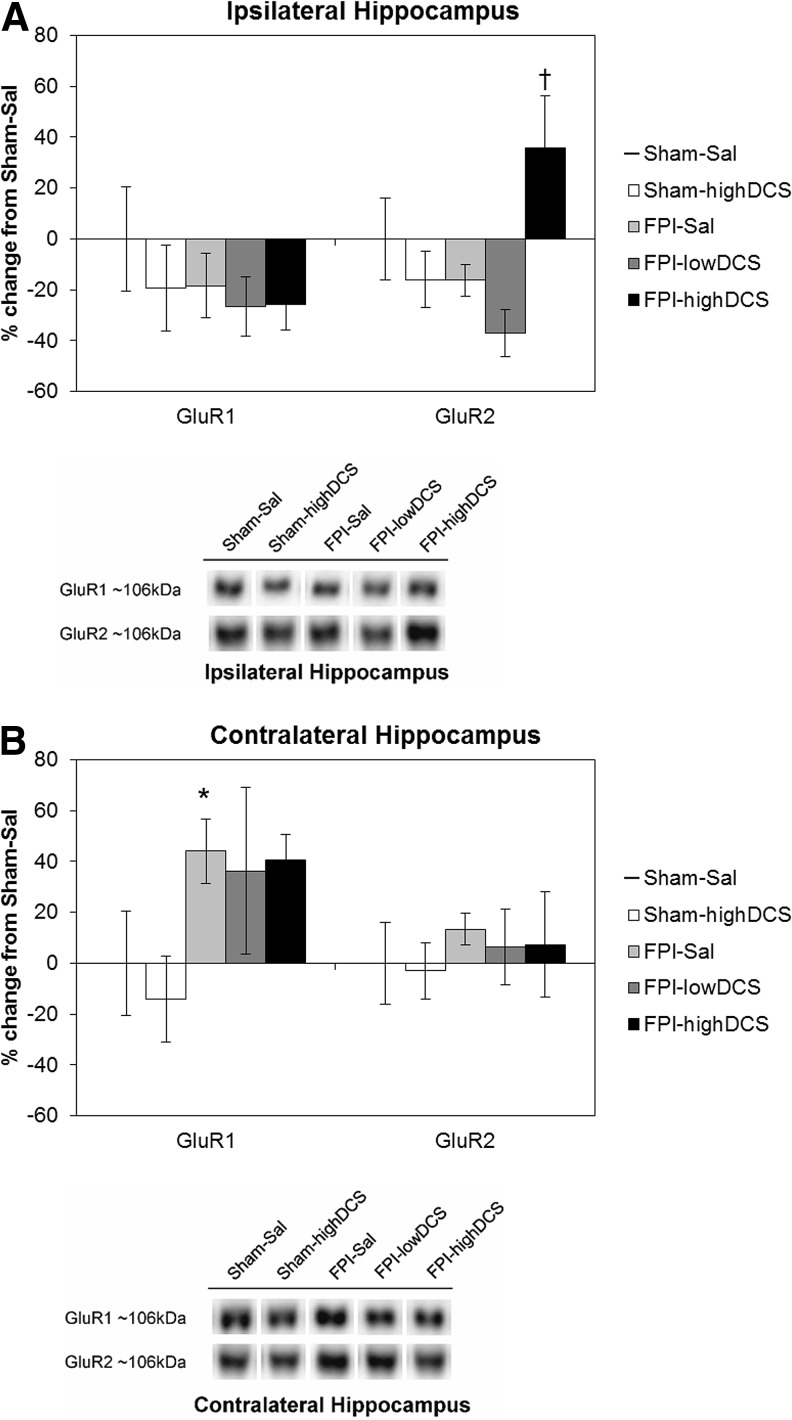
For AMPARs, there was no overall effect of injury for GluR1 and GluR2 subunits in the ipsilateral hippocampus (Fig. 3A). There was a significant main effect of DCS only for ipsilateral GluR2 (F1,39 = 4.772, p = 0.015), as well as a significant injury-by-drug interaction (F1,39 = 6.223, p = 0.017). Post-hoc testing showed that FPI-highDCS animals had significantly higher levels of ipsilateral hippocampal GluR2 (+54%) than did FPI-lowDCS animals (p = 0.006). This result suggests that DCS had a different effect on ipsilateral GluR2 based on dose levels. In the contralateral hippocampus (Fig. 3B), there was a significant main effect of injury on GluR1 (F1,38 = 6.140, p = 0.018). FPI to P19 rats resulted in increased GluR1 levels by ∼ +45%, compared with Sham-Sal animals, regardless of drug treatment. No main effect of DCS was observed in GluR1. Contralateral hippocampal GluR2 levels were unaffected by injury or DCS.
FIG. 3.
Post-injury day (PID)4 α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunits. (Mean ± standard error of the mean [SEM]) AMPAR GluR1 and GluR2 subunit levels in the hippocampus, (A) ipsilateral and (B) contralateral to the fluid percussion injury (FPI) site. Representative Western blots are shown below the graphs. D-cycloserine (DCS) treatment significantly increased GluR2 levels in the ipsilateral hippocampus. FPI resulted in increased levels of GluR1 in the contralateral hippocampus. The asterisk indicates a significant difference from Sham-Sal (*p < 0.05), and the dagger denotes a significant difference from FPI-Sal (†p < 0.05).
Experiment 2: FPI and DCS subacute effects on novel object recognition
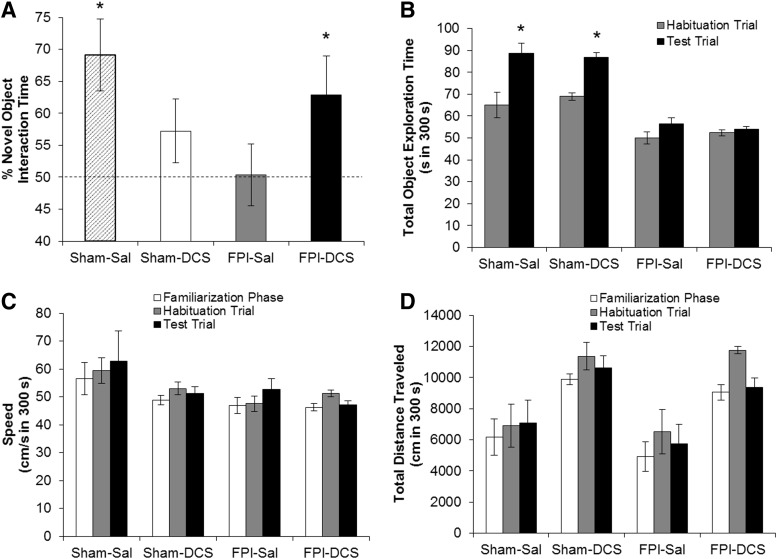
On PID4, the effects of FPI and DCS on novel object recognition memory were measured using the NOR task (Fig. 4A). Sham-Sal (n = 15) animals significantly spent more time with the novel object (66%, p = 0.015), whereas FPI-Sal rats (n = 15) were unable to distinguish between novel and familiar objects (57%, p = 0.069). Although Sham-DCS animals (n = 9) showed only trends for intact NOR (62%, p = 0.077), DCS restored novel object recognition in the FPI-DCS group (n = 9) (71%, p = 0.022). Sham-Sal and Sham-DCS animals showed significant increases in total object exploration time in the testing phase. However, although FPI animals did not significantly spend more time with both objects during the testing phase, FPI-DCS pups were still able to discern between the novel and familiar objects. No overall effect of FPI or DCS was observed in the speed of the animals (Fig. 4C). The total distance traveled by the animals was unaffected by FPI across the different phases (Fig. 4D). We did observe a DCS main effect in the total distance traveled in all three phases (familiarization: F1,42 = 11.642, p = 0.002; habituation: F1,42 = 8.414, p = 0.004; testing: F1,42 = 6.188, p = 0.017).
FIG. 4.
Post-injury day (PID)4 novel object recognition (NOR) performance. (A) Percent time spent with the novel object during the first 3 min of the NOR test trial. Dotted horizontal line shows 50% (chance level) of NOR performance. Sham-Sal pups displayed intact NOR (*p < .05), whereas fluid percussion inury (FPI)-Sal animals only exhibited chance performance. D-cycloserine (DCS) treatment restored NOR in FPI-DCS animals (*p < 0.05). (B) Total object exploration time. Sham animals showed significant increase in object exploration in the testing phase, when the novel object was presented. However, although FPI animals did not significantly spend more time during the testing phase, FPI-DCS pups were still able to discern between the novel and familiar objects. Locomotion during the habituation and test trials: (C) speed in cm/sec, and (D) total distance traveled in cm. There was no overall effect of FPI or DCS in the speed of the animals. A main effect of DCS was observed in the total distance traveled, regardless of injury. Values are expressed as mean ± standard error of the mean (SEM).
Experiment 3: FPI and DCS effects on experience-dependent plasticity
In Experiment 3, we induced experience-dependent plasticity by rearing animals in an EE for 17 days beginning on PID1. We then investigated the effects of FPI and DCS treatment on EE-induced experience-dependent plasticity by evaluating the performance of the animals in the MWM task 30 days after surgery.
MWM acquisition: latency
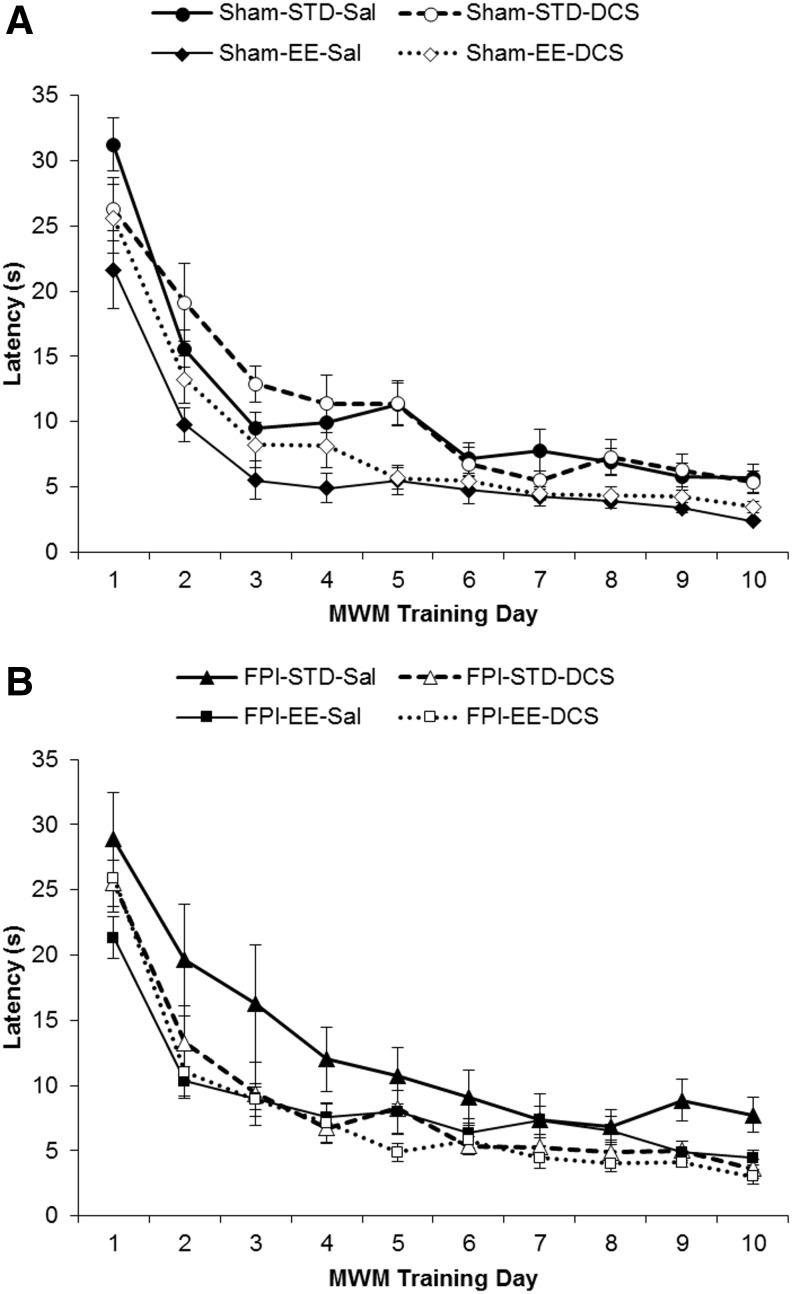
Linear mixed-effects model showed that EE rearing is an important predictor of MWM acquisition (Fig. 5A and B). Using group as a fixed effect (latency by group), EE rearing significantly improved latencies in Sham-EE-Sal but not in FPI-EE-Sal animals. Only when FPI-EE pups were treated with DCS did the acquisition latencies of injured animals significantly decrease to sham values (Sham-STD-Sal > FPI-EE-DCS, p < 0.05).
FIG. 5.
Morris water maze (MWM) acquisition – latency. (Mean ± standard error of the mean [SEM]) Latency in seconds (s) for (A) Sham and (B) fluid percussion injury (FPI) groups.
MWM acquisition: trials to criterion
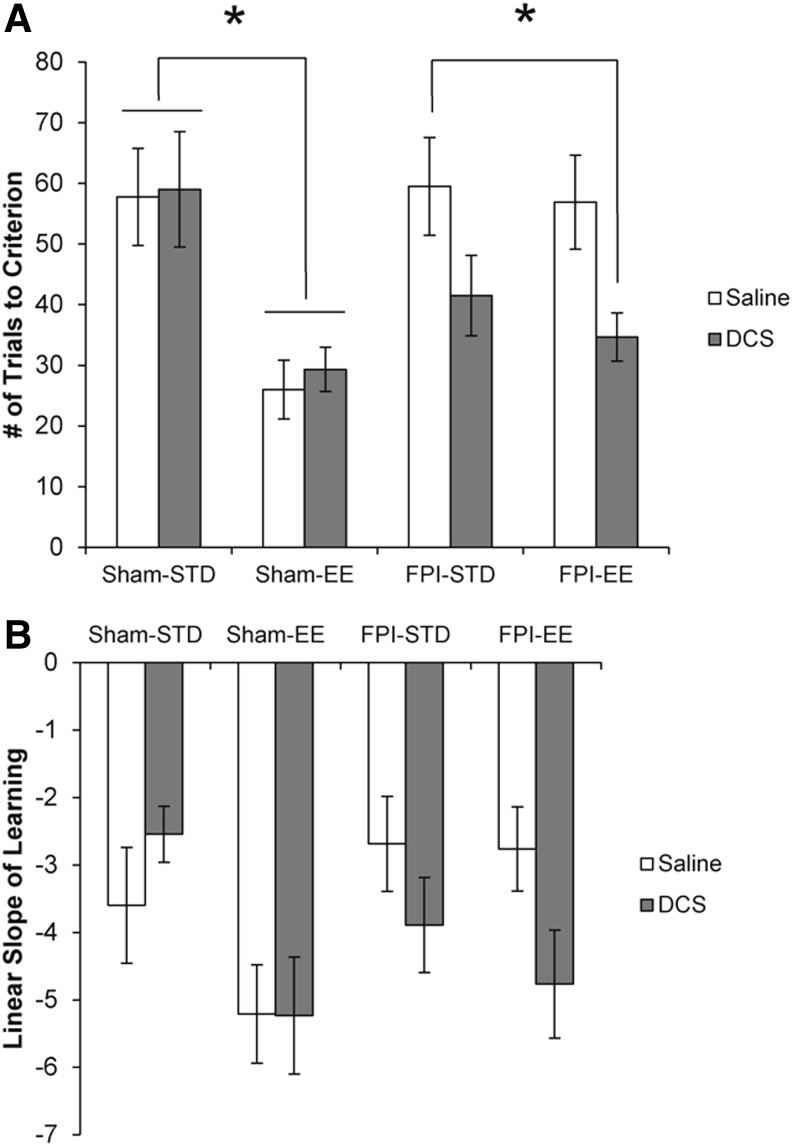
Concordant with previously published work,16,17 EE rearing significantly improved the “trials to criterion” in shams (F1,33 = 20.102, p = 0.000) but not in FPI animals (F1,33 = 0.489, p = 0.490) (Fig. 6A). Additionally, we found that DCS treatment only significantly affected FPI animals (F1,33 = 8.869, p = 0.006) and not the sham groups (F1,33 = 0.110, p = 0.742). We observed significantly improved “trials to criterion” in FPI-EE-DCS pups (FPI-EE-DCS < FPI-STD-Sal, p = 0.012).
FIG. 6.
Morris water maze (MWM) acquisition – trials to criterion and slope of learning. (A) Trials to criterion is a measure of MWM acquisition mastery, defined as the number of trials the subject acquired to reach the hidden platform in ≤5 sec, in four consecutive trials. (B) Slope of learning measures the linear rate of learning the MWM task. Values are expressed as mean ± standard error of the mean [SEM].
MWM acquisition: slope of learning
We defined the slope of learning as the slope from the linear regression of the mean latencies between the first block and the block when the animal reached “trials to criterion.” With learning slopes all< −1.0, this study demonstrated that young rats can learn the MWM task well, to levels comparable with those of adult rats (Fig. 6B).17 Similarly to the “trials to criterion” results, EE rearing significantly enhanced slopes of learning only in shams (F1,33 = 8.005, p = 0.008), and DCS treatment only significantly affected FPI pups (F1,33 = 5.032, p = 0.032).
MWM retention: probe trial
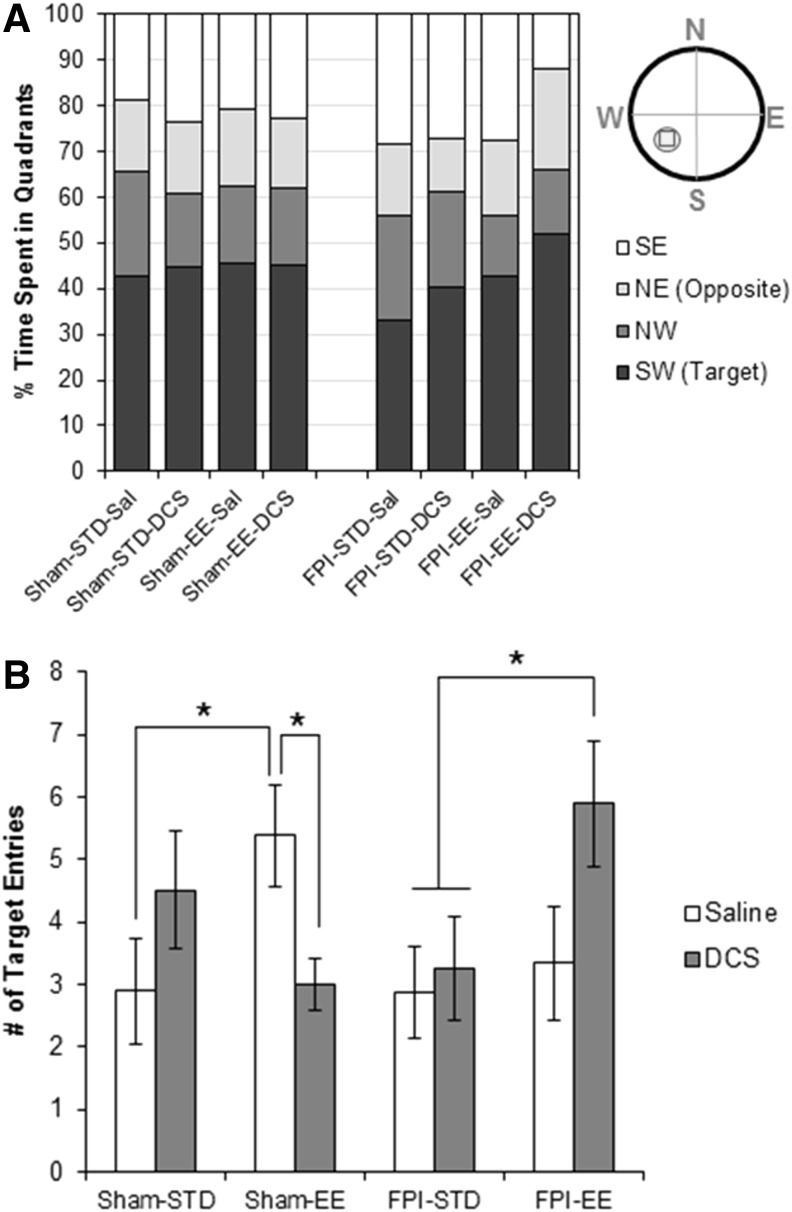
The probe trial was administered after a 1 week delay following the MWM training. The percent time spent in the target quadrant (SW) was significantly greater than that spent in any of the other quadrants (p < 0.001) across all groups (Fig. 7A). ANOVA showed a three way significant interaction effect of injury-by-housing-by-drug (F1,67 = 6.530, p = 0.013) in the number of target entries. Independent post-hoc tests showed that the FPI-STD-Sal group had a comparable number of target entries compared with their Sham-STD-Sal counterparts (Fig. 7B). There was a robust trend toward increased number of entries to the target zone in Sham-EE-Sal (Sham-STD-EE vs. Sham-EE-Sal, p = 0.055), and no effect of EE was detected in FPI-EE-Sal animals (NS, p = 0.7). DCS had a differential effect in shams and FPI animals. With the DCS treatment, FPI-EE-DCS animals significantly increased their number of target entries comparable with Sham-EE-Sal levels, which was significantly higher than entries made by Sham-STD-Sal (FPI-EE-DCS vs. Sham-STD-Sal, p = 0.037) and FPI-STD-Sal (FPI-EE-DCS vs. FPI-STD-Sal, p = 0.037). Sham-EE-DCS, on the other hand, had significantly fewer entries to the target zone than did the Sham-EE-Sal group (Sham-STD-DCS vs. Sham-EE-Sal, p = 0.022).
FIG. 7.
Morris water maze (MWM) retention – probe trial. (A) Percent time spent in MWM quadrants, and (B) the (mean ± standard error of the mean [SEM]) number of entries into the target zone (includes the small circle shown in SW quadrant).
Discussion
In this study, we showed that FPI on P19 rats failed to exhibit EE rearing induced enhancements in learning and memory when tested later in adulthood, consistent with previous studies. In FPI rats, there was a persistent downregulation of hippocampal NMDAR (NR2A) subunits ipsilateral to the injury site 4 days after the insult, and impaired performance in the NOR, an NMDAR hippocampal-dependent task. Timely use of DCS can restore NMDAR-mediated molecular and behavioral plasticity within 1 week after developmental TBI, and can rescue experience-dependent plasticity later in life.
Dichotomy of plasticity
The benefits of increased plasticity may be contingent upon the nature of the stimuli or experience and the elaborate interplay of molecular, cellular, and physiological responses. Not all plastic responses are beneficial. Depending upon the concentration of DCS in relation to what is present in the synaptic milieu, DCS can act as an agonist or an antagonist in the intact brain.43,44 For example, DCS treatment had a differential effect in sham animals. DCS has been described as a “cognitive enhancer,” and DCS treatment improved MWM memory retention in standard housed shams (Sham-STD-DCS). This is demonstrated by an increase in target zone entries during the probe trial. However, in EE housed shams, DCS treatment significantly reduced the number of target entries in Sham-DCS rats compared with saline-treated Sham-Sal rats. There are at least two possible explanations for this seemingly contradictory finding: 1) DCS has a positive effect by enhancing learning in Sham-EE-DCS animals that recognized the absence of the platform quickly, or 2) DCS has a negative effect in the Sham-EE-DCS group by acting as an NMDAR antagonist rather than an agonist, and inhibiting the recall of the platform location. All sham animals spent comparable times in the target quadrant, which is greater than the amount of time spent in the other three quadrants. Therefore, Sham-EE-DCS animals recalled the general location of the hidden platform but did not spend substantial time in the target zone in particular because they had already learned that the platform no longer existed there. Alternatively, it has been reported that differential housing in EE significantly increases glycine levels in the cerebral cortex.45 DCS activates NMDAR to a maximum of 40–50% of the activation induced by glycine or D-serine. In this situation, DCS may play an antagonist role through competitive inhibition, and reduces the NMDAR activation efficacy.43,44
Lost potential caused by lost plasticity
Experience-dependent plasticity has previously been shown to result in anatomical and cognitive enhancements in normally developing animals,46–48 and similar effects were measured in the current study, in which enhanced learning was measured in Sham-EE-Sal animals. However, FPI-EE-Sal animals failed to acquire the benefits of prolonged exposure to EE. Although FPI-EE-Sal pups did perform to the level of standard housed animals and learned and recalled the MWM task, these FPI pups did not demonstrate the same potential as their uninjured cohorts in the trials to criterion and the probe trial. Only when DCS was administered did the FPI-EE animals demonstrate comparable performance to the Sham-EE-Sal group in the MWM.
It is widely believed that a greater potential for plasticity and increased rates of spontaneous recovery from injury or disease occur in the immature brain. For example, cortical lesions early in life result in better recovery and sparing of function compared with lesions that were given to adult rats49 and monkeys.50–52 Although adult rats demonstrated significant deficits in the MWM after FPI, we demonstrated that standard housed injured weanlings (FPI-STD) showed no deficits in MWM acquisition and recall when compared with shams, consistent with data from P17 rats that received the same FPI.53 It is the FPI animals' capacity for induced experience-dependent plasticity that was impaired, and this loss of plastic capacity is referred to as “lost potential.”
Glutamatergic-mediated transmission and TBI
Neural responsiveness to a spectrum of stimuli, from physiologic to pathologic, has been closely linked with glutamatergic transmission. On one hand, physiologic stimulation of NMDAR and AMPAR glutamatergic receptors promotes plasticity and cell survival, and has been shown to be crucial for normal development and learning and memory.4,6,7,54–58 On the other hand, pathological glutamatergic activation via the AMPARs and NMDARs has been demonstrated to be a critical, pathologic consequence of TBI that may underlie chronic deficits in behavior and cognition. Experimental TBI enhances neuronal calcium permeability by increasing expression of calcium permeable AMPARs and by decreasing calcium impermeable AMPARs that contain GluR2 subunits. These changes in AMPARs may be NMDAR mediated, and correlate with increased neuronal death.21,22 A dramatic increase of NMDAR activation via the release of glutamate59,60 has been observed as early as 15 min after experimental TBI in rodents, followed by diminished NMDAR binding12 and expression (hours to days)11,13,14 that is long lasting in developing rats.15
NMDAR as target for therapeutic intervention
More recently, the use of NMDAR agonists has been shown to promote neuroprotection, and has facilitated more rapid recovery only when the treatment is delayed, missing the very early critical window of hyperactivity after TBI.11,32–34 Low doses of NMDA have been shown to preferentially activate synaptic NMDA receptors because of a significant increase in action potential firing and activation of a pro-survival molecular cascade. In contrast, toxic levels of NMDA suppress firing rates below baseline, and extrasynaptic NMDA signaling dominates.61 Failure of NMDAR blockade may have been attributable to mis-timed delivery during periods of already downregulated NMDAR function, missing the critical window of hyperactivity of the receptors. Global NMDA antagonism may therefore be triggering a pro-apoptotic cascade, which may underlie worsened outcome after treatment with NMDAR blockers.27 Use of appropriate levels of NMDA agonists during the appropriate time window would then foster more synaptic, pro-survival sequelae. Additionally, another alternative to NMDAR activation is to target its co-agonist glycine binding site. Historically, this modulatory site was named for the binding of glycine and its interaction with the NMDAR complex; however, the site has been recently shown to have a more natural affinity for the endogenous amino acid D-serine.62 Further, this glycine-binding site has been shown to not be saturated in hippocampal neurons in culture and in slice preparation,63 and, therefore, it allows modulation of NMDAR function without posing direct risks of excitotoxicity.
In this study, we demonstrated that the subacute molecular response to FPI in the young brain is reduced hippocampal NR2A ipsilateral to the injury, and that DCS treatment soon after the insult resolved NR2A levels to sham levels. There was also a dose-dependent restoration of hippocampal NR2A levels ipsilateral to FPI injury at lower doses of DCS. We did not observe any changes in NR1 and NR2B levels after FPI. DCS significantly decreased NR2B in FPI-EE-DCS animals in the contralateral hippocampus. During normal brain development, the ratio of NR2A to NR2B increases, which involves the increase in NR2A levels but also a decrease in NR2B.5,6,8–10 After FPI, in the ipsilateral hippocampus, DCS treatment resolves the NMDAR impairment by increasing the NR2A:NR2B ratio by increasing NR2A levels, whereas in the contralateral hippocampus, DCS increases this ratio by reducing NR2B levels.
AMPAR subunits were unaffected in this TBI model in our hands; we observed neither increases in GluR1, nor decreases in GluR2 levels in the ipsilateral hippocampus that characterized previous reports following TBI.21,22 This could be because of differences in the developmental time window investigated or differences in the injury models used. We observed increased GluR2 levels in the ipsilateral hippocampus on PID4 after DCS treatment in FPI rat pups. One possible explanation is that DCS treatment upregulated Ca+2 impermeable GluR2-containing AMPARs in a neuroprotective response in order to counter excitotoxicity. Interestingly, our observed upregulation of GluR1 on the contralateral hippocampus follows previous reports on TBI-induced increase of GluR1-containing AMPAR in regions not directly in the injury site.23 Our data support a contralateral enhancement of neural activation that is not NMDAR mediated, as a compensatory response to depressed ipsilateral activity.
The diminished NMDAR molecular response following developmental TBI corresponded with impaired performance on the NOR task. FPI resulted in abolished NOR, which posited the inability of injured animals to take in the benefits of EE rearing. Here, we found that DCS treatment significantly affected FPI injured pups and restored NOR performance to sham levels.
Lastly, and most importantly, administering DCS during the period of reduced NMDAR function after developmental FPI can restore EE-induced experience-dependent plasticity in injured rat pups. We showed that mastery (measured in trials to criterion) and recall of the MWM task tested during early adulthood was restored to that of their age-matched, sham counterparts.
Conclusion
In pediatric TBI, “younger is not always better,” and the effects of TBI can be worse and longer lasting in children. Injured youth may even have altered developmental profiles. Although the FPI-EE-Sal group in our study in the end learned the MWM trained task, they never achieved criterion in the same number of trials as their Sham-EE-Sal counterparts. This impairment even lasted into their early adulthood. Although the persisting belief that there is better restitution of function when brain damage occurs early in life, which has been ascribed as the “Kennard Principle” historically, Kennard would posit that age would not have been the sole predictor of recovery. Depending on the features of the injury, post-injury reorganization, staging of the lesion, and the timing and method of outcome measure assessment, early brain injury would be as equally devastating as damage to the mature brain.64 Here we demonstrated that timely administration of an NMDAR agonist can reverse subacute hippocampal NMDAR pathological activation and reinstate experience-dependent plasticity in developing FPI injured rats. Fostering excitatory transmission during recovery in conjunction with a behavioral stimulus offers an innovative new perspective on restoration of function after TBI. This mechanism-based therapeutic strategy has potential for enhancing plasticity in other paradigms of intervention and recovery from developmental brain injury.
Acknowledgments
We thank David Garfinkel for his excellent technical assistance. This work was supported by the UCLA Brain Injury Research Center (BIRC), NS27544, NS057420, NIH NINDS 1R01NS091222-01, and the Winokur Family Foundation/Child Neurology Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gilchrist J., Thomas K.E., Xu L., McGuire L.C., and Coronado V.G. (2011). Nonfatal traumatic brain injuries related to sports and recreation activites among persons aged <19 years — United States, 2001–2009. In: National Center for Injury Prevention and Control, CDC, MMWR Morbidity and Mortality Weekly Report, www.cdc.gov/mmwr/pdf/wk/mm6039.pdf pps. 1337–1342 [PubMed]
- 2.Ewing-Cobbs L., Fletcher J.M., Levin H.S., Francis D.J., Davidson K., and Miner M.E. (1997). Longitudinal neuropsychological outcome in inrants and preschoolers with traumatic brain injury. J. Int. Neuropsychol. Soc. 3, 581–691 [PubMed] [Google Scholar]
- 3.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2005). Functional plasticity or vulnerability after early brain injury? Pediatrics 116, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 4.Flint A.C., Maisch U.S., Weishaupt J.H., Kriegstein A.R., and Monyer H. (1997). NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J. Neurosci. 17, 2469–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts E.B., and Ramoa A.S. (1999). Enhanced NR2A subunit expression and decreased NMDA receptor decay time on the onset of ocular dominance plasticity in the ferret. J. Neurophysiol. 81, 2587–2591 [DOI] [PubMed] [Google Scholar]
- 6.Cull–Candy S., Brickley S., and Farrant M. (2001). NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 11, 327–335 [DOI] [PubMed] [Google Scholar]
- 7.Cull–Candy S., Kelly L., and Farrant M. (2006). Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 16, 288–297 [DOI] [PubMed] [Google Scholar]
- 8.Flint A.C., Maisch U.S., Weishaupt J.H., Kriegstein A.R., and Monyer H. (1997). NR2A subunit expression shortens NMDA receptor synptic currents in developing neocortex. J. Neurosci. 17, 2469–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurie D.J., Bartke I., Schoepfer R., Naujoks K., and Seeburg P.H. (1997). Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res. Mol. Brain Res. 51, 23–32 [DOI] [PubMed] [Google Scholar]
- 10.Monyer H., Burnashev N., Laurie D.J., Sakmann B., and Seeburg P.H. (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 [DOI] [PubMed] [Google Scholar]
- 11.Biegon A., Fry P.A., Paden C.M., Alexandrovich A., Tsenter J., and Shohami E. (2004). Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. U. S. A. 101, 5117–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller L.P., Lyeth B.G., Jenkins L.W., Oleniak L., Panchision D., Hamm R.J., Phillips L.L., Dixon C.E., Clifton G.L., and Hayes R.L. (1990). Excitatory amino acid receptor subtype binding following traumatic brain injury. Brain Res. 526, 103–107 [DOI] [PubMed] [Google Scholar]
- 13.Osteen C.L., Giza C.C., and Hovda D.A. (2004). Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience 128, 305–322 [DOI] [PubMed] [Google Scholar]
- 14.Kumar A., Zou L., Yuan X., Long Y., and Yang K. (2002). N-methyl-D-aspartate receptors: transient loss of NR1/NR2A/NR2B subunits after traumatic brain injury in a rodent model. J. Neurosci. Res. 67, 781–786 [DOI] [PubMed] [Google Scholar]
- 15.Giza C.C., Santa Maria N.S., and Hovda D.A. (2006). N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J. Neurotrauma 23, 950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fineman I., Giza C.C., Nahed B.V., Lee S.M., and Hovda D.A. (2000). Inhibition of neocortical plasticity during development by a moderate concussive brain injury. J. Neurotrauma 17, 739–749 [DOI] [PubMed] [Google Scholar]
- 17.Giza C.C., Griesbach G.S., and Hovda D.A. (2005). Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 157, 11–22 [DOI] [PubMed] [Google Scholar]
- 18.Ip E.Y., Giza C.C., Griesbach G.S., and Hovda D.A. (2002). Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. J. Neurotrauma 19, 573–585 [DOI] [PubMed] [Google Scholar]
- 19.Prins M.L., Lee S.M., Cheng C.L., Becker D.P., and Hovda D.A. (1996). Fluid percussion brain injury in the developing and adult rat: a comparative study of mortality, morphology, intracranial pressure and mean arterial blood pressure. Brain Res. Dev. Brain Res. 95, 272–282 [DOI] [PubMed] [Google Scholar]
- 20.Gurkoff G.G., Giza C.C., and Hovda D.A. (2006). Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 1077, 24–36 [DOI] [PubMed] [Google Scholar]
- 21.Bell J.D., Ai J., Chen Y., and Baker A.J. (2007). Mild in vitro trauma induces rapid Glur2 endocytosis, robustly augments calcium permeability and enhances susceptibility to secondary excitotoxic insult in cultured Purkinje cells. Brain 130, 2528–2542 [DOI] [PubMed] [Google Scholar]
- 22.Bell J.D., Park E., Ai J., and Baker A.J. (2009). PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ. 16, 1665–1680 [DOI] [PubMed] [Google Scholar]
- 23.Schumann J., Alexandrovich A.G., Biegon A., and Yaka R. (2008). Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J. Neurotrauma 25, 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han R.Z., Hu J.J., Weng Y.C., Li D.F., and Huang Y. (2009). NMDA receptor antagonist MK-801 reduces neuronal damage and preserves learning and memory in a rat model of traumatic brain injury. Neurosci. Bull. 25, 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao V.L., Dogan A., Todd K.G., Bowen K.K., and Dempsey R.J. (2001). Neuroprotection by mementine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 911, 96–100 [DOI] [PubMed] [Google Scholar]
- 26.Muir K.W. (2006). Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr. Opin. Pharmacol. 6, 53–60 [DOI] [PubMed] [Google Scholar]
- 27.Ikonomidou C., and Turski L. (2002). Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 1, 383–386 [DOI] [PubMed] [Google Scholar]
- 28.Albers G.W., Goldstein L.B., Hall D., and Lesko L.M. (2001). Aptiganel hydrochloride in acute ischemic stroke. J.A.M.A. 286, 2673–2682 [DOI] [PubMed] [Google Scholar]
- 29.Morris G.F., Bullock M.R., Marshall S.B., Marmarou A., Maas A., The Selfotel Investigators, and Marshall L.F. (1999). Failure of the competitive N-methyl-D-aspartate antagonist selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. J. Neurosurg. 91, 737–743 [DOI] [PubMed] [Google Scholar]
- 30.Narayan R.K., Michel M.E., Ansell B., Baethmann A., Biegon A., Bracken M.B., Bullock M.R, Choi S.C., Clifton G.L., Contant C.F., Coplin W.M., Dietrich W.D., Ghajar J., Grady S.M., Grossman R.G., Hall E.D., Heetderks W., Hovda D.A., Jallo J., Katz R.L., Knoller N., Kochanek P.M., Maas A.I., Majde J., Marion D.W., Marmarou A., Marshall L.F., McIntosh T.K., Miller E., Mohberg N., Muizelaar J.P., Pitts L.H., Quinn P., Riesenfeld G., Robertson C.S., Strauss K.I., Teasdale G., Temkin N., Tuma R., Wade C., Walker M.D., Weinrich M., Whyte J., Wilberger J., Young A.B. and Yurkewicz L. (2002). Clinical trials in head injury. J Neurotrauma 19, 503–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas A., Roozenbeek B., and Manley G.T. (2010). Clinical trials in traumatic brain injury: past exprerience and current developments. Neurotherapeutics 7, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temple M.D., and Hamm R.J. (1996). Chronic, post-injury administration of D-cycloserine, an NMDA partial agonist, enhances cognitive performance following experimental brain injury. Brain Res. 741, 246–251 [DOI] [PubMed] [Google Scholar]
- 33.Yaka R., Biegon A., Grigoriadis N., Simeonidou C., Grigoriadis S., Alexandrovich A.G., Matzner H., Schumann J., Trembovler V., Tsenter J., and Shohami E. (2007). D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J. 21, 2033–2041 [DOI] [PubMed] [Google Scholar]
- 34.Adeleye A., Shohami E., Nachman D., Alexandrovich A.G., Trembovler V., Yaka R., Shoshan Y., Dhawan J., and Biegon A. (2010). D-cycloserine improves functional outcome after traumatic brain injury with wide therapeutic window. Eur. J. Pharmacol. 629, 25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark R.E., Zola S.M., and Squire L.R. (2000). Impaired recognition memory in rats after damage to the hippocampus. J. Neurosci. 20, 8853–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ennaceur A., and Delacour J. (1988). A new one-trial test for neurobiological studies of memory in rats. I: behavioral data. Behav. Brain Res. 31, 47–59 [DOI] [PubMed] [Google Scholar]
- 37.Hammond R.S., Tull L.E., and Stackman R.W. (2004). On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 82, 26–34 [DOI] [PubMed] [Google Scholar]
- 38.Dere E., Huston J.P., and De Souza Silva M.A. (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci. Biobehav. Rev. 31, 673–704 [DOI] [PubMed] [Google Scholar]
- 39.Johnson M.W. (1997). Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J. Neurosci. Methods 77, 151–156 [DOI] [PubMed] [Google Scholar]
- 40.Reger M.L., Hovda D.A., and Giza C.C. (2009). Ontology of rat recognition memory measured by the novel object recognition task. Dev. Psychobiol. 51, 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maei H.R., Zaslavsky K., Teixeira C.M., and Frankland P.W. (2009). What is the most sensitive measure of water maze probe test performance? Front. Integr. Neurosci. 3, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R Development Core Team (2010). R: A Language and Enviroment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 43.Hood W.F., Compton R.P., and Monahan J.B. (1989). D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci. Lett. 98, 91–95 [DOI] [PubMed] [Google Scholar]
- 44.Lanthorn T.H. (1994). D-cycloserine: agonist turned antagonist. Amino Acids 6, 247–260 [DOI] [PubMed] [Google Scholar]
- 45.Cordoba F., Yusta B., and Munoz–Blanco J. (1984). Changes in neurotransmitter amino acids and protein in CNS areas of mice subjected to differential housing conditions. Pharmacol. Biochem. Behav. 21, 349–352 [DOI] [PubMed] [Google Scholar]
- 46.Rosenzweig M.R., Bennet E.L., and Diamond M.C. (1972). Brain changes in response to experience. Scientific American 226, 22–295062027 [Google Scholar]
- 47.Rosenzweig M.R., and Bennett E.L. (1996). Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65 [DOI] [PubMed] [Google Scholar]
- 48.Greenough W.T. (1976). Enduring brain effects of differential experience and training. Rosensweig M.R., Bennett E.L. (eds), pps. Neural Mechanisms of Learning and Memory, 255–278 [Google Scholar]
- 49.Kolb B., and Tomie J. (1988). Recovery from early cortical damage in rats. IV. Effects of hemidecortication at 1, 5 or 10 days of age on cerebral anatomy and behavior. Behav. Brain Res. 28, 259–274 [DOI] [PubMed] [Google Scholar]
- 50.Kennard M.A. (1936). Age and other factors in mortor recovery from preecentral lesions in monkeys. Am. J. Physiol. 115, 138–146 [Google Scholar]
- 51.Kennard M.A. (1938). Reorganization of motor function in the cerebral cortex of monkeys deprived of motor and prepmotor areasin infance. J. Neurophysiol. 1, 377–397 [Google Scholar]
- 52.Kennard M.A., and Fulton J.F. (1942). Age and reorganization of central nervous system. Journal of the Mount Sinai Hospital 9, 594–606 [Google Scholar]
- 53.Prins M.L., and Hovda D.A. (1998). Traumatic brain injury in the developing rat: effects of maturation on Morris water maze acquisition. J. Neurotrauma 15, 799–811 [DOI] [PubMed] [Google Scholar]
- 54.Quinlan E.M., Olstein D.H., and Bear M.F. (1999). Bidirectional, experience-dependent regulation of N-methyl-d-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc. Natl. Acad. Sci. U.S.A. 96, 12,876–12,880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quinlan E.M., Philpot B.D., Huganir R.L., and Bear M.F. (1999). Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 352–357 [DOI] [PubMed] [Google Scholar]
- 56.Liu D., Day J.C., Francis D.D., and Meaney M.J. (2000). Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 3, 799–806 [DOI] [PubMed] [Google Scholar]
- 57.Wenzel A. (1997). NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem. 68, 469–478 [DOI] [PubMed] [Google Scholar]
- 58.Sheng M., Cummings J., Roldan L.A., Jan Y.N., and Jan L.Y. (1994). Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368, 144–147 [DOI] [PubMed] [Google Scholar]
- 59.Faden A.L., Demediuk P., Panter S.S., and Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244, 798–800 [DOI] [PubMed] [Google Scholar]
- 60.Katayama Y., Becker D.P., Tamura T., and Hovda D.A. (1990). Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73, 889–900 [DOI] [PubMed] [Google Scholar]
- 61.Soriano F.X., Papadia S., Hofmann F., Hardingham N.R., Bading H., and Hardingham G.E. (2006). Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 26, 4509–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolosker H. (2007). NMDA Receptor regulation by D-serine: new findings and perspectives. Mol. Neurobiol. 36, 152–164 [DOI] [PubMed] [Google Scholar]
- 63.Wilcox K.S., Fitzsimonds R.M., Johnson B., and Dichter M.A. (1996). Glycine regulation of synpatic NMDA receptors in hippocampal neurons. J. Neurophysiol. 76, 3415–3424 [DOI] [PubMed] [Google Scholar]
- 64.Dennis M. (2010). Margaret Kennard (1899–1975): not a 'principle' of brain plasticity but a founding mother of develomental neuropsychology. Cortex 46, 1043–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]