Abstract
Tissue-engineered vascular grafts that are based on reconstituted extracellular matrices have been plagued by weak mechanical strength that prevents handling or anastomosis to native vessels. In this study, we devise a method for making dense, suturable collagen tubular constructs of diameter ≤1 mm for potential microsurgical applications, by dehydrating tubes of native rat tail type I collagen and crosslinking them with 20 mM genipin. Crosslinked dense collagen tubes with 1 mm inner diameter yielded ultimate tensile strength of 342 ± 15 gF and burst pressure of 1313 ± 156 mm Hg, comparable to the strength of a rat femoral artery, and supported endothelial cell adhesion and growth. End-to-end anastomosis of 0.5-mm-diameter tubes to explanted arteries displayed anastomotic strength of 82 ± 21 gF, which is sufficient for surgical applications. In vivo implantation of cell-free tubes as interpositional grafts in the rat femoral circulation yielded stable anastomosis with blood flow for 20 min. Seeded dense collagen tubes represent a promising alternative to venous graft that can potentially be used to bridge between short artery stubs in replantation surgeries.
Keywords: : endothelial cells, genipin, vascular tissue engineering, vascular graft, microsurgery
Introduction
Tissue-engineered vascular grafts (TEVGs) have been studied for decades as an alternative to autologous artery and vein grafts.1 Compared with autologous grafts, TEVGs are more readily available and more flexible in dimensions. As TEVGs can have a functional endothelial lumen, they are less likely to cause the acute thrombosis and chronic inflammation that are commonly associated with synthetic polymer grafts.1 Much has been done to fabricate “small-diameter” grafts 3–6 mm in outer diameter that provide the strength and compliance of a native vessel,2–4 with some designs already in clinical trials.5,6 In comparison, little is known about making grafts with 1-mm-scale outer diameter. Such “very small-diameter” vessels are often employed for microsurgical peripheral revascularization, such as in the replantation of amputated fingers. In recent years, roughly 15% of the patients who had upper extremity amputation underwent replantation surgery.7 In avulsion injuries, the wound surface is often mangled, leaving short artery stubs insufficient for arterial anastomosis. In principle, a vascular graft of diameter ≤1 mm could be used in place of a traditional venous bypass to bridge between the proximal and distal artery stubs.
Because they are designed for different implantation environments, grafts in the 3–6 mm diameter range and those of ∼1 mm diameter require different design criteria. Most prominently, compliance matching between the arterial wall and graft is absolutely crucial in ensuring long-term patency in the larger grafts made for coronary bypass,8 but is less relevant at the microsurgical scale, where collaterals will promptly develop and only short-term patency is absolutely required.9–11 The requirement for anastomotic strength and burst pressure will likely differ for the two diameter scales as well.
Type I collagen is a common material for making tissue constructs because it has superior biocompatibility and promotes cell adhesion. Weinberg and Bell made the first TEVG by seeding endothelial cells on the lumen of a collagen gel tube.12 Notably, the construct was too fragile and had to be supported by synthetic sleeves. Attempts by other groups to increase the strength of TEVGs include: incorporating smooth muscle cells into the collagen,13 substituting collagen with fibrin,14,15 and stiffening collagen using glycation.16 Although these attempts led to increased graft strength, the gels typically still required a supporting synthetic sleeve to obtain surgically acceptable mechanical strength.
Recently, Nazhat and coworkers produced dense collagen tube constructs with an inner diameter of 3.4 mm by compressing native collagen gel into a sheet, which was subsequently rolled around a mandrel.17 These dense collagen tubes displayed circumferential tensile strength and suture knot retention strength similar to that of human saphenous vein and human mammary artery. Similarly, Chaikof and coworkers showed that dried collagen–elastin tubes could achieve ultimate tensile strength and Young's modulus comparable to that of a native blood vessel.18 Although these tubular constructs lacked a complete endothelial lumen, their high mechanical strength suggested that high matrix densities may be required to form vascular grafts without supporting sleeves.
In the current study, we produced dense collagen tubes by drying native rat tail type I collagen around a mandrel 0.5–1 mm in diameter. After the dried collagen tube was further crosslinked with 20 mM genipin, it yielded anastomotic strength comparable to rat femoral artery and could withstand the pressure of arterial flow. Moreover, we showed that human umbilical vein endothelial cells (HUVECs) grew into a confluent lumen that remained patent for 7 days inside these tubes. Finally, we implanted dried-and-crosslinked cell-free tubes in the rat femoral circulation, and found that they were mechanically stable and sustained blood flow in the acute (∼20 min) setting. To our knowledge, this work provides the first successful demonstration of end-to-end suture anastomosis of artery and collagen tubes at the 1-mm scale in vitro and in vivo.
Materials and Methods
Cell culture
HUVECs (BioWhittaker) were grown on gelatin-coated dishes in MCDB131 media (Caisson Labs) with 10% fetal bovine serum (Atlanta Biologicals), 1% glutamine–penicillin–streptomycin (Invitrogen), 5 U/mL heparin (Sigma), and 25 μg/mL endothelial cell growth supplement (Biomedical Technologies). Cells were routinely cultured under 5% carbon dioxide (CO2) at 37°C and passaged at a ratio of 1:4 using 0.005% trypsin in phosphate-buffered saline (PBS; Invitrogen). Cells were used up to passage 7.
Formation of flat collagen gels
To screen for the preparation condition that best improves the mechanical strength of collagen gel, we formed a variety of flat gels. Every 160 μL of acid-solubilized rat tail type I collagen (BD Biosciences) was neutralized with 28 μL of PBS, 20 μL of 10× PBS, and 20 μL of 0.2 M sodium hydroxide to yield a final collagen concentration of 6.3 mg/mL. Neutralized collagen was pipetted into rectangular polydimethylsiloxane (PDMS) molds 12 × 7 mm2 in length and width, and allowed to gel for 1 h at room temperature. The amount of neutralized collagen varied between 100 and 600 μL, depending on the mechanical test performed. After gelation, collagen was removed from the mold and washed in water for 1 h to remove salts, yielding a highly hydrated native collagen gel. To produce crosslinked collagen gel, the native gel was then submerged in 20 mM genipin in PBS for 2 h and washed three times with PBS for 10 min each. To produce dried collagen gel, the native gel was dehydrated at 37°C under steady air flow for 2 h, thereby removing over 99% of water, and then rehydrated in PBS. The percent dehydration was determined by measuring the weight of collagen gel before and after drying. To produce gel that was both dried and crosslinked, dried gel was rehydrated in PBS for 30 min, crosslinked in 20 mM genipin for 2 h, and washed three times with PBS for 10 min each. Dried and dried-and-crosslinked flat gels were used within 1 h after rehydration and crosslinking, respectively. All mechanical experiments were performed with gels in the hydrated state.
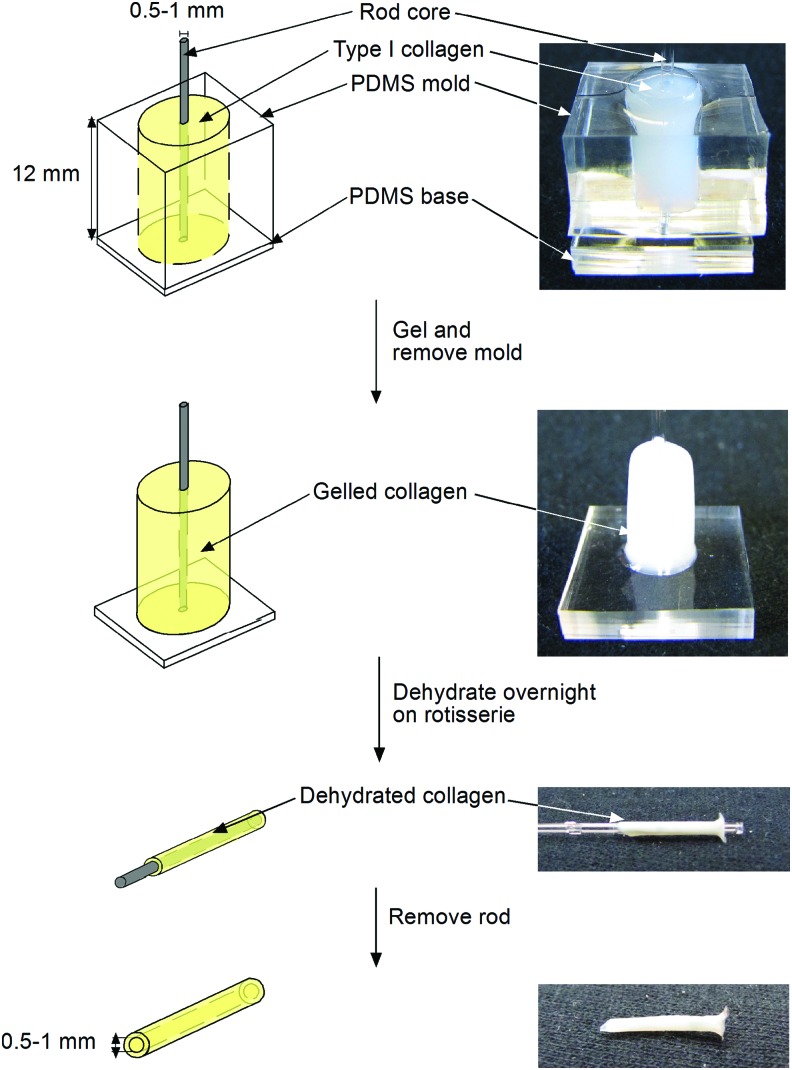
Formation of dense collagen tubes
Collagen tubes were formed under conditions that yielded highest mechanical strength in flat gels (Fig. 1). Briefly, 1 mL of neutralized collagen was pipetted into a cylindrical PDMS mold 8 mm in diameter and 12 mm in height, with a core of 0.5- or 1-mm-diameter glass or stainless steel coated with bovine serum albumin. After gelling for 2 h at room temperature, the collagen gel was removed from the mold, but with the rod in place, washed for 2 h in water, and dried at 37°C with minimal airflow overnight, removing over 99% of water. The dried collagen tube was rehydrated for 30 min in PBS and carefully removed from the rod. To produce tubes that were both dried and crosslinked, the rehydrated collagen tube was placed in 20 mM genipin for 2 h, and washed three times in PBS for 10 min each. Dried tubes were used within 1 h after rehydration; dried-and-crosslinked tubes were used within 1 day after crosslinking. All mechanical and implantation experiments were performed with the tubes in the hydrated state.
FIG. 1.
Schematic diagram of the formation of dried collagen tubes with ≤1 mm inner diameter. PDMS, polydimethylsiloxane. Color images available online at www.liebertpub.com/tea
Harvesting rat femoral vessels
To compare the strength of collagen tube-to-vessel anastomosis with that of vessel-to-vessel anastomosis, we harvested the femoral arteries from five female Sprague-Dawley rats (Charles River Laboratories). Each rat (∼250 g) was anesthetized with 5% isoflurane and maintained with 2% isoflurane with a nose cone. The lower abdomen was opened, and the femoral artery and vein were exposed by blunt dissection. The proximal and distal ends of the femoral vessels and any small vessel branches were ligated with 10-0 microsurgical nylon sutures (AROSurgical), and ∼1 cm lengths of both the left and right femoral arteries were dissected. The rat was euthanized with CO2 while still under anesthesia, followed by bilateral thoracotomy. All surgical procedures were performed in accordance with the Institutional and National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
In vitro anastomosis of collagen tubes and/or explanted vessels
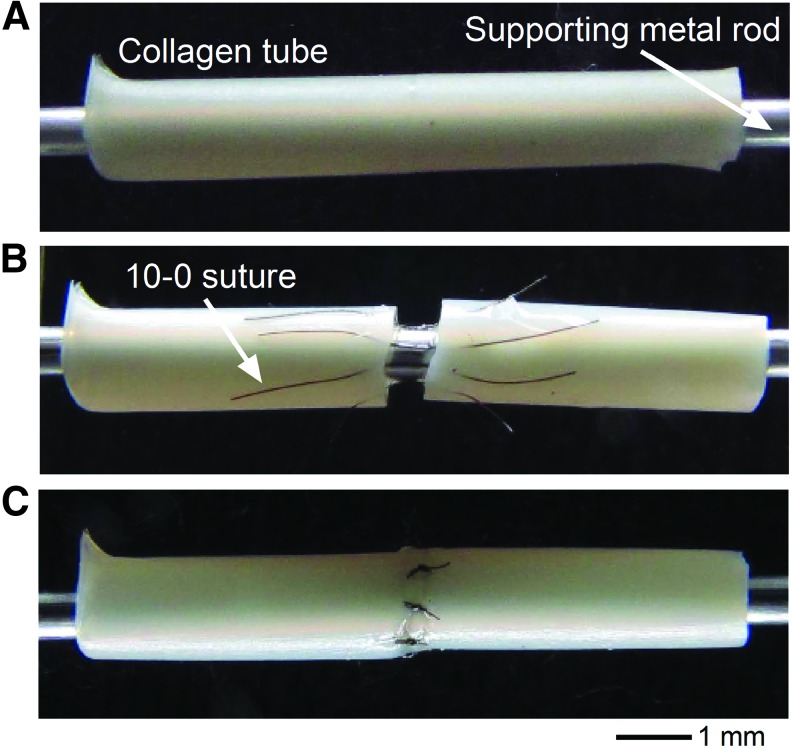
Collagen tubes (1 mm inner diameter) were trimmed to expose a flat edge, and were supported on a metal rod during the process of anastomosis (Fig. 2A). Eight 10-0 sutures were inserted 1.5 mm from the edges of the tube at an even spacing along the circumference (Fig. 2B). Once all eight sutures were inserted, simple square knots were used to tie each suture (Fig. 2C).
FIG. 2.
Anastomosis of collagen tubes. (A) Collagen tubes before anastomosis. (B) Suture-threaded collagen tubes. (C) Completed anastomosis. A similar process was used for anastomosis of collagen tube to an explanted rat femoral artery and for anastomosis of two arteries. Color images available online at www.liebertpub.com/tea
A similar procedure was used to perform artery-to-artery and artery-to-collagen tube anastomoses. Here, the collagen tubes were 0.5 mm in inner diameter to better match the diameter of a femoral artery.
Morphological measurement
Cross-sections of flat and tubular collagen gels were imaged with an Axiovert 200 M microscope (Zeiss) at 5× magnification. The thickness of flat collagen gels and the wall thickness of tubular collagen gels were measured using Axiovision version 4.5 software (Zeiss).
Tensile tests
Ultimate strength was measured for flat gels and intact dense collagen tubes (1 mm inner diameter). The specimen was attached to a force gauge (Jonard) at one end, and pulled at the other end at a rate of 1 mm/s until the specimen broke. The ultimate strength was measured as the strength at failure in grams-force (gF).
Anastomotic strength was measured for anastomosed dense collagen tubes (1 mm inner diameter) and for anastomosed dense collagen tubes (0.5 mm inner diameter) and rat femoral arteries in a similar manner.
Suture retention strength was measured for flat gels and intact tubes by first cutting one end of the sample with a razor blade to expose a flat edge. A 10-0 suture was then thrown 1.5 mm from the edges and a single square knot was tied. The suture was secured to a force gauge and the specimen was pulled at 1 mm/s. The maximum load achieved was measured in gF.
All tensile tests were performed at room temperature.
Burst and compliance tests
Burst pressure was measured for intact dense collagen tubes (1 mm inner diameter). One end of the tubular sample was cinched onto a 20-gauge needle with 6-0 prolene suture and overlap of 3 mm, and the other end was clamped shut with a hemostat. The needle was connected to pressurized nitrogen gas through a plastic tube (inner diameter of 1/16 inch) that was filled with PBS. The pressure of the system was gradually increased until the sample burst.
Compliance was measured for dried-and-crosslinked tubes of 0.5 mm inner diameter. Tubes were pressurized as described above in five increments from 0 to 477 mm Hg. Compliance was calculated by linear regression between pressure and percentage change in outer diameter.
All burst and compliance tests were performed at room temperature.
Collagenase resistance assay
The biochemical stability of dried and dried-and-crosslinked tubes of 0.5 mm inner diameter was determined by assessing their resistance to digestion by collagenase. Collagen tubes were exposed to 30 U/mL bacterial collagenase (type I from Clostridium histolyticum, dissolved in Dulbecco's modified Eagle's medium; Worthington) for 4 h at 37°C, and the digested tubes were dried and weighed every 30 min.
Cytotoxicity assay
Dried-and-crosslinked collagen tubes (1 mm inner diameter) were conditioned in media overnight. A suspension of 50 million HUVECs/mL was injected into the lumen of the collagen tube. The tubes were incubated at 37°C for 10 min to allow cells to adhere, and were then washed with fresh media to remove nonadherent cells. Seeded tubes were cultured at 37°C, and were assessed for confluence and viability of endothelial cells 2 and 7 days after seeding. Seeded tubes were cut open lengthwise and the inner lumen was stained with 10 μg/mL Hoechst 33342, 20 μg/mL calcein AM (Invitrogen), and 10 μM ethidium homodimer-1 (Invitrogen) for 30 min.
Scanning electron microscopy
Scanning electron microscopy (SEM) was used to examine the ultrastructure of dried-and-crosslinked collagen tubes (0.5 mm inner diameter). The rehydrated sample was fixed in 2.5% glutaraldehyde for 1 h, dehydrated with series exchange of ethanol, and dried with series exchange of hexamethyldisilazane. The dried sample was sputter coated with gold and imaged with a scanning electron microscope (Zeiss Supra 55VP).
In vivo implantation of collagen tubes as interpositional arterial graft
The right femoral arteries of two Sprague-Dawley rats were exposed using the same procedure for vessel harvest. The blood flow in the femoral artery was temporarily stopped with a microvascular clamp. A 5-mm-long segment of femoral artery was explanted, exposing two artery stubs. Each artery stub was flushed with heparinized saline and had adventitia trimmed. A dried-and-crosslinked collagen tube (0.5 mm inner diameter) was anastomosed to the artery stubs with four stay sutures, two on either end, followed by 12 interrupted sutures. Implants were performed with tubes that had been rehydrated. After all 16 sutures were tied, the microvascular clamp was released to restore blood flow in the femoral circulation. The ipsilateral epigastric fat pad was used to aid in hemostasis.19 Blood flow in the collagen tube was confirmed by milking the distal anastomosis as described by Acland.20 Rats were sacrificed 20 min after establishing blood flow.
Statistical analysis
All statistical tests were performed using Prism version 5 software (GraphPad). Mann–Whitney U test was performed for each comparison. For single pairwise comparisons, a p < 0.05 was considered to be statistically significant. When multiple comparisons were made, a p < 0.01 was considered to be statistically significant. Comparisons with the ultimate strength of native or crosslinked gels (which were effectively zero) used Wilcoxon signed-rank test, with a significance condition of p < 0.01.
Results
Native collagen gels did not withstand suturing
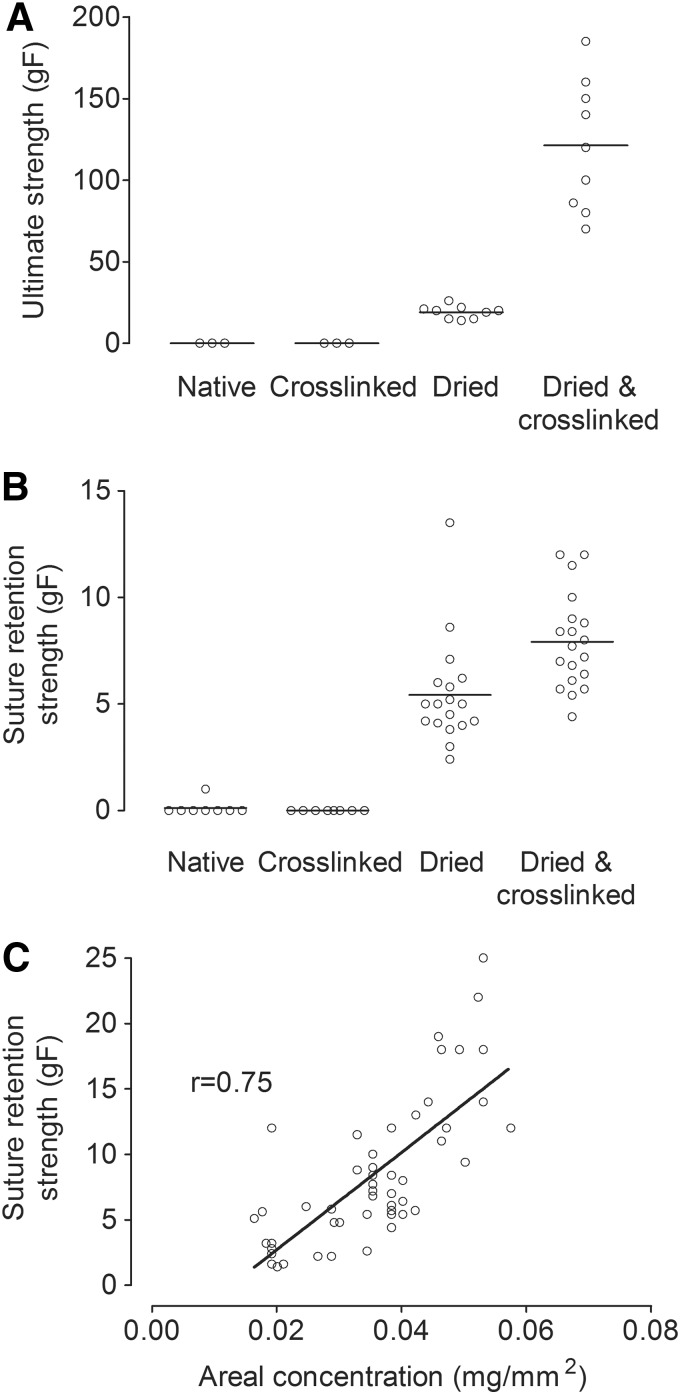
Twenty microliters of neutralized collagen mixture were used to produce flat 12 × 7 × 0.2 mm3 slabs of native collagen gel, with the thickness of 0.2 mm approximating that of a human radial arterial wall.21 In this highly hydrated state, the collagen gel was too fragile to be handled. Puncturing the gel with the curved needle from a 10-0 suture invariably tore apart the entire gel. Thus, the ultimate tensile strength and suture retention strength were effectively zero for native gels (Fig. 3A, B); in only one of eight samples could we measure a nonzero suture retention strength.
FIG. 3.
Screening for collagen gel treatments that favor suturability. (A) Ultimate strengths and (B) suture retention strengths for flat collagen gels of four gel preparation conditions (native, crosslinked, dried, and dried-and-crosslinked). (C) Plot of suture retention strength versus areal collagen concentration for dried-and-crosslinked gels.
Drying followed by crosslinking increased strength of flat collagen gels
Flat collagen gels were used to screen for preparation conditions that yielded the greatest mechanical strength. Flat 0.2-mm-thick collagen gels were compared for ultimate strength and suture retention strength in four preparation conditions: native, crosslinked, dried, and dried-and-crosslinked. To obtain similar final thicknesses, 20 μL of neutralized collagen mixture were used to produce native and crosslinked gels, and 400 μL were used to produce dried and dried-and-crosslinked gels. All gels were tested in their rehydrated state. Even after crosslinking with genipin, collagen gels that had not been dried could not be sutured, and their ultimate strength was effectively zero. Dried collagen gels of the same thickness showed significant increases in both ultimate strength (19.1 ± 3.9 gF, p = 0.009) and suture retention strength (5.4 ± 2.5 gF, p = 0.0002) compared with native gels that had not been dried. Unlike native gels, dried gels displayed significant increases in ultimate strength (121.2 ± 40.0 gF, p = 0.0004) and suture retention strength (7.9 ± 2.2 gF, p = 0.0006) upon crosslinking (Fig. 3A, B).
Suture retention strength positively correlated with areal concentration of collagen
To better understand the factors that control suture retention strength, we correlated the measured strengths with the areal concentration of collagen (i.e., mass of collagen per unit area of scaffold). Our reasoning was that increasing the density of collagen fibers or increasing the thickness of the collagen gel should independently and synergistically result in higher suture retention strength. Areal concentration quantifies the amount of collagen that resists suture pullout, since it equals the density of collagen fibers multiplied by the thickness (or wall thickness, in the case of collagen tubes) of the sample. Suture retention strength was measured on dried-and-crosslinked flat gels made with 0.2–0.6 mL of neutralized collagen mixture, which corresponded to hydrated gel thickness of 0.1–0.5 mm. Spearman's correlation between areal concentration and suture retention strength yielded a correlation coefficient of 0.75 (p < 0.0001) (Fig. 3C).
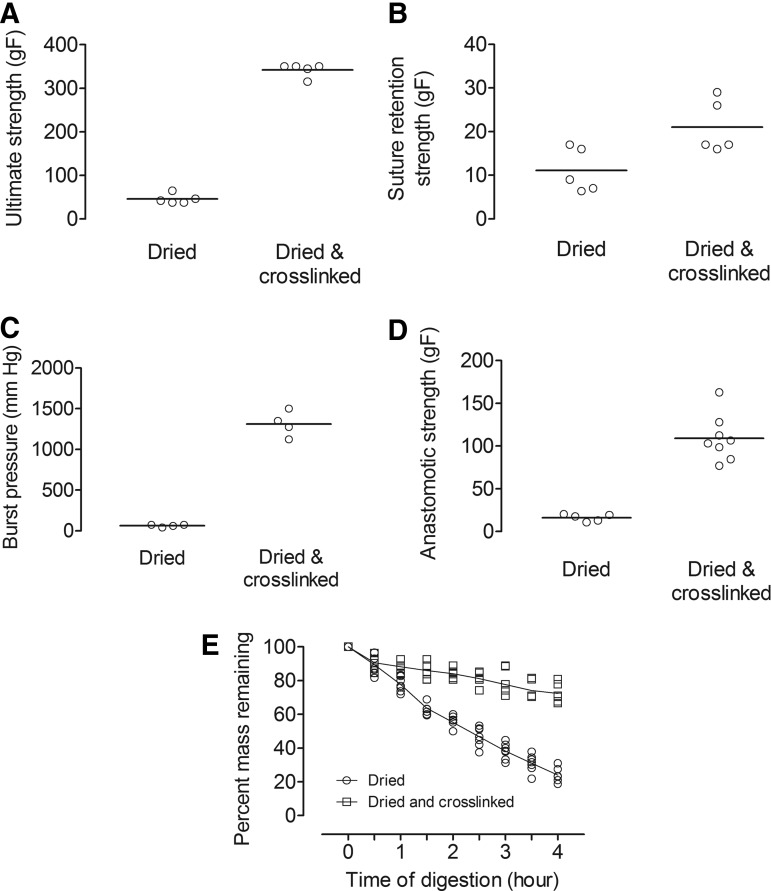
Drying and crosslinking collagen tubes yielded strengths and biochemical stability far greater than those of dried tubes
Based on the results from testing flat gels, collagen tubes of ∼1 mm inner diameter were prepared under either dried or dried-and-crosslinked condition by molding gels around a thin mandrel and then dehydrating (and in some cases, crosslinking) them (Figs. 1 and 2A). The wall thickness of these tubes was 0.45 ± 0.19 mm for dried gels and 0.28 ± 0.10 mm for dried-and-crosslinked gels. Dried-and-crosslinked tubes yielded significantly higher ultimate tensile strength (342.1 ± 15.1 gF vs. 45.7 ± 11.3 gF, p = 0.0079), suture retention strength (21.0 ± 6.0 gF vs. 11.1 ± 5.1 gF, p = 0.044), anastomotic strength (109.1 ± 26.8 gF vs. 16.2 ± 4.1 gF, p = 0.0016), and burst pressure (1313 ± 156 mm Hg vs. 63 ± 16 mm Hg, p = 0.029) than for collagen tubes that were only dried (Fig. 4). Surprisingly, even though crosslinking caused a doubling of suture retention strength, it resulted in nearly order of magnitude increases in ultimate strength, anastomotic strength, and burst pressure.
FIG. 4.
Characterization and comparison of dried and dried-and-crosslinked collagen tubes. (A) Ultimate tensile strengths. (B) Suture retention strengths. (C) Burst pressures. (D) Anastomotic strengths. (E) Percent mass remaining after collagenase digestion.
Crosslinking also significantly increased the stability of collagen tubes. After being digested for 4 h in 30 U/mL collagenase, dried and dried-and-crosslinked tubes (inner diameter of 0.5 mm) had 24.0% ± 4.1% and 72.4% ± 5.7% (p = 0.0034) residual mass, respectively (Fig. 4E).
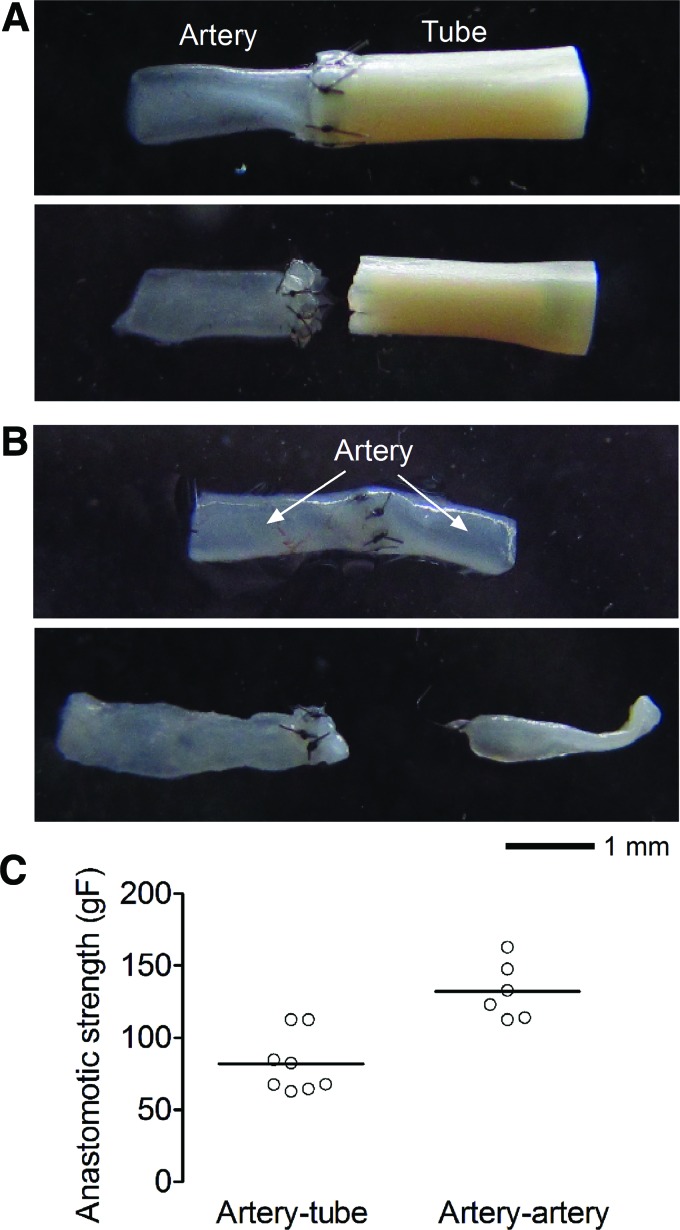
Anastomosis of artery and collagen tube was robust and withheld >80 gF tension
As the mechanical strengths of dried collagen tubes were significantly lower than those of dried-and-crosslinked collagen tubes, only the latter were used for artery-to-collagen tube anastomosis. The dimensions of the tubes were adjusted to ∼0.5 mm inner diameter and ∼1 mm outer diameter to better match the size of rat femoral artery (Fig. 5A). The artery-to-collagen tube anastomotic strength was 81.9 ± 20.6 gF, which was lower than the 132.1 ± 19.9 gF we obtained from artery-to-artery anastomosis (p = 0.003) (Fig. 5C). Nevertheless, the artery–tube anastomosis displayed strengths that should suffice for surgical implantation as an interpositional graft.22 We noticed that the artery–artery anastomosis always failed at one end of the artery (i.e., the artery tore apart; Fig. 5B), whereas the artery–tube anastomosis always failed with suture tearing through the collagen tube (Fig. 5A). This finding indicated that the dried-and-crosslinked collagen tube is weaker than the native artery.
FIG. 5.
Anastomosis of explanted rat femoral arteries and collagen tubes. (A) Artery–tube and (B) artery–artery anastomosis before (top) and after (bottom) failure. (C) Plot of artery–tube and artery–artery anastomotic strengths. Color images available online at www.liebertpub.com/tea
Endothelial cells grew to confluence in the lumen of collagen tubes
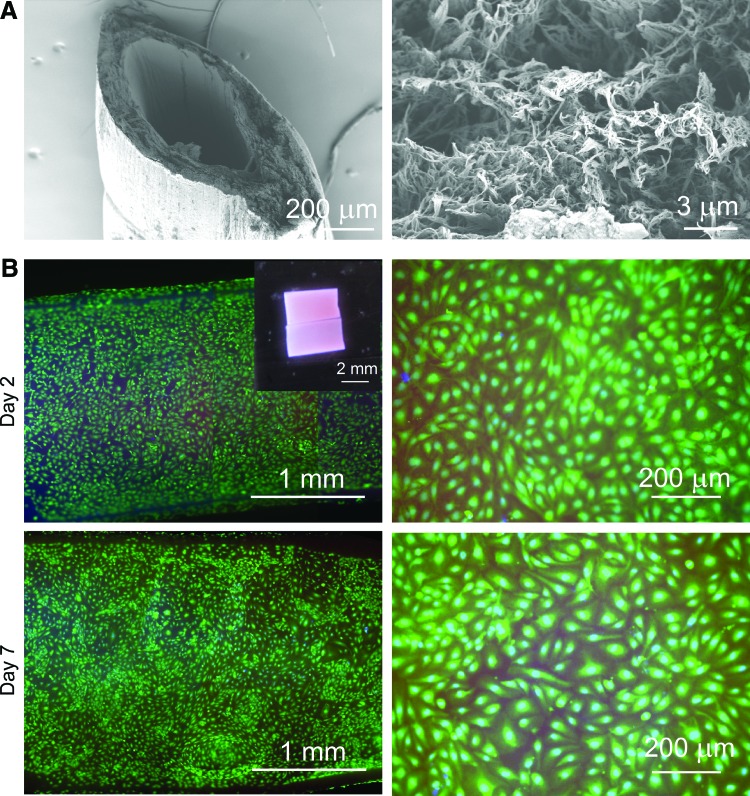
SEM showed that the tubes consisted of dense fibrous mats (Fig. 6A). The lumen surface was particularly dense, which should provide a favorable environment for endothelial cell adhesion and growth. Indeed, the live/dead stains of the lumen of seeded collagen tubes showed that confluent endothelial coverage could be achieved 2–3 days after seeding. Furthermore, confluence was maintained up to 7 days, and almost all endothelial cells remained viable (Fig. 6B).
FIG. 6.
Endothelialization of dried-and-crosslinked collagen tubes. (A) Scanning electron micrographs of tube wall and lumen surface. (B) Representative nuclear and viability stains of endothelial cells in the lumen of collagen tubes at day 2 and 7; images at low and high magnification are shown. Inset, a seeded collagen tube that was cut open for imaging. Color images available online at www.liebertpub.com/tea
Dried-and-crosslinked tube successfully withstood implantation and supported blood flow
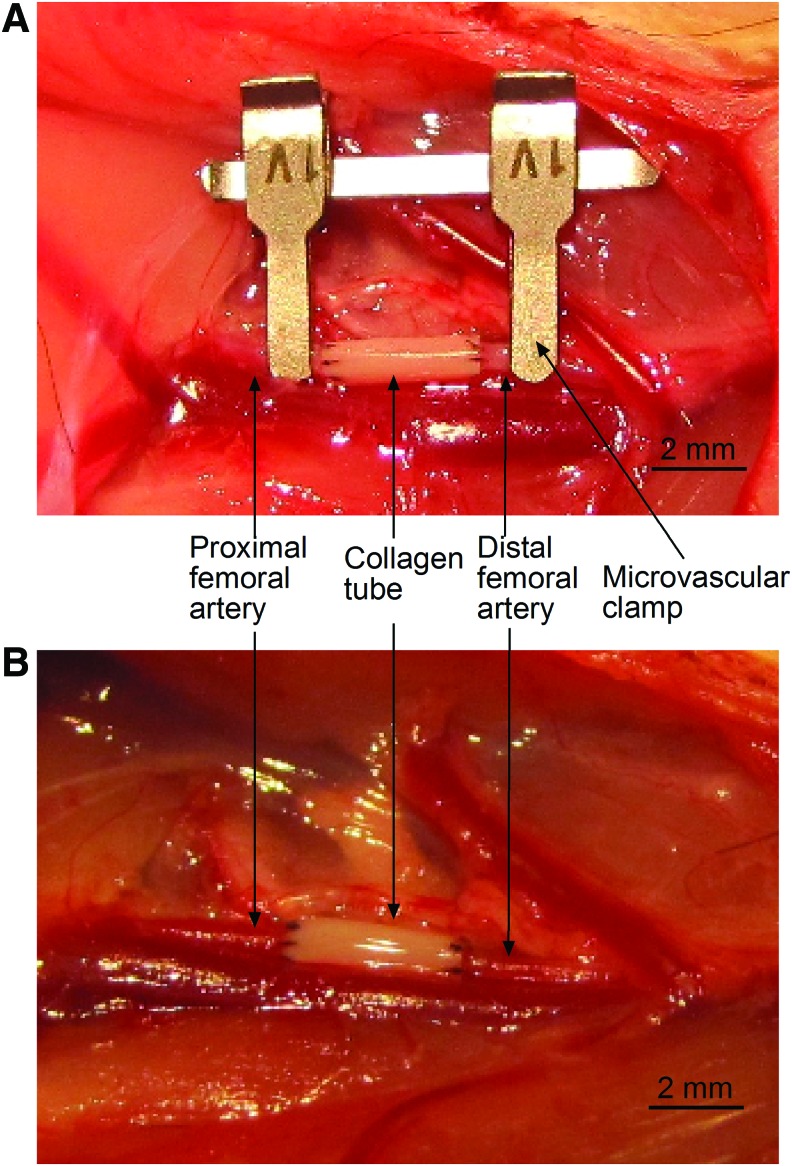
To determine whether our tubes could withstand in vivo arterial flow, we implanted unseeded dried-and-crosslinked tubes as interpositional grafts in the rat femoral circulation (Fig. 7A). After releasing the microvascular clamp, the anastomosis proved to be strong enough to hold arterial flow (Fig. 7B). Pulsation was observed when femoral circulation was restored and for up to 20 min (when the rats were sacrificed), indicating flow in the collagen tube. During this period, patency was further confirmed by performing the Acland milking test,20 where we observed refilling of the distal femoral artery after emptying it of blood.
FIG. 7.
Images of an implanted dried-and-crosslinked collagen tube before (A) and after (B) release of microvascular clamp. Color images available online at www.liebertpub.com/tea
Although (as mentioned earlier) compliance matching between graft and vessel is less crucial at the microsurgical level, we measured the compliance of dried-and-crosslinked tubes, and obtained a value of 1.72% ± 0.42%/100 mm Hg.
Discussion
In the current study, we formed collagen tubes with ≤1 mm inner diameter that could withstand anastomosis and arterial pressurization and support endothelial cell adhesion and growth. We found that condensing native, hydrated collagen tubes by nearly 100-fold through dehydration, followed by rehydration and crosslinking for 2 h with genipin yielded a scaffold with ultimate tensile strength, burst pressure, and anastomotic strength comparable to those of a rat femoral artery of similar diameter. Furthermore, endothelial cells were able to grow to confluence in these tubes and maintain patency for 7 days. To our knowledge, this result represents the first collagen-based tubular scaffold of ≤1 mm diameter that could be microsurgically anastomosed with sufficient strength for implantation in vivo using a standard end-to-end suturing technique without a need for external mechanical support.
Early attempts to form vascular grafts from highly hydrated collagen were not able to produce mechanically resilient constructs that could withstand handling and suture, even with crosslinking.12,23 Indeed, we were not able to put a suture through native collagen gels that were 0.2 mm in thickness without tearing the rest of the gel. Genipin, a less cytotoxic crosslinker than formaldehyde,23 has been shown to increase the mechanical strength of collagen scaffolds and tissue. Nevertheless, crosslinking native gel with genipin did not yield suturable constructs, either.
Recent studies showed that dense tubular collagen could be produced in hours by rolling up a compressed sheet, and that these tubes exhibited mechanical properties comparable to those of native vessels.17,18 In the current study, we showed that drying a rectangular block of hydrated collagen gel into a flat sheet increased the collagen density by ∼20-fold, which is comparable to the result obtained by Nazhat and coworkers.17 A 0.2-mm-thick dense collagen sheet yielded ∼20 gF in ultimate strength and ∼5 gF in suture retention strength. Crosslinking a dried collagen gel further increased ultimate strength and suture retention strength to ∼120 and 8 gF, respectively. It should be noted that in the current study, the suture retention strengths were measured with 10-0 suture instead of the much thicker 4-0 suture used by previous groups. This difference may partly explain why the suture retention strengths we obtained were one order of magnitude lower than those reported by other groups (∼100 gF); differences in the scaffold thickness and measurement technique may also play a role.
In previous studies, hydrated collagen was either compressed or dried into a sheet, and was subsequently rolled around a mandrel to produce a tubular construct.17,18 Despite the claim that there was no void space in rolled sheets, the published micrographs appeared to show separation between layers in the collagen.17 Chaikof and coworkers improved the design by applying recombinant elastin-like protein between collagen layers, followed by liquefying and regelling the scaffold to fill the void space.18 Very recently, Hu and coworkers showed that vacuum-assisted compression could greatly reduce the void space in rolled sheets.24 In the current study, we dried tubular collagen directly on the mandrel, followed by rehydration. The resulting single-layered collagen tubes were monolithic, which proved to be easier to suture compared with layered tubes. In preliminary experiments using multilayered tubes, we found that it was often easy to split the layers when puncturing the tube with sutures (data not shown).
Drying a tubular collagen gel as a whole piece yielded higher density than rolling up a dried collagen sheet. While we obtained density of 14 wt.% for flat collagen sheets, similar to the result by Nazhat and coworkers,17 our tubular collagen had density of up to 30 wt.%. The difference likely results from constraints on the dehydration process that differ between the two gel geometries. Since the collagen gel contracts perpendicular to a contact surface but not along it, the tubular form (which has large outer to inner diameter ratio before drying) resulted in higher density increase. Dried collagen tubes yielded ultimate tensile stress (UTS) of 0.21 ± 0.11 MPa, which is comparable to the result obtained by other groups.17,18 The UTS of dried-and-crosslinked tubes was 2.1 ± 0.6 MPa, which was much closer to the value of mammalian arteries (1.4–11.1 MPa for mammalian artery).18
The burst pressure of dried collagen tubes did not exceed 75 mm Hg, and we, therefore, did not further consider this preparation condition to be suitable for artery–tube anastomosis. On the other hand, dried-and-crosslinked collagen tubes yielded average burst pressures of ∼1300 mm Hg, which is comparable to the value obtained by Chaikof and coworkers for tubes of 1.3 mm inner diameter.18 While this value is still shy of the burst pressure of native arteries (which can be as high as 3000 mm Hg)25, it is close to the value for native vein (∼1200 mm Hg)26 and more than sufficient to withstand pressure of arterial flow. Compliance of dried-and-crosslinked tubes averaged ∼1.7%/100 mm Hg, which is comparable to the value (0.7–2.6%/100 mm Hg) of mammalian vein.18
As expected, crosslinked collagen scaffolds were significantly more resistant to collagenase degradation than uncrosslinked scaffolds were. Nevertheless, it is difficult to extrapolate from collagenase digestion rates to in vivo stability; we note that other studies of crosslinked collagen-based scaffolds have obtained similar mass loss rates.27 Future studies with long-term in vivo implantation will be needed to better assess the stability of our crosslinked tubes.
We note that the artery–tube anastomotic strength was lower than that of artery–artery anastomosis. Although arteries are considered the gold standard for vascular grafts, the value we obtained for artery–tube anastomotic strength should be sufficient for microsurgical anastomosis.22 Indeed, when dried-and-crosslinked collagen tubes were implanted as interpositional graft in the rat femoral circulation, they were able to sustain arterial flow, with short-term patency confirmed by the Acland milking test and presence of pulsation.
In the current study, we made a few modifications to facilitate end-to-end anastomosis of collagen tubes. In standard microsurgical suture anastomosis, two stay sutures are first tied at opposite points on the vessel circumference, and pulled apart to create a fishmouth field that immobilizes the apposing ends and aids in placement of the remaining sutures.28 Because the collagen tubes were much less elastic than vessels were, we did not pull the stay sutures and risk dissection of the tube wall. Instead, the collagen tubes were anastomosed as a free graft between artery stubs without extra stabilization. It should also be noted that the native vessels in the current study were directly anastomosed to collagen scaffold with suture, which is the current clinical standard for graft implantation. To the best of our knowledge, nearly all other studies that claim anastomosis between an engineered collagen-based scaffold and native vessels ≤1 mm outer diameter used a polymer mesh to reinforce the anastomosis,29 or surgical glue in place of suture.26
Only a few groups have engineered implantable vascular grafts at the ≤1 mm scale. Narita and coworkers reported making grafts of 0.7 mm inner diameter from poly-ɛ-caprolactone.30,31 Nakayama and coworkers developed biotubes of 0.7 mm inner diameter by using a natural in vivo foreign body encapsulation process.32 Yim and coworkers produced multilayered poly(vinyl alcohol) with 0.9–1.0 outer diameter.33 While these grafts also achieved mechanical strengths suitable for implantation, the methods of Narita and Yim lacked preformed endothelial layers, and the method of Nakayama was inherently slow (8 weeks to create one tube). Lack of complete endothelialization leads to early thrombus formation and is the major mode of failure of small-caliber vascular graft.2 Although we did not implant endothelialized tubes in this study, we did demonstrate that seeding the collagen tubes with endothelial cells led to full endothelial coverage within 7 days as expected, given the reported biocompatibility of genipin-treated collagen scaffold.34 It should be noted that the endothelium in the experiments was not subjected to rough surgical handling; we expect some endothelium to be removed during an implantation procedure.
The logical next step is to implant these endothelialized collagen tubes as interpositional grafts in vivo. Our in vitro, ex vivo, and in vivo data have demonstrated that this scaffold design has sufficient mechanical strength and endothelial coverage for implantation. We believe this design provides a straightforward and promising route to ≤1-mm-diameter vascular grafts for peripheral revascularization, and possibly for connecting engineered tissues to a host circulation.
Acknowledgments
The authors thank Dr. Yelena Akelina (Columbia University Medical Center) for microsurgical advice, Le Li and Dr. Kamil Ekinci (Boston University) for assistance with SEM, and Tyler Ryan, Dr. John Jiang, Dr. Ed Damiano, Dr. Mark Grinstaff, and Dr. Li Liu (Boston University) for experimental assistance. This work was supported by award EB018851 and the Translational Research in Biomaterials training program (award EB006359) from the National Institute of Biomedical Imaging and Bioengineering, and by the Undergraduate Research Opportunities Program and a Clare Boothe Luce Scholarship (to J.T.M.) at Boston University.
Disclosure Statement
No competing financial interests exist.
References
- 1.L'Heureux N., Dusserre N., Marini A., Garrido S., de la Fuente L., and McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med 4, 389, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Isenberg B.C., Williams C., and Tranquillo R.T. Small-diameter artificial arteries engineered in vitro. Circ Res 98, 25, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Koch S., Flanagan T.C., Sachweh J.S., Tanios F., Schnoering H., Deichmann T., Ella V., Kellomaki M., Gronloh N., Gries T., Tolba R., Schmitz-Rode T., and Jockenhoevel S. Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation. Biomaterials 31, 4731, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Niklason L.E., Gao J., Abbott W.M., Hirschi K.K., Houser S., Marini R., and Langer R. Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., and Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60, 1353, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Friedrich J.B., Poppler L.H., Mack C.D., Rivara F.P., Levin L.S., and Klein M.B. Epidemiology of upper extremity replantation surgery in the United States. J Hand Surg Am 36, 1835, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Ballyk P.D., Walsh C., Butany J., and Ojha M. Compliance mismatch may promote graft-artery intimal hyperplasia by altering suture-line stresses. J Biomech 31, 229, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Black M.J., Chait L., O'Brien B.M., Sykes P.J., and Sharzer L.A. How soon may the axial vessels of a surviving free flap be safely ligated: a study in pigs. Br J Plast Surg 31, 295, 1978 [DOI] [PubMed] [Google Scholar]
- 10.Wise S.R., Harsha W.J., Kim N., and Hayden R.E. Free flap survival despite early loss of the vascular pedicle. Head Neck 33, 1068, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Kissun D., Shaw R.J., and Vaughan E.D. Survival of a free flap after arterial disconnection at six days. Br J Oral Maxillofac Surg 42, 163, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Weinberg C.B., and Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 231, 397, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Hirai J., and Matsuda T. Self-organized, tubular hybrid vascular tissue composed of vascular cells and collagen for low-pressure-loaded venous system. Cell Transplant 4, 597, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Grassl E.D., Oegema T.R., and Tranquillo R.T. A fibrin-based arterial media equivalent. J Biomed Mater Res A 66, 550, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Swartz D.D., Russell J.A., and Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 288, H1451, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Girton T.S., Oegema T.R., Grassl E.D., Isenberg B.C., and Tranquillo R.T. Mechanisms of stiffening and strengthening in media-equivalents fabricated using glycation. J Biomech Eng 122, 216, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Ghezzi C.E., Marelli B., Muja N., and Nazhat S.N. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater 8, 1813, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Kumar V.A., Caves J.M., Haller C.A., Dai E., Liu L., Grainger S., and Chaikof E.L. Acellular vascular grafts generated from collagen and elastin analogs. Acta Biomater 9, 8067, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akelina Y., and Danilo P. Endogenous adipose tissue as a hemostatic: use in microsurgery. Microsurgery 28, 192, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Acland R. Signs of patency in small vessel anastomosis. Surgery 72, 744, 1972 [PubMed] [Google Scholar]
- 21.Myredal A., Osika W., Li Ming G., Friberg P., and Johansson M. Increased intima thickness of the radial artery in patients with coronary heart disease. Vasc Med 15, 33, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Colen L.B., Gonzales F.P., and Buncke H.J. The relationship between the number of sutures and the strength of microvascular anastomoses. Plast Reconstr Surg 64, 325, 1979 [DOI] [PubMed] [Google Scholar]
- 23.Madhavan K., Belchenko D., Motta A., and Tan W. Evaluation of composition and crosslinking effects on collagen-based composite constructs. Acta Biomater 6, 1413, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Tuan-Mu H.Y., Lu P.C., Lee P.Y., Lin C.C., Chen C.J., Huang L.L., Lin J.H., and Hu J.J. Rapid fabrication of a cell-seeded collagen gel-based tubular construct that withstands arterial pressure: rapid fabrication of a gel-based media equivalent. Ann Biomed Eng 44, 3384, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Konig G., McAllister T.N., Dusserre N., Garrido S.A., Iyican C., Marini A., Fiorillo A., Avila H., Wystrychowski W., Zagalski K., Maruszewski M., Jones A.L., Cierpka L., de la Fuente L.M., and L'Heureux N. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials 30, 1542, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B., Montgomery M., Chamberlain M.D., Ogawa S., Korolj A., Pahnke A., Wells L.A., Masse S., Kim J., Reis L., Momen A., Nunes S.S., Wheeler A.R., Nanthakumar K., Keller G., Sefton M.V., and Radisic M. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat Mater 15, 669, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helling A.L., Tsekoura E.K., Biggs M., Bayon Y., Pandit A., and Zeugolis D.I. In vitro enzymatic degradation of tissue grafts and collagen biomaterials by matrix metalloproteinases: improving the collagenase assay. ACS Biomater Sci Eng 2016. DOI: 10.1021/acsbiomaterials.5b00563 [DOI] [PubMed] [Google Scholar]
- 28.Cooley B. A Laboratory Manual for Microvascular and Microtubal Surgery. Reading, MA: Surgical Specialties Corporation, 2001 [Google Scholar]
- 29.Hooper R.C., Hernandez K.A., Boyko T., Harper A., Joyce J., Golas A.R., and Spector J.A. Fabrication and in vivo microanastomosis of vascularized tissue-engineered constructs. Tissue Eng Part A 20, 2711, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuwabara F., Narita Y., Yamawaki-Ogata A., Kanie K., Kato R., Satake M., Kaneko H., Oshima H., Usui A., and Ueda Y. Novel small-caliber vascular grafts with trimeric Peptide for acceleration of endothelialization. Ann Thorac Surg 93, 156, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Kuwabara F., Narita Y., Yamawaki-Ogata A., Satake M., Kaneko H., Oshima H., Usui A., and Ueda Y. Long-term results of tissue-engineered small-caliber vascular grafts in a rat carotid arterial replacement model. J Artif Organs 15, 399, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Ishii D., Enmi J.I., Moriwaki T., Ishibashi-Ueda H., Kobayashi M., Iwana S., Iida H., Satow T., Takahashi J.C., Kurisu K., and Nakayama Y. Development of in vivo tissue-engineered microvascular grafts with an ultra small diameter of 0.6 mm (MicroBiotubes): acute phase evaluation by optical coherence tomography and magnetic resonance angiography. J Artif Organs 19, 262, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Cutiongco M.F., Kukumberg M., Peneyra J.L., Yeo M.S., Yao J.Y., Rufaihah A.J., Le Visage C., Ho J.P., and Yim E.K. Submillimeter diameter poly(vinyl alcohol) vascular graft patency in rabbit model. Front Bioeng Biotechnol 4, 44, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C., Lau T.T., Loh W.L., Su K., and Wang D.A. Cytocompatibility study of a natural biomaterial crosslinker—genipin with therapeutic model cells. J Biomed Mater Res B Appl Biomater 97, 58, 2011 [DOI] [PubMed] [Google Scholar]