ABSTRACT
Lysine acetylation is known as a post translational modification (PTM) by histone acetyltransferases (HAT) that modifies histones and non-histone proteins to regulate gene expression. Serine acetylation, however, is reported in mammalian hosts by serine acetyltransferase of Yersinia pestis (YopJ) during infection. The protein target and cellular function of bacterial YopJ in mammalian systems are not fully addressed. Here we report dual acetylation at the serine and lysine residues by transiently expressed serine acetyltransferase YopJ mimicking Y. pestis infection in HeLa cells. Using shotgun proteomics followed by label-free quantification, we demonstrate an increase of dual acetylation in YopJ transfected human cells, including 10 Ser- (YopJ/non-YopJ 1.3-fold, p = 0.02) and 8 Lys- (YopJ/non-YopJ 3.5-fold, p = 0.00003) acetylation sites. Specifically, YopJ expression augments acetylation of membrane-associated E3 ubiquitin ligase MARCH8 at the serine residue Sac44, Sac71 and Sac253, and the lysine residue Kac247 and Kac252. YopJ-mediated Ser- and Lys-acetylation of MARCH8 is further confirmed by Western blotting using the specific antibodies against MARCH8 Sac71 and pan-acetyl lysine. Functional study demonstrates that YopJ-mediated Ser- and Lys-acetylation affects the auto-ubiquitination of MARCH8. The mutant C172A of YopJ previously shown to abolish the acetyltransferase activity also reduces Ser- and Lys-acetylation and diminishes the auto-ubiquitination of MARCH8. In support, MARCH8 is indeed acetylated at serine and lysine in vitro by purified YopJ but the activity is reduced by the C172A mutant in YopJ. Our study provides evidence that bacterial serine acetyltransferase YopJ mediates Ser- and Lys-acetylation and affects auto-ubiquitination of ubiquitin ligase MARCH8 in human cells.
KEYWORDS: acetylation, acetyltransferase, histone deacetylase, infection, ubiquitination, Yersinia pestis
Introduction
Human Yersinia pestis infection causes the deadly disease called bubonic plague commonly spreading through rodents and fleas.1,2 To establish infection, Y. pestis expresses multiple outer membrane proteins that are secreted and adhesive to host epithelial cells capable of suppressing or resisting host immune responses by various mechanisms.1,2 Generally, Yersinia outer proteins (Yops) are effectors counteracting host innate immune responses including the pro-inflammatory signaling pathways of mitogen-activated protein (MAP) kinase (MAPK) and nuclear factor Kappa B (NF-κB).3,4 Among them, YopJ is a 32 kD protein with 288 amino acids known to block cytokine production and inducing apoptosis of the infected cells. YopJ is also a serine acetyltransferase5,6 that is known to counteract the aforementioned inflammatory responses by acetylation of the serine residues in I kappa B kinase (IKK) and MAPK kinases (MKKs). YopJ-mediated serine acetylation is shown to prevent serine phosphorylation, and subsequently block MAPK signaling and NF-κB activation, leading to significantly reduced production of both pro-inflammatory and anti-apoptotic host cytokines.5,6 YopJ is also reported to inhibit NF-κB activation and pro-inflammatory cytokine production by reducing the activation of eukaryotic initiation factor 2 (eIF2) in yeast and mammalian cells.7 Thus, YopJ-mediated serine acetylation has a great impact on the innate immune responses of the infected hosts.
Notably, the endogenous mammalian counterpart of serine acetyltransferase is not yet identified, although low-level of serine acetylation on histone H3 is reported in human cells.8 More recently, 2 proteomics-based studies have detected serine acetylation on non-histone proteins in higher eukaryotes,9,10 suggesting the existence of serine/threonine acetyltransferases and their possible roles in human systems.
Lys-acetylation is a well-known post translational modification (PTM) in higher eukaryotes and has a great impact on a wide range of biological processes such as metabolism, RNA modification, nuclear morphology, gene expression and mitochondrial function.11,12 Lysine acetyltransferases and their counterparts for the reverse reaction, best-known as histone acetyltransferases (HATs) and histone deacetylases (HDACs), act cooperatively to tightly regulate the acetylation of histone and non-histone proteins for gene expression, cell development and cancer development11-13 However, the interaction between lysine and serine acetyltransferases has not been examined.
In this study, we aim to characterize the molecular effects of bacterial acetyltransferase YopJ on serine and Lys-acetylation by mimicking Y. pestis infection in human cells. Using shotgun proteomics and label-free quantification, acetylation at the serine and lysine residues with and without YopJ are identified and compared. Specifically, Ser- and Lys-acetylation at different positions of membrane-associated E3 ubiquitin ligase MARCH8 was characterized. Specific antibodies directed against MARCH8 acetylated serine residues were produced. Immunoassays were used to confirm YopJ-induced Ser- and Lys-acetylation in MARCH8. YopJ catalytic mutant study indicated that YopJ-induced Ser- and Lys-acetylation in MARCH8 was dependent on its catalytic activity. Our data support the conclusion that bacterial acetyltransferase YopJ targets the protein substrates for acetylation at both the lysine and serine residues in the host cells.
Results
Bacterial serine acetyltransferase YopJ mediates protein acetylation at the serine and lysine residues in HeLa cells
To characterize protein acetylation by bacterial serine acetyltransferase, recombinant YopJ was transiently expressed in human HeLa cells (Fig. 1a). Cell lysates with or without YopJ expression were prepared, proteins were digested by trypsin in solution, and the peptides were analyzed by LC-MS/MS. The acquired MS/MS spectra (Supplemental Data: massive 000080123 raw dataVe1–3, Y1–3) were searched against the human protein database with dynamic acetylation at the serine and lysine residues by Proteome Discoverer 1.4 with Mascot and Sequest search engines. A total of 10,759 high confident peptides were identified that were grouped into 1418 proteins (Supplemental Data: massive 000080123/ YopJcellular-KS.msf, Supplemental Table 1). For label-free quantification, 9487 peptides were selected by the Progenesis LC-MS software (Score > 30, FDR < 0.01, Supplemental Table 2), 47 of which were acetylated (Supplemental Table 3). Among the acetylated peptides, 10 were found at Lys (8 internal, 2 N-termini of protein), and 37 at Ser (10 internal, 27 N-termini of protein). For instance, acetylation was found at the second serine of the peptide A(Sac)GVAVSDGVIK (YopJ/non-YopJ = 1.6, p = 0.04, Fig. 1b) and the third lysine of the peptide AD(Kac)EAAFDDAVEER (YopJ/non-YopJ = 1.7, p = 0.03, Fig. 1c). The acetylated serine (Sac) in the first peptide was determined by the b2 ion and intensified by the b3-b11, y2-y10 ions, and further defined by the y11 ion. Similarly, the acetylated lysine (Kac) in the second peptide was determined by the b3–13 ions and intensified by the y2–11 ions, and further defined by the y13 ion.
Figure 1.
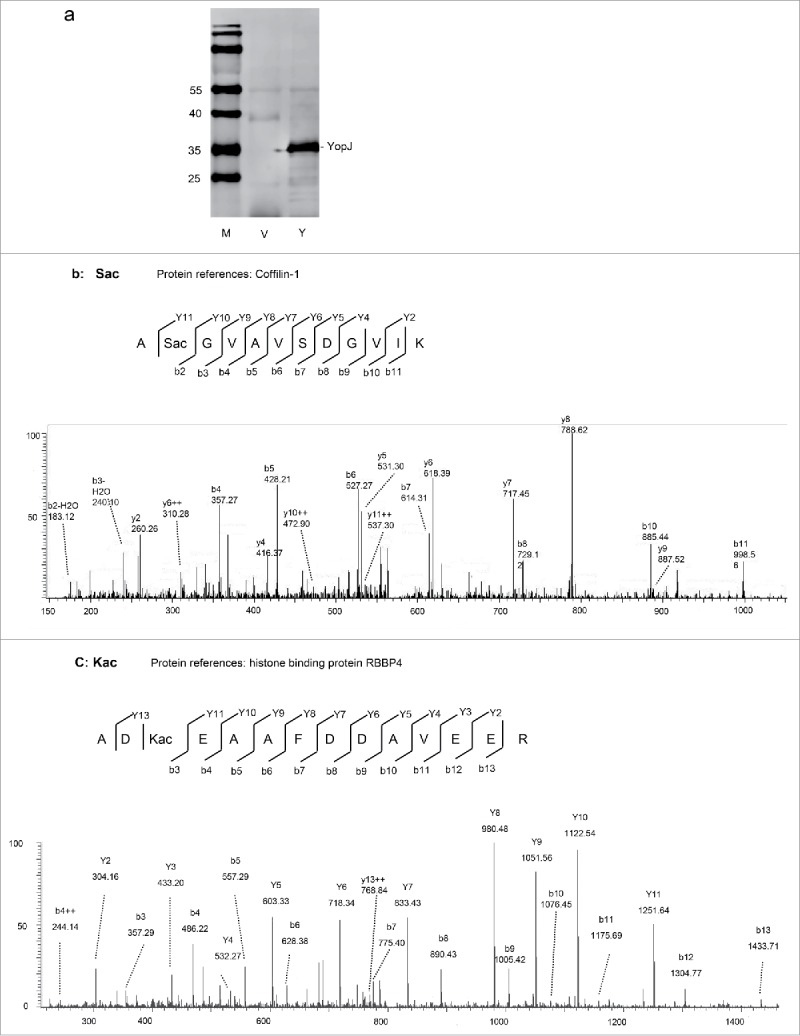
YopJ-mediated protein acetylation at the serine and lysine residues in HeLa cells. (a) Western blotting of HeLa cell lysates with transiently expressed YopJ (Y) or control (V). The blots were detected with the anti-flag monoclonal antibody and stained with IRDye800CW-conjugated rabbit anti-mouse IgG. M, low molecular weight protein marker. (b) MS-MS spectrum of a Sac peptide from protein coffilin-1. (c) MS-MS spectrum of a Kac peptide from RBBP4.
Peptide intensities were used to quantify the levels of Ser- and Lys-acetylation with or without YopJ expression. A medium intense peptide from α-actin, HQGVMVGMGQK (Uniprot # P60709), was used as an internal reference to normalize between experiments. The average acetylation levels were calculated on the basis of triplicate experiments, and the ratios between acetylated peptides with and without YopJ were obtained (Supplemental Table 3). The total intensities of the 10 internal Ser-acetylated peptides were significantly increased by YopJ (YopJ/non-YopJ = 1.3, P = 0.02) while the intensities of N-terminal Ser-acetylated peptides were unaltered (YopJ/non-YopJ = 1.008, P = 0.89). The intensities of 8 Lys-acetylated peptides were dramatically enhanced by YopJ (YopJ/non-YopJ = 3.5, p = 0.000006). The acetylated peptides significantly altered by YopJ treatment were listed in Table 1. For instance, in comparison with non-acetylated peptides, ASQSacQGIQQLLQAEK increased 15.9 folds, NIFEKacSPLTEPNFENK increased 22.1 folds, and KacNIFEK increased 16.2 folds. Note that all of the internal Ser-acetylation was found in non-histone proteins and the most Lys-acetylated peptides were found from histones. In summary, our data indicated a basal level of endogenous serine acetylation without bacterial YopJ, suggesting the existence of human serine acetyltransferase in HeLa cells. Importantly, bacterial serine acetyltransferase YopJ mediated protein acetylation at both the β-hydroxyl group of serine and the epsilon-amino group of lysine, suggesting a mechanism of bacterial acetyltransferase YopJ mediated dual acetylation at the serine and lysine residues. In addition, YopJ-mediated upregulation of Ser-acetylation occurred apparently at the internal protein positions, whereas protein N-terminal acetylation was not significantly affected.
Table 1.
Relative intensities1 of S/K acetylated peptides with/without YopJ.
| Peptide modification |
||||||
|---|---|---|---|---|---|---|
| site | score | acetylated sequence | without YopJ | with YopJ | Ratio w/o YopJ2 | P value |
| Sac | 33.47 | ALSacAVSacTQQK | 0.019 | 0.029 | 1.547 | 0.021 |
| 88.7 | ASacGADSKGDDLSTAILK | 0.009 | 0.024 | 2.577 | 0.001 | |
| 81.21 | ASacGVAVSDGVIK | 0.305 | 0.472 | 1.547 | 0.043 | |
| 39.69 | ASQSacQGIQQLLQAEK | 0.001 | 0.023 | 15.91 | 0.197 | |
| 52.48 | MERPQPDSacMPQDLSEALK | 0.006 | 0.019 | 3.031 | 0.019 | |
| 34.05 | TADDPSLSacLIK | 0.003 | 0.000 | 0.045 | 0.292 | |
| 36.23 | VQVSacYK | 0.042 | 0.017 | 0.408 | 0.005 | |
| Subtotal | 0.387 | 0.584 | 1.510 | 0.011 | ||
| Kac | 59.23 | ADKacEAAFDDAVEER | 0.022 | 0.037 | 1.696 | 0.032 |
| 32.16 | GGKacGLGKacGGAK | 0.016 | 0.036 | 2.332 | 0.007 | |
| 43.68 | GLGKacGGAKacR | 0.042 | 0.158 | 3.773 | 0.002 | |
| 34.12 | KacNIFEK | 0.001 | 0.010 | 16.186 | 0.006 | |
| 35.92 | KacQLATKacAAR | 0.005 | 0.020 | 3.837 | 0.013 | |
| 45.41 | KacSTGGKacAPR | 0.002 | 0.015 | 6.556 | 0.000 | |
| 34.8 | NIFEKacSPLTEPNFENK | 0.002 | 0.036 | 22.115 | 0.008 | |
| 37.93 | QLATKacAAR | 0.015 | 0.056 | 3.612 | 0.012 | |
| Subtotal | 0.104 | 0.368 | 3.529 | 0.000 | ||
Relative intensities: normalized to the control actin peptides.
Ratio w/o YopJ: average intensity of YopJ divided by average intensity without YopJ.
Acetylation of ubiquitin E3 ligase MARCH8 at the serine and lysine residues by bacterial serine acetyltransferase YopJ
The peptides from ubiquitin E3 ligase were frequently identified for Ser- and Lys-acetylation (Supplemental Table 1). To further confirm YopJ-mediated acetylation at the serine and lysine residues, ubiquitin E3 ligase MARCH814–16 was co-transfected with YopJ in HeLa cells. The full-length MARCH8 (M8) was known to mediate ubiquitination of CD86 and MHC class II proteins and promote the subsequent endocytosis via multi-vesicular bodies.16 To increase the solubility, a truncated MARCH8 (ΔM8, 1-156aa) lacking the trans-membrane domains was prepared for comparison. The full-length and truncated MARCH8 were fused with the flag tag, and their co-expression with or without YopJ was confirmed in HeLa cells by Western blotting (Fig. 2a) and the tagged-proteins were immunoprecipitated with the antibody against the flag and separated on SDS-PAGE (Fig. 2b). To confirm Ser- and Lys-acetylation, the tagged proteins were excised from the gel, digested with trypsin, and analyzed by LC-MS/MS (Supplemental Data: massive 000080123 raw data M8 1–3, YopJ 1–3, M8D1–3, M8DY-1–3) (Table 2). Ser-acetylation was identified at Sac44, Sac71 and Sac253, and Lys-acetylation was found at Kac247 and Kac252 (Supplemental Fig. 1a, 1b, 1c, 1d, 1e). Note that acetylation of MARCH8 was not previously reported.
Figure 2.

Ubiquitin E3 ligase MARCH 8 was acetylated at the serine and lysine residues by YopJ in HeLa cells. (a) Western blotting of the transiently expressed MARCH 8 (M8) and a truncated form of MARCH8 (ΔM8). The blots were detected with the anti-flag monoclonal antibody and stained with IRDye680CW-conjugated rabbit anti-mouse IgG. M, low molecular protein marker; con, control. (b) Immunoprecipitation-Western blotting (IP-WB) of March 8 (M8) and the truncated form (ΔM8). IP: mouse anti-flag antibody. WB: anti-flag antibody. M, low molecular protein marker. HC: IgG heavy chain. LC: IgG light chain. (c) Intensity ratios of the Ser-acetylated peptides at Sac44, Sac71 and Sac253 with or without YopJ. The intensities were normalized to non-acetylated peptides. (d) Intensity ratios of the Lys-acetylated peptides at K247 and K252 with or without YopJ.
Table 2.
Acetylated peptide sequences identified in MARCH8 by LC-MS/MS.
| type | Ac-sites | Peptide Sequences | Score |
|---|---|---|---|
| Sac | Sac253 | S(ac)PLTEPNFENK | 33.52 |
| Sac44 | TLGHFMS(ac)HSSNISK | 27.49 | |
| Sac71 | TS(ac)ITPSSQDICR | 46.53 | |
| Kac | Kac247 | K(ac)NIFEK | 38.62 |
| Kac252 | NIFEK(ac)SPLTEPNFENK | 47.83 |
For label-free quantification, intensities between the acetylated and non-acetylated peptides were calculated and used to evaluate the levels of acetylation with or without YopJ. Statistical analysis was based on triplicate experiments. Markedly, acetylation at Sac253 and Sac44 increased by 28 ± 11 (p = 0.002) and 31 ± 8 (p = 0.002) folds in YopJ transfected HeLa cells, respectively (Fig. 2c). In consistent, the acetylated lysines at Kac252 and Kac247 were augmented for 36 ± 18 (p = 0.0003) and 18 ± 8 (p = 0.01) times (Fig. 2d), respectively. Thus, these data confirmed that ubiquitin E3 ligase MARCH8 was indeed one of the substrates of YopJ-mediated Ser- and Lys-acetylation in human cells.
YopJ-mediated dual acetylation of MARCH8 at serine and lysine is confirmed by immunoassays in vivo and in vitro
To further confirm YopJ-mediated acetylation of MARCH8 at the serine and lysine residues, the antibodies were produced against Sac71 of MARCH8 by immunization of rabbits with a synthetic peptide Ac-T(Sac)ITPSSQDICRICH CEGDC-NH2. Acetylation of the peptide at the required position was confirmed by LC-MS/MS (Fig. 3a). The antisera from immunized rabbits were negatively selected with the peptide without acetylation (Ac-TSITPSSQDICRICHCEGDC-NH2) followed by affinity-purification using the acetylated peptide. In the dot blotting assay, the MARCH8 Sac71 antibody specifically detected the Sac71 peptide, but did not detect the same peptide lacking the acetylated serine, or the different MARCH8 peptides with or without serine acetylation (Fig. 3b). In the competition assay, the positive signal was efficiently competed away by the Sac71 peptide, but it was not affected by the same peptide lacking the acetylated serine (Fig. 3c). To detect MARCH8 with Ser-acetylation, the truncated MARCH8 was co-transfected with YopJ in HeLa cells, immunoprecipitated by anti-flag antibody, and detected with the Sac71 antibody on Western blots. A positive signal was clearly detected and was efficiently competed away with the Sac71 peptide; however, the signal was slightly affected by the same peptide lacking the acetylated serine (Fig. 3d), proving the specificity of Sac71 antibody.
Figure 3.
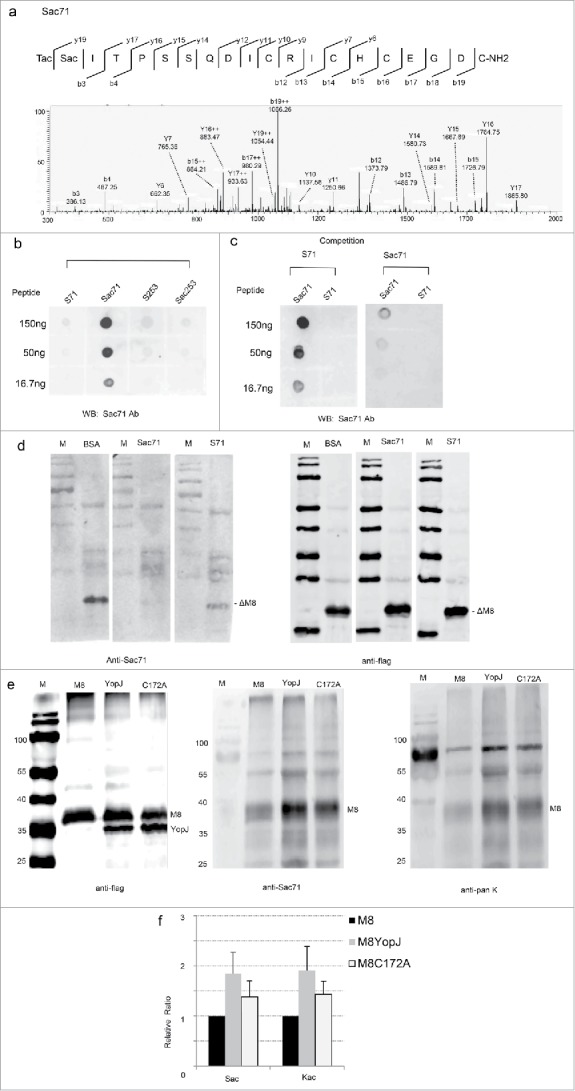
Immunoassays for YopJ-mediated serine and Lys-acetylation of MARCH8. (a) MS/MS spectrum of MARCH8 synthetic peptide containing Sac71. (b) Specificity of the Sac71 antibody demonstrated by dot blotting. Two acetylated peptides Ac-T(Sac)ITPSSQDICRICHCEGDC-NH2 (Sac71), Ac-KS(ac)PLTEPNFEN KC-NH2 (Sac253), and unmodified peptide Ac-TSITPSSQDICRICHCEGDC-NH2 (S71), Ac-KSPLTEPNFENKC-NH2 (S253) were blotted on NC membrane, detected with the anti-Sac71 polyclonal antibody and stained with IRDye680-conjugated goat anti-rabbit IgG. Anti-Sac71 antibody recognize only Sac71 peptide. (c) Dot-blotting for competition: The acetylated peptide Ac-T(Sac)ITPSSQDICRICHCEGDC-NH2 (Sac71) and non-acetylated peptide Ac-TSITPSSQDICRICHCEGDC-NH2 (S71) were blotted on NC membrane, detected with the anti-Sac71 polyclonal antibody pre-incubated with Sac71 or S71 peptide and visualized with IRDye680-conjugated goat anti-rabbit IgG. Pre-incubation with Sac71 antibody competed away the dot-blot signals while pre-incubation with S71 preserved the signals. (d) Western-blotting for competition. HeLa cell lysates transfected with the truncated MARCH8 (ΔM8) was resolved by SDS-PAGE. Western blots were detected with anti-Sac71 (the left panel) or anti-flag (the right panel) antibody with the competition of the acetylated peptide (Sac71) or the non-acetylated peptide (S71). M: low molecular protein marker (e) Immune assays to prove YopJ-mediated dual acetylation at serine and lysine. HeLa cells were co-transfected with MARCH8 in the presence (+) or absence (−) of YopJ /C172A mutant. M8: transfection with MARCH8 recombinant plasmid. YopJ: cotransfection with both March8 and YopJ recombinant plasmids. C172A: cotransfection with both March8 and YopJ catalytic mutant C172A recombinant plasmids. The MARCH8 was immunoprecipitated with the anti-flag antibody and Western blots were detected with the anti-flag (the left panel), anti-Sac71 (the middle panel) or pan-acetyl - lysine antibodies(the right panel). M: low molecular protein marker (f) YopJ-mediated dual acetylation of MARCH8 was quantified by LI-COR quantitation software. The intensities obtained with anti-Sac71 (Ser-acetylation) and anti-pan-acetyl lysine (Lys-acetylation) antibodies were normalized to that of the anti-flag antibody, data from 3 replicate experiments.
To confirm YopJ-mediated Ser- and Lys-acetylation, the MARCH8 were co-transfected with YopJ into HeLa cells, cell lysates were immunoprecipitated with anti-flag antibody, Ser-/Lys-acetylation was analyzed by the antibodies against Sac71 and pan-acetyl lysine by Western blotting. While the expression level of the MARCH8 was not affected (Fig. 3e, the left panel), the Ser-acetylated MARCH8 were significantly increased by YopJ expression (1.8 ± 0.5, p = 0.027, n = 3) (Fig. 3e, the middle panel, Fig. 3f). Similarly, the level for Lys-acetylation in MARCH8 was also significantly upregulated (1.9 ± 0.4, p = 0.012, n = 3) (Fig. 3e, the right panel, Fig. 3f). Consistently, our data from independent immunoassays also confirmed YopJ-mediated acetylation of MARCH8 at both the Ser and Lys residues in HeLa cells. In support, the C172A mutant, which was known to reduce the acetyltransferase activity of YopJ,5 was also demonstrated to reduce the dual acetylation at Ser and Lys (Fig. 3e, Fig. 3f), indicating that the augmentation of Ser- and Lys- acetylation was dependent on the catalytic activity.
Next we examined the dual acetylation activity of YopJ in vitro using the purified MARCH8 protein and recombinant YopJ (Fig. 4a). The flag-tagged MARCH8 protein was immobilized on the resin by immune-affinity purification and incubated with recombinant YopJ and YopJ mutant C172A cell lysates in the presence of acetyl-coenzyme A and HDAC inhibitors. The immobilized MARCH8 was recovered and examined by anti-flag antibody (Fig. 4a, the upper panel, Fig. 4b); the levels of Ser- and Lys-acetylation were examined by Western-blotting using MARCH8 Sac71 (Fig. 4b, the lower panel, Fig. 4b) and Pan-acetyl lysine (Fig. 4a, the middle panel, Fig. 4.b) antibodies. Consistently, acetylation at the Ser- as well as Lys- residues was enhanced in comparison with the control; however, both activities were reduced when YopJ was replaced by the C172A mutant. Thus, YopJ was consistently responsible for the acetylation at the Ser and Lys residues. To further prove the direct acetylation of YopJ at the Ser- and Lys- residues, we repeated the in vitro acetylation assay using the purified MARCH8 protein and affinity-purified YopJ, the result is consistent with that using recombinant YopJ cell lysate (Fig. 4c, Fig. 4d).
Figure 4.
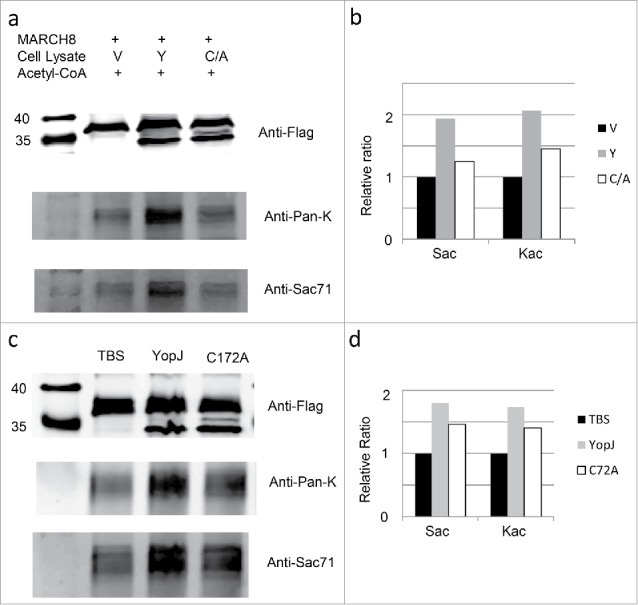
In vitro acetylation of MARCH8 by YopJ. HeLa cell lysate with MARCH8 transfection was aliquoted equally into 3 fractions; the recombinant MARCH8 in each fraction was immobilized on the resin by incubation with the anti-flag antibody; the immobilized MARCH8 was washed 4 times by PBS and prepared for in vitro acetylation reaction. As controls, the immobilized MARCH8 was incubated with 200µl TBS in the presence of 50µM acetyl-CoA, protease inhibitor cocktail, 1 mM PMSF, 10 mM Nicotinamide and 10 mM Sodium Butyrate. For in vitro acetylation reaction with recombinant YopJ cell lysates, the immobilized MARCH8 was incubated with the Hela cell lysate transfected with vector(V), YopJ(Y),YopJ C172A mutant (C/A) (Fig. 4a, Fig. 4b). For in vitro acetylation reaction with affinity-purified YopJ, the immobilized MARCH8 was incubated with TBS (TBS), YopJ (YopJ) and YopJ C172A mutant (C172A) (Fig. 4c, Fig. 4d). After incubation at 37°C for 1h, the immobilized MARCH8 was washed by PBS 4 times, recovered by boiling in SDS-loading buffer and analyzed by Western-blotting using antibodies against the flag(Fig. 4a, upper panel Fig. 4c, upper panel)MARCH8 Sac71 (Fig. 4a, lower panel, Fig. 4c, lower panel), or anti-Pan-K (Fig. 4a, middle panel, Fig. 4c, middle panel). Signal intensities of the Ser-acetylated MARCH8, and the Lys-acetylated MARCH8 were quantified by Li-COR software and normalized against the control levels of MARCH8 (Fig. 4b, Fig. 4d).
YopJ-mediated dual acetylation at serine and lysine elevates auto-ubiquitination of MARCH8 and the elevation is catalytic activity-dependent
To address whether YopJ-mediated dual acetylation was involved in any cellular or biological functions, auto-ubiquitination of MARCH8 was tested in vitro using ubiquitin activating enzyme (UBE1), the E2 ubiquitination conjugating enzyme (UbcH5a) and the transiently expressed E3 ubiquitin ligase MARCH8. Briefly, the flag-tagged MARCH8 was co-expressed with or without YopJ/C172A, and immune-purified with the anti-flag antibody. The expressions of the flag-tagged MARCH8 and YopJ/C172A were confirmed by Western blotting using the anti-flag antibody. The auto-ubiquitination activity of the flag-tagged MARCH8 was examined by in vitro ubiquitination followed by Western blotting using the P4D1 antibody against ubiquitin. For comparison, the purified MARCH8 was splitted into 3 equal fractions and detected with Western blotting (Fig. 5). The first fraction was detected with the anti-flag antibody to demonstrate the loading quantity of each sample (Fig. 5, the middle panel), the second fraction was incubated with the ubiquitination enzyme UBE1 and UbcH5a and ubiquitin for 6 hours and detected with the P4D1 antibody against ubiquitin (Fig. 5, the left panel). The third fraction was incubated in the ubiquitination buffer in the presence of ubiquitin for 6 hours without the ubiquitination enzyme; the result was examined with anti-P4D1 antibody (Fig. 5, the right panel). Because the 3 fractions were equally splitted, the relative levels of the purified MARCH8 with or without YopJ/C172A expression (Fig. 5, the right panel) were considered the same in the second and third fractions. Therefore, the auto-ubiquitination level of MARCH8 was significantly increased in the presence of YopJ (Fig. 5, the left panel). In addition, the C172A mutant of YopJ reduced auto-ubiquitination of MARCH8 comparison with the MARCH8 control (Fig. 5, the left panel), implying this process was also dependent on the catalytic activity. Thus, these data demonstrated that bacterial YopJ-mediated dual acetylation at serine and lysine simultaneously enhanced auto-ubiquitination of MARCH8.
Figure 5.
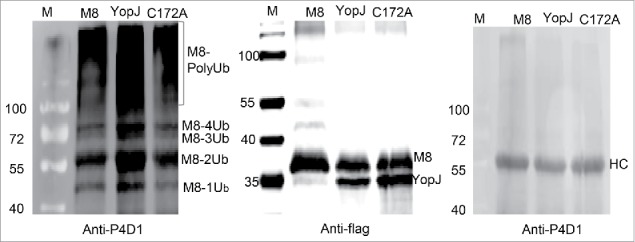
YopJ-mediated dual acetylation enhanced auto-ubiquitination of MARCH8. HeLa cells were transfected with flag-tagged MARCH8 (M8) in the presence or absence of flag-tagged YopJ or YopJ C172A Mutant. The purified MARCH8 were splitted into 3 equal fractions, one fraction was detected with anti-flag antibody by Western blots (the middle panel), another fraction was used for in vitro auto-ubiquitination assay (the left panel). The MARCH8 proteins were immunoprecipitated with anti-flag antibody, and incubated with ubiquitin-activating enzyme E1 (UBE1) and ubiquitin-conjugating enzyme E2 (UbcH5a). Formation of the poly-ubiquitinated MARCH8 (PolyUb) was detected by Western blotting using the anti-P4D1 antibody (the left panel). The third fraction was incubated with ubiquitination buffer without addition of E1 (UBE1) or E2 (UbcH5a), Formation of the poly-ubiquitinated MARCH8 (PolyUb) was not detected (the right panel). Ub: ubiquitin; HC: heavy chain of IgG. M8: transfection with only MARCH8: YopJ: cotransfection of YopJ with MARCH8; C172A: cotransfection of YopJ C172A mutant with MARCH8.
Discussion
Using shotgun proteomics and label-free quantification, we demonstrate in this study a dual acetylation at serine and lysine mediated by bacterial acetyltransferase YopJ in human HeLa cells. By mimicking Y. pestis infection, our data indicate that bacterial YopJ is involved in the regulation of Ser- and Lys-acetylation that transfers the acetyl group to the β-hydroxyl group of serine and/or the epsilon-amino group of lysine. As Lys-acetylation, it is known to acetylate Histones and considered as an important mechanism to regulate gene transcription,12 our finding anticipates a great impact of YopJ-mediated acetylation during Y. pestis infection on human cell functions. For the Ser-acetylation feature, YopJ is previously shown to acetylate the serine residues in MEK2 and I kappa B kinase (IKK) during Y. pestis infection, leading to inactivation of the MAPK and NF-κB signaling pathways and the downregulation of pro-inflammatory cytokine production.2-6,17
One consequence of YopJ-mediated dual acetylation is the cross interaction and possible competition between Ser- and Lys-acetylation, particularly when a protein is acetylated at either the lysine and serine residues. Notably, the peptide KacVASacGHR is acetylated at 2 proximal serine and lysine sites on the same peptide (Fig. S2), which indicated the possible interactions between Ser- and Lys-acetylation. In addition, as the β-hydroxyl group of serine is also the site for phosphorylation and glycosylation, our finding suggests possible interactions between the networks of YopJ-mediated serine acetylation with serine phosphorylation and glycosylation through the Ser-acetylated proteins. Indeed, YopJ is reported to acetylate the critical serine residues in MAPKK6, MEK2 and TGFβ-activated kinase (TAK1) that blocks phosphorylation, prevents activation of these kinases, and inhibits innate immune signaling.5,6,17
Notably, ubiquitin E3 ligase is identified as one of the substrates of YopJ-mediated dual acetylation at serine and lysine. Immunoprecipitation followed by LC-MS/MS analysis confirms acetylation of ubiquitin E3 ligase MARCH8 at Kac247, Kac252, Sac44, Sac71 and Sac253. Label-free quantification indicates that overexpression of YopJ augments the acetylation levels of Sac44, Sac71 and Sac253 more than 28 times and Kac247, Kac252 more than 17 times. Moreover, further immunoassays using the anti-Sac71 and pan-acetyl-lysine antibodies confirm YopJ-mediated upregulation of Ser- and Lys-acetylation of MARCH8. Note that, although phosphorylation at S71 and ubiquitination at K252 of MARCH8 are reported (www.phosphosite.org), Ser- and/or Lys-acetylation at these sites has not been documented. Potential competitions between acetylation, phosphorylation and ubiquitination may be anticipated at these positions. In support, MARCH8 is indeed acetylated at serine and lysine in vivo and in vitro by YopJ but the activity is abolished by the C172A mutant in YopJ. Finally, our data demonstrate that YopJ-mediated Ser- and Lys-acetylation is involved in auto-ubiquitination of MARCH8. As auto-ubiquitination is an important step of ubiquitination activation,18 our data suggest that YopJ-mediated dual acetylation participates the regulation of MARCH8-mediated ubiquitination signal transduction. Nevertheless, MARCH8 is known for its function in antigen presentation,14-16,19,20 cell apoptosis,21 and cell transportation.22 Recently, MARCH8 is also reported to inhibit HIV infection by reducing virion incorporation of envelope glycoproteins.23 Our data shed lights to the potential role of YopJ-mediated MARCH8 dual acetylation in regulating innate and adaptive immune responses during Y. pestis infection.
In conclusion, our study provides evidence for YopJ-mediated dual acetylation at serine and lysine, suggesting a connection between Ser- and Lys-acetylation networks. Specifically, YopJ-mediated dual acetylation is involved in auto-ubiquitination of ubiquitin ligase MARCH8, suggesting a general role in regulation of MARCH8-mediated ubiquitination in human cells.
Materials and methods
Reagents and cell lines
Human HeLa cells (ATCC-CCL-2™) were used for transient protein expression. K562 cells (ATCC-CCL-243™) were used to obtain cDNA of MARCH8. BamHI, XhoI, HindIII, T4 DNA ligase, and Q5a DNA polymerase were purchased from New England Biolabs. IRDye800CW or IRDye680 conjugated IgGs were from LI-COR Biosciences. Low molecular protein marker was purchased from Thermofisher Scientific(Cat # 26616). Protein G plus-agarose (Cat # Sc-2002) and normal mouse IgG1 (Cat # Sc-3877) for immunoprecipitation were from Santa Cruz. Trypsin for mass spectrometry came from Sigma (Cat#T6567). Sodium butyrate and nicotinamide were also purchased from Sigma. BCA protein assay kit (NCI3225CH) was from Thermofisher Scientific.
Recombinant plasmid construction and expression in HeLa cells
The YopJ cDNA (Genbank accession # KP 641301) was chemically synthesized, MARCH8 cDNA (GenBank accession # NM_001002266, 1–291 aa) was amplified from the total RNA of K562 cells by RT-PCR. A truncated MARCH8 (GenBank accession # NM_001002266, 1–156 aa) was amplified by RT-PCR. The cDNAs were sub-cloned into the pcDNA3.0-flag expression vector and their sequences verified by DNA sequencing. YopJ catalytic mutant C172A was generated by site-specific mutagenesis and confirmed by DNA sequencing. The recombinant plasmids were purified using Omega Maxi-Kits (OMEGA bio-tek, D6926–03) and then transfected into HeLa cells by Lipofectamine (Invitrogen, CA, USA) according to the manufacturer's protocol. HeLa cells were harvested 24–48 hrs post transfection.
Protein extraction
The transfected cells were rinsed by PBS 3 times and lysed in RIPA lysis buffer (Millipore, Billerica, MA) with protease and phosphotase inhibitor cocktails (Roche, Switzerland), HDAC inhibitors (sodium butyrate and nicotinamide), leucine, pepstatin and aprotinin, either sonicated for 15 seconds, or kept on ice for 40 min with vigorous vortex for 20 s per 10 minutes. The cell lysates were centrifuged for 10 min at 14000rpm, and the supernatants were collected, protein concentration were determined by BCA kit (Pierce, thermo scientific, NCI3225CH) and stored at −80°C until further use.
In-solution tryptic digestion
Cell lysates from different transfected cells or the proteins from immunoprecipitation were digested with trypsin as described previously.24 Briefly, protein samples were dissolved in 200μl of 0.1 M Tris/HCl (PH 8.5) containing 8 M Urea (Sigma, cat # U5128) and loaded on a filter unit (Microcon YM-30, Millipore, cat# 42410). After centrifugation, the proteins on the filter were washed twice with 100μl of 50 mM ammonium bicarbonate, re-suspended in 40μl of 50 mM ammonium bicarbonate with trypsin (Sigma-Aldrich, USA), incubated at 37°C for 24 h in a wet chamber. The digested peptides were collected by centrifugation and desalted with a C18 spin column (Millipore, USA) following manufacturer's protocol. The purified peptides were dried in SpeedVac and stored at −80°C until further use.
Immunoprecipitation and in-gel tryptic digestion
HeLa cells were co-transfected with MARCH8 and YopJ; cell lysates were prepared. The flag-tagged MARCH8 was immunoprecipitated with the anti-flag antibody following manufacture's standard protocol. The precipitated proteins were separated on SDS-PAGE and stained by Coomassie Bright Blue. The protein bands corresponding to MARCH8 were excised from the gel, cut into 1 mm slices, and digested overnight with trypsin following the modified in-gel digestion protocol.25 The digested peptides were desalted by C18 column ZipTip (Millipore, Billerica, MA) for LC-MS/MS analysis.
LC-MS/MS analysis
∼2 μg of the trypsin-digested peptides were injected into a C18 column (200x ϕ0.075 mm) and eluted with a 120 min linear gradient (5–35% acetonitrile with 0.1% formic acid) followed by the Orbitrap Elite mass spectrometer. Data were acquired in a data-dependent mode, in which MS/MS fragmentation was performed for the 20 most intense peaks of every full MS scan. MS/MS spectra were searched against the human protein database (UniProt:human-20151224, 149,731 sequences, 47,136,486 residues) using Mascot and Sequest, parts of the Proteome Discoverer 1.4 data analysis package (Thermo Scientific, San Jose, CA). MS/MS spectra were searched with a maximum mass tolerance of 10 ppm for the precursors, 0.8 Da for fragments, dynamic modifications of lysine and serine acetylation and methionine oxidation, and missed cleavage of 2. The thresholds for Mascot IonScore and Sequest XCorr as accepting individual MS/MS spectra were 38 and 1.22 × charges,24 respectively. All modification site assignments were confirmed by manual spectrum interpretation.
Label-free quantification
The abundance of the acetylated peptides was quantified by label-free method using Progenesis LC-MS software (version 4.1; Nonlinear Dynamics, UK). Briefly, the acquired raw data were transformed to the mzxml format, searched by Proteome Discoverer 1.4 with mascot, high confident peptides were imported into Progenesis LC-MS software. The ion intensity maps from multiple analysis were examined for defects. The best data set was selected as the reference for data alignment. Peptide ions with charge state of +1 or > 4 were excluded. Peptides were also searched against a decoy database to determine False Discovery Rate (FDR). For quantification, the unique peptide validated by MS (high confident peptide, label-free score>30) was chosen and calculated by summing the abundance of all peptides allocated to a specific site. Statistics analysis was based on the ratio of the sum of all acetylated peptides at a specific site to that of the non-acetylated peptides to that of a set of reference peptides (HQGVMVGMGQK) from α- actin (P60709).
MARCH8 Sac71 polyclonal antibody production
Polyclonal antibody specific to MARCH8 Sac71 was produced following a protocol modified from pan-acetyl antibody production.26 A peptide encompassing acetylated Sac71 of MARCH8, Ac-T(Sac)ITPSSQDICRICHCEGDC-NH2, was synthesized and verified by LC-MS/MS that more than 90% of the peptides were the acetylated peptide. The corresponding non-acetylated peptide, Ac-TSITPSSQDICRICHCEGDC-NH2, was also synthesized and verified by LC-MS/MS. The acetylated peptide was conjugated to BSA or KLH by a conjugation kit and verified by Coomassie Blue staining that the peptide was successfully conjugated. The New Zealand rabbits were first immunized by injection (s.c.) of 400 μg conjugated-proteins with CFA (Freund's Adjuvant, Complete, Sigma, F5881). Four weeks later, another 400μg conjugated-proteins with IFA (Freund's Adjuvant, Incomplete, Sigma, F5506) was immunized. The last injection (i.v.) was performed 3 weeks later with 200μg conjugated-proteins in PBS. 10 d after the last injection, blood samples were collected from rabbit ear and the antibody titer was determined by ELISA. All blood samples were collected in 15 d after the last injection. The anti-sera were passed twice through the affinity chromatography column coupled with the unmodified peptide to deplete the antibodies for the same peptide lacking the acetylated Sac71. The depleted anti-sera were affinity purified with the acetylated Sac71 peptide. Finally, the specificity of the Sac71 antibody was confirmed by the dot-blot peptide competition assay and western-blot competition assay. All animal work was permitted by Ethic Committee on Animal Experiments of Medical School of Shandong University and conducted according to relevant national and international guidelines.
Immunoprecipitation-Western blotting (IP-WB)
The flag-tagged MARCH8 was first immunoprecipitated with the anti-flag antibody following the standard protocol from manufacture. The immunoprecipitated proteins were then separated by SDS-PAGE, transferred to nitrocellulose membranes, and blocked by 5% BSA. For detection, the membrane was incubated with the primary antibodies against the flag-tag (mouse), pan-acetyl lysine (cell signaling,#9441), or MARCH8 Sac71. After washing, the targeted proteins were stained by fluorescence-conjugated secondary antibody (IRDye800CW or IRDye680 conjugated IgG, LI-COR). The membranes were scanned with Odyssey infrared imaging system (Li-COR).
In-vitro acetylation assay with recombinant YopJ Cell lysates
HeLa cell lysate with MARCH8 transfection was aliquoted equally into 3 fractions; the recombinant MARCH8 in each fraction was immobilized on the resin by incubation with the anti-flag antibody; the immobilized MARCH8 was washed 4 times by PBS and prepared for in vitro acetylation reaction. As controls, the immobilized MARCH8 was incubated with 200µl cell lysate transfected with pcDNA3.0 vector (V) in the presence of 50µM acetyl-CoA, protease inhibitor cocktail, 1mM PMSF, 10mM Nicotinamide and 10mM Sodium Butyrate. For in vitro acetylation reaction, the immobilized MARCH8 was incubated with the cell lysate transfected with YopJ (Y) or YopJ C172A mutant (C/A), respectively. After incubation at 37°C for 1h, the immobilized MARCH8 was washed by PBS 4 times, recovered by boiling in SDS-loading buffer and analyzed by Western-blotting using antibodies against the flag, MARCH8 Sac71, or anti-Pan-K. Signal intensities of the Ser-acetylated MARCH8, and the Lys-acetylated MARCH8 were quantified by Li-COR software and normalized against the control levels of MARCH8.
In-vitro acetylation assay with affinity-purified YopJ
HeLa cell lysate with flag-tagged YopJ or YopJ C172A mutant transfection was incubated with the anti-flag antibody and affinity-purified by the protein G resin. The bound YopJ was washed with PBS, eluted with 0.2 M Glycine (PH = 3.0) , neutralized with 1 M Tris-Cl (PH = 8.5) and dialyzed against autoclaved TBS for in vitro acetylation reaction. HeLa cell lysate with flag-tagged MARCH8 transfection was aliquoted equally into 3 fractions; the recombinant MARCH8 in each fraction was immobilized on the protein G resin after incubation with the anti-flag antibody; the immobilized MARCH8 was washed 4 times by PBS and prepared for in vitro acetylation reaction. For in vitro acetylation reaction, the immobilized MARCH8 was incubated with the affinity-purified YopJ or YopJ C172A mutant, respectively, in the presence of 50µM acetyl-CoA, protease inhibitor cocktail, 1 mM PMSF, 10 mM Nicotinamide and 10 mM Sodium Butyrate. As controls, the immobilized MARCH8 was incubated in the same buffer without YopJ. After incubation at 37°C for 1h, the immobilized MARCH8 was washed by PBS 4 times, recovered by boiling in SDS-loading buffer and analyzed by Western-blotting using antibodies against the flag, MARCH8 Sac71, or anti-Pan-K. Signal intensities of the Ser-acetylated MARCH8, and the Lys-acetylated MARCH8 were quantified by Li-COR software and normalized against the control levels of MARCH8.
In vitro ubiquitination assays
The flag-tagged E3 ubiquitin ligase MARCH8 was purified from the cell lysates by immunoprecipitation using anti-flag antibody, and used directly for in vitro ubiquitination. The E2 ubiquitination conjugating enzyme (UbcH5a) and ubiquitin activating enzyme (UBE1) were purchased from Boston Biochem (Cat # E2–616, Cat # E-305). Briefly, the in vitro reaction mixture (40μl) included 50 nM rabbit UBE1, 2 μM UbcH5a (E2), 28 μM ubiquitin (Calbiochem, Cat # 662060), 5 mM ATP, 50 mM Tris-HCl (pH 7.5), 5 mM MgCl2 and 2 mM DTT, 25 μM MG-132 proteasome inhibitor (Calbiochem # 474790). The mixture was incubated at 37°C for 6 hrs and stopped by boiling in SDS-loading buffer. Samples were separated by 12% SDS-PAGE and immunoblotted with anti-ubiquitin antibody P4D1 (Cell signaling technology, Cat # 3936s).
Statistics analysis
SPSS 19.0 software (SPSS Inc., USA) was used for statistical analysis. P-value < 0.05 or FDR < 0.01 was considered statistically significant. Statistics between 2 groups was performed using a 2-tailed student's t- test.
Supplementary Material
Abbreviations
- CFA
Freund's Adjuvant, complete
- eIF2
eukaryotic initiation factor 2
- FDR
False Discovery Rate
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HDACi
histone deacetylase inhibitors
- IFA
Freund's Adjuvant, Incomplete
- IKK
I kappa B kinase
- IP
immunoprecipitation
- i.v
injection by vein
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- MARCH8
membrane-associated RING-CH 8
- MS
mass spectrometry
- NF-κB
nuclear factor Kappa B
- PTM
post translational modification
- s.c
subcutaneous injection
- WB
western blotting
- YopJ
Yersinia pestis outer protein J
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Boquan Jin from the Fourth Military Medical University for providing an effective immunization protocol to produce antibody, and Zhen Yan to provide the plasmid vector containing YopJ full length gene. It was the result of work partially supported with resources and the use of facilities at VA Boston Healthcare System, USA, Cancer Research Center, Shandong University, China and the Innovation Project of Shandong Academy of Medical Sciences.
Funding
This work was supported by grants from National Natural Science Foundation of China (#31471322 and #30928031) and grants from Department of Public Health (2014WS0069) and Department of Science and Technology (2016CYJS01A01) in Shandong Province.
References
- [1].Forman S, Wulff CR, Myers-Morales T, Cowan C, Perry RD, Straley SC. yadBC of Yersinia pestis, a new virulence determinant for bubonic plague. Infect Immun 2008; 76:578-87; PMID:18025093; http://dx.doi.org/ 10.1128/IAI.00219-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pradel E, Lemaître N, Merchez M, Ricard I, Reboul A, Dewitte A, Sebbane F. New insights into how Yersinia pestis adapts to its mammalian host during bubonic plague. PLoS Pathog 2014; 10:e1004029; PMID:24675805; http://dx.doi.org/ 10.1371/journal.ppat.1004029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rosenzweig JA, Chopra AK. Modulation of host immune defenses by Aeromonas and Yersinia species: convergence on toxins secreted by various secretion systems. Front Cell Infect Microbiol 2013; 3:70; PMID:24199174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Matsumoto H, Young GM. Translocated effectors of Yersinia. Curr Opin Microbiol 2009; 12:94-100; PMID:19185531; http://dx.doi.org/ 10.1016/j.mib.2008.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mukherjee S, Keitany G, Li Y, Wang Y, Ball HL, Goldsmith EJ, Orth K. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 2006; 312:1211-4; PMID:16728640; http://dx.doi.org/ 10.1126/science.1126867 [DOI] [PubMed] [Google Scholar]
- [6].Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and I kappa B kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc Natl Acad Sci U S A 2006; 103:18574-9; PMID:17116858; http://dx.doi.org/ 10.1073/pnas.0608995103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shrestha N, Bahnan W, Wiley DJ, Barber G, Fields KA, Schesser K. Eukaryotic initiation factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. J Biol Chem 2012; 287:28738-44; PMID:22761422; http://dx.doi.org/ 10.1074/jbc.M112.375915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Britton LM, Newhart A, Bhanu NV, Sridharan R, Gonzales-Cope M, Plath K, Janicki SM, Garcia BA. Initial characterization of histone H3 serine 10 O-acetylation. Epigenetics 2013; 8:1101-13; PMID:23949383; http://dx.doi.org/ 10.4161/epi.26025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lu Z, Cheng Z, Zhao Y, Volchenboum SL. Bioinformatic analysis and post-translational modification crosstalk prediction of lysine acetylation. PLoS One 2011; 6:e28228; PMID:22164248; http://dx.doi.org/ 10.1371/journal.pone.0028228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang K, Chen Y, Zhang Z, Tao S, Zhu H, Zhao Y. Unrestrictive identification of non-phosphorylation PTMs in yeast kinases by MS and PTMap. Proteomics 2010; 10:896-903; PMID:20049863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Montgomery DC, Sorum AW, Meier JL. Defining the orphan functions of lysine acetyltransferases. ACS Chem Biol 2015; 10:85-94; PMID:25591746; http://dx.doi.org/ 10.1021/cb500853p [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Menzies KJ, Zhang H, Katsyuba E, Auwerx J. Protein acetylation in metabolism - metabolites and cofactors. Nat Rev Endocrinol 2016; 12:43-60; PMID:26503676; http://dx.doi.org/ 10.1038/nrendo.2015.181 [DOI] [PubMed] [Google Scholar]
- [13].Haery L, Thompson RC, Gilmore TD. Histone acetyltransferases and histone deacetylases in B- and T-cell development, physiology and malignancy. Genes Cancer 2015; 6:184-213; PMID:26124919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lapaque N, Jahnke M, Trowsdale J, Kelly AP. The HLA-DRalpha chain is modified by polyubiquitination. J Biol Chem 2009; 284:9; http://dx.doi.org/ 10.1074/jbc.M805736200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Goto E, Ishido S, Sato Y, Ohgimoto S, Ohgimoto K, Nagano-Fujii M., Hotta H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J Biol Chem 2003; 278:14657-68; PMID:12582153 [DOI] [PubMed] [Google Scholar]
- [16].Bartee E, Mansouri M, Hovey Nerenberg BT, Gouveia K, Fruh K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J Virol 2004; 78:1109-20; PMID:14722266; http://dx.doi.org/ 10.1128/JVI.78.3.1109-1120.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Paquette N, Conlon J, Sweet C, Rus F, Wilson L, Pereira A, Rosadini CV, Goutagny N, Weber AN, Lane WS, et al.. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc Natl Acad Sci U S A 2012; 109:12710-5; PMID:22802624; http://dx.doi.org/ 10.1073/pnas.1008203109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weissman AM, Shabek N, Ciechanover A. The predator becomes the prey: regulating the ubiquitin system by ubiquitylation and degradation. Nat Rev Mol Cell Biol 2011; 12:605-20; PMID:21860393; http://dx.doi.org/ 10.1038/nrm3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jahnke M, Trowsdale J, Kelly AP. Ubiquitination of human leukocyte antigen (HLA)-DM by different membrane-associated RING-CH (MARCH) protein family E3 ligases targets different endocytic pathways. J Biol Chem 2012; 287:7256-64; PMID:22247549; http://dx.doi.org/ 10.1074/jbc.M111.305961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jahnke M, Trowsdale J, Kelly AP. Ubiquitination of HLA-DO by MARCH family E3 ligases. Eur J Immunol 2013; 43:1153-61; PMID:23400868; http://dx.doi.org/ 10.1002/eji.201243043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van de Kooij B, Verbrugge I, de Vries E, Gijsen M, Montserrat V, Maas C, Neefjes J, Borst J. Ubiquitination by the membrane-associated RING-CH-8 (MARCH-8) ligase controls steady-state cell surface expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) receptor 1. J Biol Chem 2013; 288:6617-28; PMID:23300075; http://dx.doi.org/ 10.1074/jbc.M112.448209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fujita H, Iwabu Y, Tokunaga K, Tanaka Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J Cell Sci 2013; 126:2798-809; PMID:23606747; http://dx.doi.org/ 10.1242/jcs.119909 [DOI] [PubMed] [Google Scholar]
- [23].Tada T, Zhang Y, Koyama T, Tobiume M, Tsunetsugu-Yokota Y, Yamaoka S, Fujita H, Tokunaga K. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins. Nat Med 2015; 21:1502-7; PMID:26523972; http://dx.doi.org/ 10.1038/nm.3956 [DOI] [PubMed] [Google Scholar]
- [24].Krey JF, Wilmarth PA, Shin JB, Klimek J, Sherman NE, Jeffery ED, Choi D. David LL and Barr-Gillespie PG Accurate label-free protein quantitation with high- and low-resolution mass spectrometers. J Proteome Res 2014; 13:1034-44; PMID:24295401; http://dx.doi.org/ 10.1021/pr401017h [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao Z, Wu F, Ding S, Sun L, Liu Z, Ding K, Lu J. Label-free quantitative proteomic analysis reveals potential biomarkers and pathways in renal cell carcinoma. Tumour Biol 2015; 36:939-51; PMID:25315187; http://dx.doi.org/ 10.1007/s13277-014-2694-2 [DOI] [PubMed] [Google Scholar]
- [26].Guan KL, Yu W, Lin Y, Xiong Y, Zhao S. Generation of acetyllysine antibodies and affinity enrichment of acetylated peptides. Nat Protoc 2010; 5:1583-95; PMID:21085124; http://dx.doi.org/ 10.1038/nprot.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


