How are physiological signals integrated into a biochemical switch governing cell division? Cells enter mitosis and divide due to regulated activation of the cyclin-dependent kinase Cdk1. During interphase, Cdk1 is inactive due to inhibitory phosphorylation at a conserved tyrosine by the protein kinase Wee1. To trigger mitotic entry, the protein phosphatase Cdc25 removes this inhibitory phosphate to activate Cdk1. Once activated, Cdk1 inhibits Wee1 and activates Cdc25, generating a bistable switch. The timing of Cdk1 activation depends on cellular surveillance mechanisms that detect cell size, DNA damage, nutrient availability, and more (Fig. 1). These mechanisms might act by altering the balance of Wee1 versus Cdc25 activity, but to understand this conceptual framework we must define the underlying molecular mechanisms. Fission yeast cells are an ideal system for this question because cell size at division is reproducible, easily measured, and highly sensitive to the balanced activities of Wee1 vs. Cdc25. However, the lack of reagents to detect endogenous untagged Wee1 protein in fission yeast prevented translating genetic pathways into biochemical mechanisms. Lucena and colleagues have now generated new antibodies that detect endogenous Wee1 and Cdc25, and further tested the conservation of regulatory mechanisms discovered in other organisms.1 Perhaps more importantly, they can now relate biochemical changes with quantifiable phenotypes in fission yeast cells, setting the stage for a systematic understanding of the highly conserved Wee1-Cdc25-Cdk1 mitotic switch.
Figure 1.
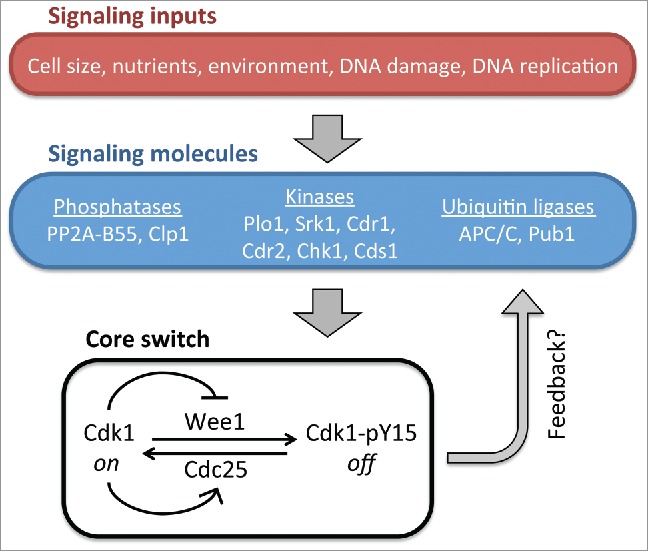
Schematic cartoon for the Wee1-Cdc25-Cdk1 mitotic entry control system. A subset of known molecular regulators is depicted, including the PP2A-B55 and Clp1 mechanisms identified by Lucena and colleagues. Cdk1 feedback to Wee1 and Cdc25 generates a bistable on/off switch for mitotic entry.
Lucena and colleagues use their new antibodies to detect Wee1 and Cdc25 proteins in synchronized cell cultures.1 The phosphorylation of both proteins changes dramatically during cell cycle progression, and both proteins are degraded at division to reset the system. The modest 3HA epitope tag impairs the dynamic hyperphosphorylation of Wee1, explaining why previous studies missed this regulation. What regulates the phosphorylation and abundance of Wee1 and Cdc25? The authors test 2 phosphatases, Clp1/Cdc14 and PP2A-B55, that are linked with dephosphorylation of Cdk1 substrates in late mitosis but also have intriguing connections with the core Wee1-Cdc25 switch. They show that both Wee1 and Cdc25 remain hyperphosphorylated throughout cell cycle progression in cells lacking either Clp1 or PP2A-B55. These results are consistent with previous studies from budding and fission yeasts,2,3 but also provide enticing new details. For example, mutants that alter Wee1 and Cdc25 phosphorylation during cell cycle progression have no effect on degradation of these proteins, suggesting separate control systems for phosphorylation vs. degradation. A caveat to this interpretation is the reliance on SDS-PAGE band shifts, which might miss some phosphorylation events; thus, additional work may uncover specific phosphorylation sites that escaped detection in this initial work. Nonetheless, the authors have uncovered an impressive phosphorylation program under the control of a conserved regulatory network.
The reagents and observation from this study open several new questions that are now experimentally feasible. First, how does this system respond to environmental changes and DNA damage? For example, nutrient limitation reduces cell size at division due to Wee1 and Cdc25; changes in this phosphorylation program may relay nutrient availability to Cdk1 activity. Second, what pathways regulate dynamic phosphorylation of Wee1 and Cdc25? Lucena and colleagues identified a role for the PP2A-B55 phosphatase complex, which was recently found to act in a multi-phosphatase relay pathway that orders the events of mitosis.4 This places regulation of Wee1 and Cdc25 within a larger phosphatase system ordering the events of cell division. Recent work also defined a Greatwall kinase-based pathway linking nitrogen availability with PP2A activity.5 We can now generate testable predictions for how this pathway might regulate cell size at division through Wee1 and Cdc25. Beyond phosphatases, the protein kinases hyperphosphorylating Wee1 and Cdc25 during cell cycle progression require investigation. Core feedback from Cdk1 will contribute, but additional kinases will be critical as well. For example, the stress-activated kinase Srk1 regulates Cdc25 localization through direct phosphorylation,6 and the protein kinase Cdr1/Nim1 directly phosphorylates and inhibits Wee1.7 These and other candidates can now be investigated in the context of a cell cycle-regulated phosphorylation program. By studying conserved pathways in fission yeast, the underlying molecular mechanisms can be connected to quantitative phenotypic changes in cell size. This new study reveals the importance of conserved regulatory pathways in a fundamental cellular process that continues to provide exciting open questions for the future.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Lucena R, Alcaide-Gavilan M, Anastasia SD, Kellogg DR. Wee1 and Cdc25 are controlled by conserved PP2A-dependent mechanisms in fission yeast. Cell Cycle 2017; 16:428-435; PMID:28103117; http://dx.doi.org/ 10.1080/15384101.2017.1281476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Harvey SL, Enciso G, Dephoure N, Gygi SP, Gunawardena J, Kellogg DR. A phosphatase threshold sets the level of Cdk1 activity in early mitosis in budding yeast. Mol Biol Cell 2011; 22:3595-608; PMID:21849476; http://dx.doi.org/ 10.1091/mbc.E11-04-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wolfe BA, Gould KL. Fission yeast Clp1p phosphatase affects G2/M transition and mitotic exit through Cdc25p inactivation. EMBO J 2004; 23:919-29; PMID:14765109; http://dx.doi.org/ 10.1038/sj.emboj.7600103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Grallert A, Boke E, Hagting A, Hodgson B, Connolly Y, Griffiths JR, Smith DL, Pines J, Hagan IM. A PP1-PP2A phosphatase relay controls mitotic progression. Nature 2015; 517:94-8; PMID:25487150; http://dx.doi.org/ 10.1038/nature14019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chica N, Rozalen AE, Perez-Hidalgo L, Rubio A, Novak B, Moreno S. Nutritional Control of Cell Size by the Greatwall-Endosulfine-PP2A.B55 Pathway. Curr Biol 2016; 26:319-30; PMID:26776736; http://dx.doi.org/ 10.1016/j.cub.2015.12.035 [DOI] [PubMed] [Google Scholar]
- [6].Lopez-Aviles S, Grande M, Gonzalez M, Helgesen AL, Alemany V, Sanchez-Piris M, Bachs O, Millar JB, Aligue R. Inactivation of the Cdc25 phosphatase by the stress-activated Srk1 kinase in fission yeast. Mol Cell 2005; 17:49-59; PMID:15629716; http://dx.doi.org/ 10.1016/j.molcel.2004.11.043 [DOI] [PubMed] [Google Scholar]
- [7].Wu L, Russell P. Nim1 kinase promotes mitosis by inactivating Wee1 tyrosine kinase. Nature 1993; 363:738-41; PMID:8515818; http://dx.doi.org/ 10.1038/363738a0 [DOI] [PubMed] [Google Scholar]


