Abstract
Environmental enrichment (EE) enhances cognition after traumatic brain injury (TBI). Galantamine (GAL) is an acetylcholinesterase inhibitor that also may promote benefits. Hence, the aims of this study were to assess the efficacy of GAL alone (standard [STD] housing) and in combination with EE in adult male rats after TBI. The hypothesis was that both therapies would confer motor, cognitive, and histological benefits when provided singly, but that their combination would be more efficacious. Anesthetized rats received a controlled cortical impact or sham injury, then were randomly assigned to receive GAL (1, 2, or 3 mg/kg; intraperitoneally [i.p.]) or saline vehicle (VEH; 1 mL/kg; i.p.) beginning 24 h after surgery and once daily for 21 days (experiment 1). Motor (beam-balance/walk) and cognitive (Morris water maze [MWM]) assessments were conducted on post-operative Days 1–5 and 14–19, respectively. Cortical lesion volumes were quantified on Day 21. Sham controls were better versus all TBI groups. No differences in motor function or lesion volumes were observed among the TBI groups (p > 0.05). In contrast, GAL (2 mg/kg) enhanced MWM performance versus VEH and GAL (1 and 3 mg/kg; p < 0.05). In experiment 2, GAL (2 mg/kg) or VEH was combined with EE and the data were compared with the STD-housed groups from experiment 1. EE alone enhanced motor performance over the VEH-treated and GAL-treated (2 mg/kg) STD-housed groups (p < 0.05). Moreover, both EE groups (VEH or GAL) facilitated spatial learning and reduced lesion size versus STD + VEH controls (p < 0.05). No additional benefits were observed with the combination paradigm, which does not support the hypothesis. Overall, the data demonstrate that EE and once daily GAL (2 mg/kg) promote cognitive recovery after TBI. Importantly, the combined therapies did not negatively affect outcome and thus this therapeutic protocol may have clinical utility.
Keywords: : acetylcholinesterase inhibitors (AChEIs), beam-walking, behavior, controlled cortical impact (CCI), functional recovery, galantamine, hippocampus, learning and memory, Morris water maze, traumatic brain injury
Introduction
A proposed cholinergic hypothesis, based on clinical and pre-clinical data, posits that disruptions in acetylcholine (ACh) neurotransmission after traumatic brain injury (TBI) mediate many of the cognitive impairments reported.1 Support for the hypothesis is derived from numerous studies showing injury-related alterations in the cholinergic system, such as reduced ACh turnover and release, decreased expression of muscarinic and nicotinic ACh receptors, vesicular ACh transporters, and choline acetyltransferase (ChAT), the rate-limiting enzyme for the synthesis of ACh.2–10 In parallel with the aforementioned cholinergic disruptions, cognitive performance also is diminished.11–15 As such, several therapeutic strategies have been used to attenuate cognitive deficits or improve performance after TBI.15,16 Among them, acetylcholinesterase inhibitors (AChEIs), which extend the effect of ACh on neurons and restore cholinergic tone, have received the most attention.12–15
AChEIs have shown improvement in memory and executive function in TBI patients17–23 and mixed results in rodents.24,25 For example, donepezil, a competitive, reversible, and potent AChEI, exhibits small-to-moderate pro-cognitive effects in humans23 and in select laboratory conditions.24–28 In a murine model of cortical impact injury, donepezil was reported to improve spatial learning24; however, the effect was modest and the post hoc statistic did not correct for multiple comparisons, which may have affected the interpretation. In marked contrast, low doses of donepezil were not only ineffective in promoting spatial learning and motor function after a moderate controlled cortical impact (CCI) injury, but once daily doses of 2 mg/kg and 3 mg/kg were actually detrimental to the recovery process.25 Similarly, low doses of physostigmine, a reversible AChEI, prevented TBI-induced spatial memory impairments and attenuated locomotor deficits in a rotarod task, but higher doses led to a decline in performance.29,30 Daily administration of tacrine after moderate fluid percussion injury also resulted in a dose-related impairment of water maze performance for both TBI and sham-operated controls.31 While these AChEIs may enhance cholinergic neurotransmission, their action is ubiquitous and may lead to increased incidence of cholinergic side effects, which limit their use.32,33
However, unlike the aforementioned AChEIs, galantamine (GAL) is unique because at low doses it acts as an allosteric potentiating ligand at α4- and α7-nicotinic ACh receptors (nAChR), while at high doses it acts as a competitive and reversible AChEI with considerably weaker potency and shorter duration of action, compared with donepezil.34–36 GAL decreases AChE activity and increases nAChRs expression mainly in the frontal cortex and hippocampus,37,38 which are brain regions that often become dysfunctional after clinical or experimental TBI.39–41 Therapeutically, GAL is associated with improved episodic memory and amelioration of depressive symptoms in TBI patients.42 GAL also has been shown to improve cognitive function in patients with Alzheimer's disease and mild cognitive impairment.43–48 Moreover, a substantial proportion of patients who failed to recover with donepezil exhibited benefits when switched to GAL, suggesting that previous failures in responding to certain AChEIs do not predict responses to others, such as GAL.49
The differential findings reported with AChEIs underscore the importance of other neurotransmitters in mediating cognition and therefore evaluating these pharmacotherapies in concert with more broad-spectrum therapeutic paradigms to restore homeostasis may be more fruitful in alleviating cognitive deficits after TBI. An ideal candidate therapy to augment or complement pharmacotherapies is environmental enrichment (EE). EE is an expansive living condition affording a plethora of physical, sensory, and social stimulation.50–54 Because of its consistent and robust effectiveness in conferring motor, cognitive, and histological benefits after TBI,50–57 EE is considered a pre-clinical model of neurorehabilitation.53,54 Moreover, the EE-induced benefits are long-lasting58 and can be achieved even when initiation is delayed59,60 or abbreviated.61 EE also can provide additional benefit when combined with other therapeutic strategies that on their own also confer improvement, and thus this strategy is relevant to mimic clinical rehabilitation that often is paired with pharmacotherapies.62–64
Although GAL appears to be less toxic and more effective than other AChEIs, such as donepezil, due to its actions as a weaker AChEI and a powerful nicotinic ligand,65,66 its therapeutic efficacy on neurological recovery after TBI remains unclear. Hence, the aims of this study were to evaluate the therapeutic dose profile of chronic pharmacological treatment with GAL on motor, cognitive, and histopathological outcomes after TBI (experiment 1), then to combine the optimal dose with EE (experiment 2) to test the hypothesis that both therapies will confer benefits when provided singly, but their combination would be more efficacious than either alone.
Methods
Experiment 1: GAL dose response
Animals
Forty-eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 300–325 g on the day of surgery were pair-housed in standard (STD) steel-wire mesh cages, and maintained in temperature- (21 ± 1°C) and light- (on 7:00 a.m. to 7:00 p.m.) controlled environment with ad libitum access to rat chow and water. After 1 week of acclimatization, the rats were pre-trained on the beam-walk/balance tasks. All experimental procedures were carried out during the light cycle and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. Every attempt was made to limit the number of rats used and to minimize suffering.
Surgery
On the day of surgery, the rats were pre-assessed on the motor tasks to determine baseline performance. A controlled cortical impact (CCI) injury was subsequently produced as described previously.58–61,63,64,67–69 Briefly, surgical anesthesia was induced and maintained with inhaled concentrations of 4% and 2% isoflurane, respectively, in 2:1 N2O:O2. After endotracheal intubation, the rats were secured in a stereotaxic frame and ventilated mechanically. Core temperature was maintained at 37 ± 0.5°C with a heating blanket. Using aseptic procedures, a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6 mm in diameter) was made in the right hemisphere with a trephine. The craniectomy was enlarged further with rongeurs to accommodate the 6-mm impact tip. Subsequently, the impacting rod was extended and the impact tip was centered and lowered through the craniectomy until it touched the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm farther to produce a brain injury of moderate severity (2.8 mm tissue deformation at 4 m/sec). Immediately after the CCI, anesthesia was discontinued, the incision was sutured, and the rats were extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but did not receive the impact.
Post-surgery
After surgery, the rats were randomly assigned to four TBI groups, which are represented as TBI + STD + GAL (1 mg/kg; n = 8), TBI + STD + GAL (2 mg/kg; n = 8), TBI + STD + GAL (3 mg/kg; n = 8), TBI + STD + vehicle (VEH; 1 mL/kg; n = 8) and four sham control groups (n = 4 per group) that received the same doses of GAL and VEH as their TBI counterparts.
Acute neurological evaluation
Hind limb reflexive ability was assessed immediately after the discontinuation of anesthesia by gently squeezing the rats' paw every 5 sec and recording the latency to elicit a withdrawal response. Return of the righting reflex was determined by the time required to turn from the supine to prone position on three consecutive trials.
Drug administration
GAL (Sigma-Aldrich, St. Louis, MO) was prepared daily by dissolving in sterile saline, which also served as the VEH. GAL (1, 2, or 3 mg/kg) or a comparable volume of VEH (1 mL/kg) was administered intraperitoneally beginning 24 h after cortical impact or sham injury and once daily for 3 weeks. On the days of behavioral testing, the injections were administered 1 h prior to testing. The doses were selected based on preliminary data from our laboratory showing a narrow therapeutic range. The route of administration is standard protocol in our laboratory. 58–61,63,64,67–69
Motor function
Motor performance was assessed using well-established and well-documented beam-balance and beam-walk tasks.58–61,63,64,67,68,70 Briefly, beam-balance consisted of placing the rat on an elevated narrow beam (1.5 cm wide) and recording the time it remained on for a maximum of 60 sec. Beam-walk consisted of recording the elapsed time to traverse the beam (2.5 cm wide × 100 cm long). Pre-assessment was conducted prior to surgery (to establish a baseline measure), as well as on post-operative Days 1–5, and consisted of three trials (60 sec allotted time) per day on each task. The average daily scores for each subject were used in the statistical analyses.
Cognitive function: spatial learning
Acquisition of spatial learning was assessed using a Morris water maze (MWM) task.71 The maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26 ± 1°C) to a depth of 28 cm and was positioned in a room with salient visual cues. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat. Spatial learning consisted of providing four blocks of daily trials (4 min inter-trial interval) for 5 consecutive days (post-operative Days 14–18) in which the rat was given a maximum of 120 sec to find the hidden platform (2 cm below the water surface). On post-operative Day 19, the platform was raised 2 cm above the water surface, making it visible to the rat; this manipulation served as a control to determine the contributions of non-spatial factors (e.g., sensorimotor performance, motivation, and visual acuity) on cognitive performance. Each trial lasted until the rat climbed onto the platform or the maximum allotted time had elapsed. If the rats did not find the platform within the given time, they were manually guided to it. All rats remained on the platform for 30 sec, then were returned to a heated incubator between trials. The times of the four daily trials for each rat were averaged and used in the statistical analyses. A spontaneous motor activity recording and tracking system (San Diego Instruments, San Diego, CA) was used to record the data, which included time to locate the platform and percent time in the target quadrant.
Histology: quantification of cortical lesion volume
Three weeks after CCI or sham injury the rats were anesthetized with Fatal-Plus (0.3 mL, intraperitoneally), then perfused transcardially with 200 mL 0.1 M phosphate-buffered saline (pH 7.4) followed by 300 mL 4% paraformaldehyde. The brains were extracted, post-fixed in the perfusate for 1 week, dehydrated with alcohols, and embedded in paraffin. Coronal sections (7-μm thick) were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on Superfrost/Plus glass microscope slides. After drying at room temperature, the sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet. Cortical lesion volumes (mm3) were determined by calculating the area of the lesion (mm2), which was done by outlining the inferred area of missing cortical tissue for each section (typically 5–7) taken at 1-mm intervals (MCID, Imaging Research, Ontario, Canada), then by summing the lesions obtained from each section as previously reported.50,68,69,72,73
Experiment 2: combined therapeutic paradigm, GAL + EE
Subjects and surgery
An additional 24 adult male rats were added to this experiment, which consisted of two TBI groups (n = 8 per group) and two sham control groups (n = 4 per group) denoted as TBI + EE + VEH (1 mL/kg), TBI + EE + GAL (2 mg/kg), Sham + EE + VEH (1 mL/kg), and Sham + EE + GAL (2 mg/kg). Initial housing, motor pre-training, surgery, and acute neurological evaluation were identical to that described for experiment 1. These groups were compared with the TBI + STD + VEH and TBI + STD + GAL (2.0 mg/kg) groups and their respective sham controls from experiment 1. Given that experiment 1 and experiment 2 were conducted in close proximity (within a week) by the same personnel, we felt there were no confounds that could affect the outcomes and hence it was unnecessary to use more rats, which is in line with the University of Pittsburgh IACUC and National Institutes of Health (NIH) guidelines. Assessment of motor and cognitive function, as well as histological outcomes for experiment 2, were performed exactly as for experiment 1.
Drug administration
The dose of GAL for comparison between the STD and EE groups was selected based on the finding from experiment 1 showing that 2 mg/kg was most effective. Drug preparation and route and frequency of administration were identical to that described for experiment 1.
Housing conditions: environmental manipulation
Following surgery and after the effects of anesthesia abated (as evidenced by spontaneous movement in the holding cage), the rats were returned to the colony where those designated for enrichment were immediately placed in specifically designed steel-wire cages (91 × 76 × 50 cm). The EE cages consisted of three levels with ladders to ambulate from one level to another and contained various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), and ad libitum food and water.50,53,54,74 To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was twice per week. Ten to 12 rats, which included GAL and VEH-treated TBI and sham controls, were housed in the EE together to minimize variability among the groups. Rats in the STD conditions were placed in typical shoebox cages (37 × 25 × 18 cm, two rats per cage) with only food and water.
Statistical analysis
All analyses were performed using StatView 5.0.1 software (Abacus Concepts, Inc.) on data collected by blinded experimenters. The motor and cognitive analyses were conducted using repeated-measures analysis of variance (ANOVA). The acute neurological data (i.e., hind limb withdrawal reflex and righting reflex), as well as the data for the visible platform, probe trial, swim speed, and cortical lesion volume, were analyzed using one-factor ANOVAs. When the overall ANOVA revealed significant effects, the Newman-Keuls post hoc test was used to determine specific group differences. The results are expressed as the mean ± standard error of the mean (standard error of the mean [SEM]) and were considered significant when p ≤ 0.05.
Results
Experiment 1: GAL dose response
Sham controls did not differ from one another, regardless of treatments, and thus they were pooled into one group (denoted as SHAM).
Acute neurological function
No differences were revealed among the TBI groups in the hind limb withdrawal reflex (left range = 179.3 ± 7.3 sec to 193.8 ± 6.7 sec, p > 0.05; right range = 173.6 ± 7.2 sec to 181.8 ± 4.7 sec, p > 0.05) or for return of righting ability (range 374.7 ± 8.1 sec to 393.9 ± 9.6 sec, p > 0.05) following the discontinuation of anesthesia. However, as expected, all TBI groups were significantly different from the SHAM controls, who displayed limb withdrawal reflex times ranging from 29–34 sec and righting reflex times of 96–104 sec (p < 0.05).
Motor function: beam-balance
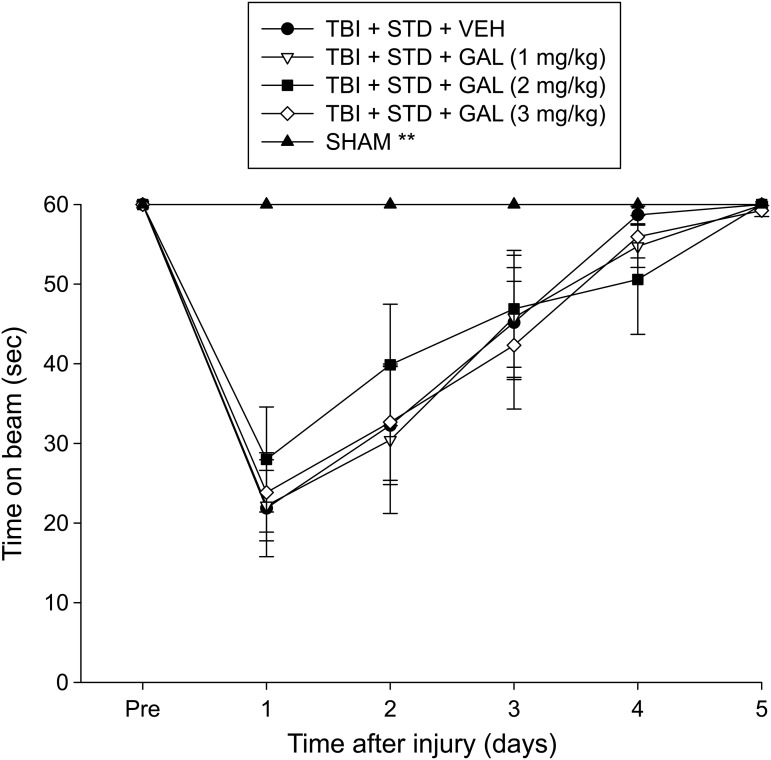
There were no pre-surgical differences among groups, as all rats were capable of balancing on the beam for the allotted 60 sec on each of the three trials (Fig. 1). Following the CCI, all TBI rats were significantly impaired, compared with the SHAM controls, which were able to maintain pre-surgical balancing ability for the entire 60 sec. The ANOVA revealed significant Group (F4,43 = 8.061, p < 0.0001) and Day (F5,215 = 63.970, p < 0.0001) differences, as well as a significant Group × Day interaction (F20,215 = 6.430, p < 0.0001). The post hoc analysis revealed that the SHAM group was better than all TBI groups (p < 0.05), which did not differ from one another (p > 0.05).
FIG. 1.
Mean (± standard error of the mean) time (sec) balancing on an elevated narrow beam prior to and after traumatic brain injury (TBI) or sham injury. **p < 0.05 vs. all TBI groups. No differences were revealed among the TBI groups. STD, standard; VEH, vehicle; GAL, galantamine.
Motor function: beam-walk
Similar to the beam-balance data, there were no differences among groups prior to surgery, as all rats proficiently traversed the entire length of the beam to reach the goal box (Fig. 2). Following TBI, there was a significant increase in beam-walking time for all TBI groups relative to SHAM controls. The ANOVA revealed significant Group (F4,43 = 17.965, p < 0.0001) and Day (F5,215 = 122.968, p < 0.0001) differences, as well as a significant Group × Day interaction (F20,215 = 11.904, p < 0.0001). Post hoc analyses revealed that the SHAM group was able to traverse the beam better than all TBI groups (p < 0.05). No differences were detected among the TBI groups, regardless of treatment (p > 0.05).
FIG. 2.
Mean (± standard error of the mean) time (sec) to traverse an elevated narrow beam prior to and after traumatic brain injury (TBI) or sham injury. **p < 0.05 vs. all TBI groups. No differences were revealed among the TBI groups. STD, standard; VEH, vehicle; GAL, galantamine.
Cognitive function: spatial learning
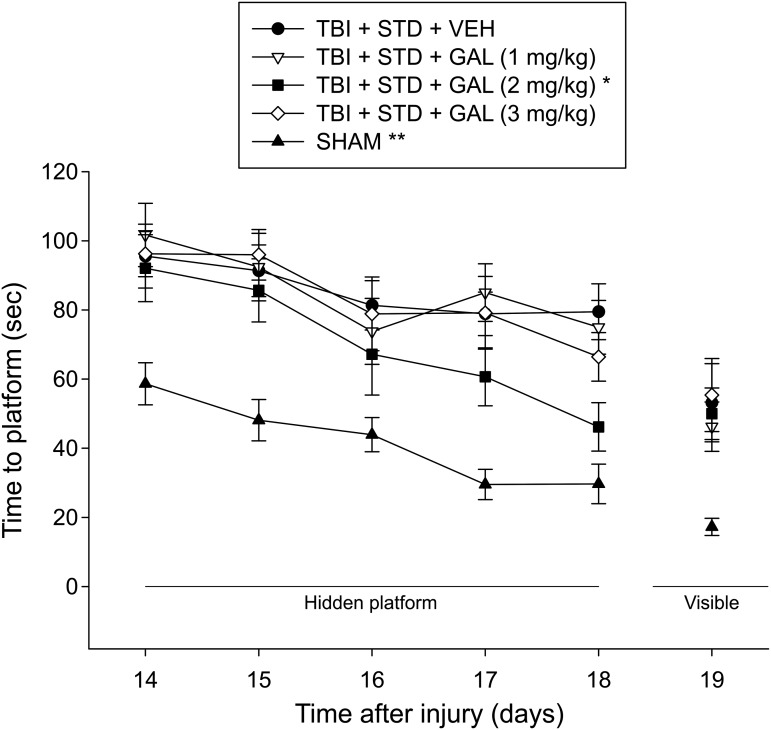
Analysis of the water maze data revealed significant Group (F4,43 = 18.218, p < 0.0001) and Day (F4,172 = 16.849, p < 0.0001) differences, but no Group × Day interaction (F16,172 = 0.771, p = 0.717), indicating that although the TBI groups became progressively better at locating the escape platform in a similar fashion regardless of treatment, they were still significantly impaired relative to the SHAM group, which learned the task at a faster rate (p < 0.05; Fig. 3). The post hoc also revealed that the TBI + GAL v(2.0 mg/kg) group displayed enhanced recovery of cognitive performance over the 5 test days of spatial learning, compared with the TBI + VEH, TBI + GAL (1 mg/kg), and TBI + GAL (3 mg/kg) groups (p's < 0.05). No other group comparisons were significant (p's > 0.05). Analysis of the visible platform data revealed a significant group effect (F4,43 = 6.527, p = 0.0003) that was attributed to the SHAM controls requiring less time to reach the platform versus the TBI groups, which did not differ from one another (p's > 0.05). There were no differences in swim speed among the groups (p > 0.05).
FIG. 3.
Mean (± standard error of the mean) time (sec) to locate either a hidden or visible platform in a water maze. For hidden platform, *p < 0.05 vs. traumatic brain injury (TBI) + STD + VEH and **p < 0.05 vs. all TBI groups. For visible platform, **p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05). STD, standard; VEH, vehicle; GAL, galantamine.
Histology: cortical lesion volume
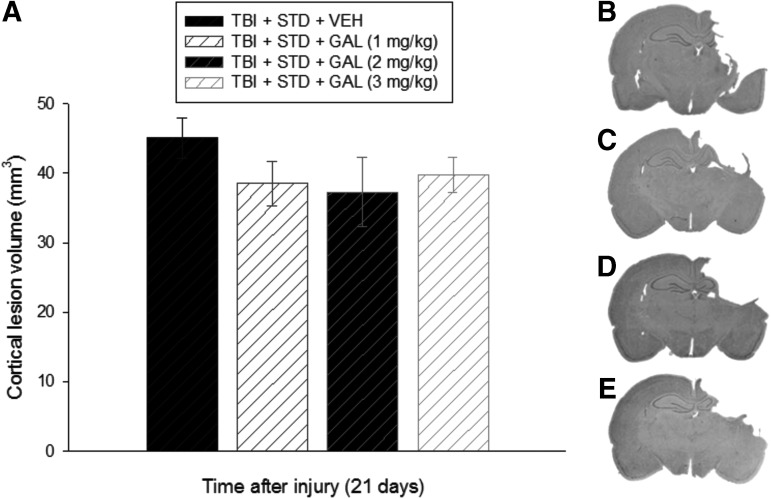
Analysis of the lesion data did not reveal a difference among the groups, regardless of treatment (p = 0.44; Fig. 4). Specifically, mean ± SEM cortical lesion volumes were 45.1 ± 2.9, 38.5 ± 3.2, 37.3 ± 4.9, and 39.7 ± 2.5 mm3 for the TBI + VEH, TBI + GAL (1 mg/kg), TBI + GAL (2 mg/kg), and TBI + GAL (3 mg/kg) groups, respectively.
FIG. 4.
Panel (A) depicts mean (± standard error of the mean) cortical lesion volume (mm3) at 21 days after traumatic brain injury (TBI). No significant differences were revealed among the groups, regardless of treatment. (B–E) Average sized lesions at the level of the dorsal hippocampus for the TBI + STD + VEH, TBI + STD + GAL (1 mg/kg), TBI + STD + GAL (2 mg/kg), and TBI + STD + GAL (3 mg/kg) groups, respectively. STD, standard; VEH, vehicle; GAL, galantamine.
Experiment 2: combined therapeutic paradigm: GAL + EE
The sham controls administered GAL or VEH and housed in EE conditions did not differ from the STD-housed shams in experiment 1, and thus they were pooled into one group. The makeup of the shams for experiment 2 consisted of STD-housed shams receiving either GAL (2 mg/kg) or VEH (from experiment 1) and EE shams receiving GAL (2 mg/kg) or VEH and are denoted as SHAM.
Acute neurological function
No differences were revealed among the TBI groups in the hind limb withdrawal reflex (left range = 182.5 ± 6.7 sec to 191.9 ± 8.31 sec, p > 0.05; right range = 176.7 ± 8.1 sec to 183.6 ± 6.3 sec, p > 0.05) or for return of righting ability (range 383.2 ± 5.8 sec to 397.3 ± 7.4 sec, p > 0.05) following the discontinuation of anesthesia. Similar to that observed in experiment 1, all TBI groups were significantly different from the SHAM controls that displayed limb withdrawal reflex times ranging from 29–33 sec and righting reflex times of 93–101 sec (p < 0.05).
Motor function: beam-balance
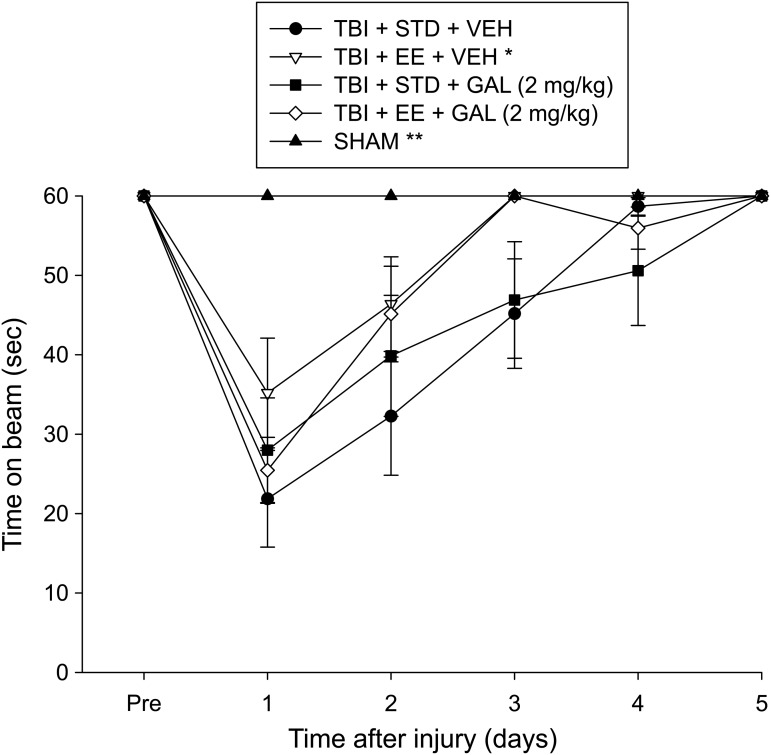
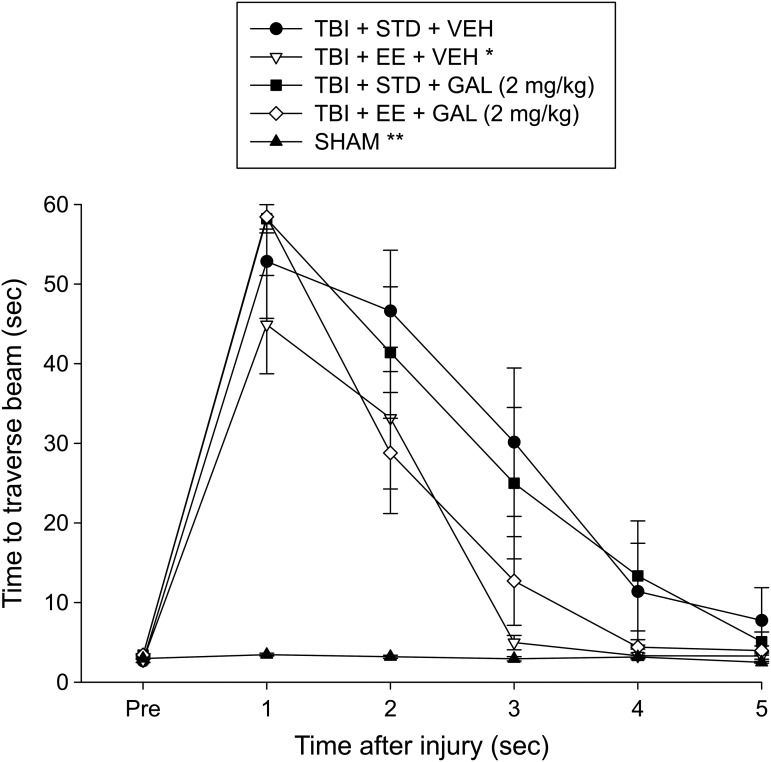
No pre-surgical differences were observed among groups as all rats were capable of balancing on the beam for the allotted 60 sec on each trial (Fig. 5). After surgery, the repeated-measures ANOVA revealed significant Group (F4,43 = 8.885, p < 0.0001) and Day (F5,215 = 59.555, p < 0.0001) differences, as well as a significant Group × Day interaction (F20,215 = 6.939, p < 0.0001). The post hoc analysis revealed that all TBI groups were significantly impaired, compared with the SHAM group, which was able to maintain balance for the full 60 sec (p < 0.05). Among the TBI groups, the TBI + EE + VEH performed markedly better than the TBI + STD + VEH group (p < 0.05). No other comparisons were significant (p > 0.05).
FIG. 5.
Mean (± standard error of the mean) time (sec) balancing on an elevated narrow beam prior to and after TBI or sham injury. *p < 0.05 vs. TBI + STD + VEH and **p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05). STD, standard; VEH, vehicle; EE, environmental enrichment; GAL, galantamine.
Motor function: beam-walk
There were no pre-surgical differences in time to traverse the beam among groups, as all rats were proficient and reached the goal box in approximately 5 sec (Fig. 6). Following surgery, there was an increase in beam-walking time for all TBI groups relative to SHAM controls. The ANOVA revealed significant Group (F4,43 = 16.787, p < 0.0001) and Day (F5,215 = 114.326, p < 0.0001) differences, as well as a significant Group × Day interaction (F20,215 = 11.916, p < 0.0001). Post hoc analyses revealed that the SHAM group was able to traverse the beam quicker than all TBI groups (p < 0.05) and that the TBI + EE + VEH group traversed the beam more rapidly than the TBI + STD + VEH and TBI + STD + GAL groups (p < 0.05). No difference was revealed between the TBI + EE + VEH and TBI + EE + GAL groups (p > 0.05). No other comparisons were significant (p > 0.05).
FIG. 6.
Mean (± standard error of the mean) time (sec) to traverse an elevated narrow beam prior to and after traumatic brain injury (TBI) or sham injury. *p < 0.05 vs. TBI + STD + VEH and TBI + STD + GAL (2 mg/kg). **p < 0.05 vs. all TBI groups. No other comparisons were significant (p > 0.05). STD, standard; VEH, vehicle; GAL, galantamine.
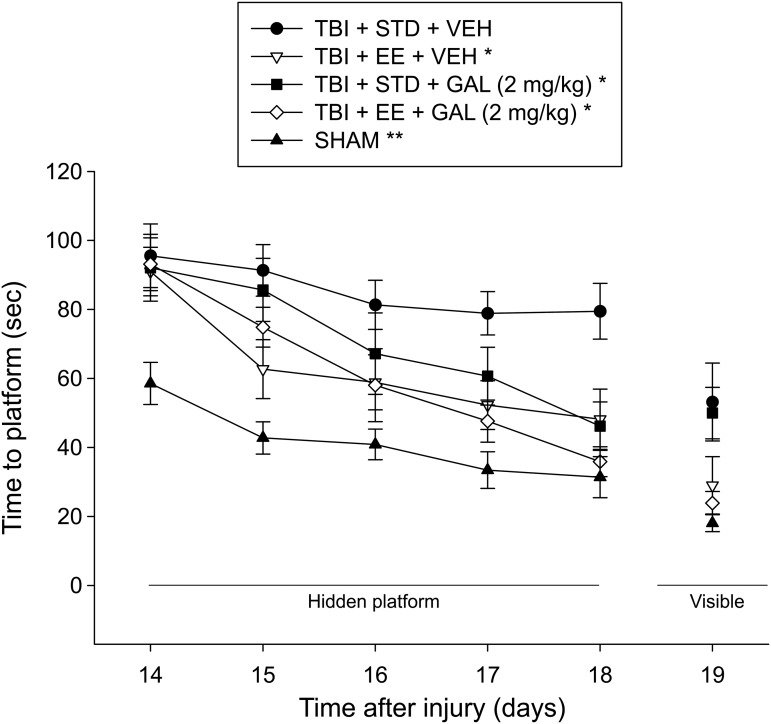
Cognitive function: spatial learning
Analysis of the water maze data revealed significant Group (F4,43 = 12.294, p < 0.0001) and Day (F4,172 = 29.984, p < 0.0001) differences, but no Group x Day interaction (F16,172 = 1.380, p = 0.156). Post hoc analysis revealed that the SHAM group learned the location of the escape platform faster than the TBI groups, regardless of treatment (p < 0.05; Fig. 7). Moreover, the TBI + EE + VEH, TBI + EE + GAL, and TBI + STD + GAL groups performed the task better than the TBI + STD + VEH group (p's < 0.05) but did not differ from one another (p > 0.05). No other group comparisons were significant (p's > 0.05). Analysis of the visible platform data revealed a significant group effect (F4,43 = 6.535, p = 0.0003). Specifically, the post hoc analysis revealed that the SHAM, TBI + EE + VEH, and TBI + EE + GAL groups reached the visible platform quicker than TBI + STD treatment groups, regardless of treatment (p < 0.05), but did not differ from each other (p > 0.05). There was no difference between the TBI + STD groups (p > 0.05). There were no differences in swim speed among the groups (p > 0.05).
FIG. 7.
Mean (± standard error of the mean) time (sec) to locate either a hidden or visible platform in a water maze. For hidden platform, *p < 0.05 vs. traumatic brain injury (TBI) + STD + VEH and **p < 0.05 vs. all TBI groups. For visible platform, the SHAM, TBI + EE + VEH, and TBI + EE + GAL groups reached the platform quicker than the TBI + STD groups, regardless of treatment (p < 0.05). No other comparisons were significant (p > 0.05). STD, standard; VEH, vehicle; EE, environmental enrichment; GAL, galantamine.
Histology: cortical lesion volume
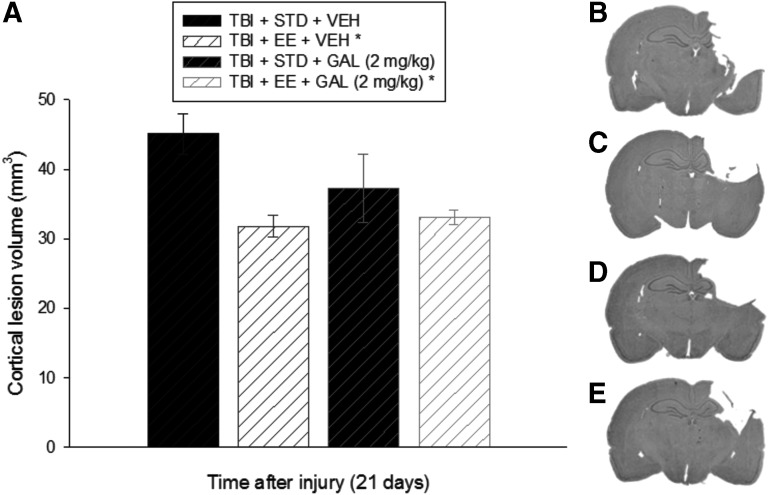
Analysis of the lesion data revealed a difference among the groups (p = 0.026; Fig. 8). Specifically, both EE groups exhibited smaller lesions relative to the TBI + STD + VEH group (p < 0.05), but did not differ from each other (p > 0.05). No other comparisons were different (p > 0.05). Mean ± SEM cortical lesion volumes were 45.1 ± 2.9, 37.3 ± 4.9, 31.8 ± 1.5, and 33.1 ± 1.0 mm3 for the TBI + STD + VEH, TBI + STD + GAL, TBI + EE + VEH, and TBI + EE + GAL groups, respectively.
FIG. 8.
Panel (A) depicts mean (± standard error of the mean) cortical lesion volume (mm3) at 21 days after TBI. *p < 0.05 vs. TBI + STD + VEH. No other comparisons were significant (p > 0.05). (B–E) Average sized lesions at the level of the dorsal hippocampus for the TBI + STD + VEH (same group from experiment 1), TBI + EE + VEH, TBI + STD + GAL (2 mg/kg; same group from experiment 1), and TBI + EE + GAL (2 mg/kg) groups, respectively. STD, standard; VEH, vehicle; EE, environmental enrichment; GAL, galantamine.
Discussion
The goal of the current study was to assess the potential benefits of GAL when presented alone (experiment 1) and when combined with EE (experiment 2) after a CCI injury of moderate severity. In experiment 1, three doses of GAL (1, 2, and 3 mg/kg) were administered to rats housed in STD conditions to determine a dose–response effect on motor, cognitive, and histological outcome. In experiment 2, the optimal dose from experiment 1 was administered to rats in conjunction with EE, a pre-clinical model of neurorehabilitation, to determine if the benefits conferred by each therapy would be potentiated by the combination paradigm. Experiment 2 is significant, because in the real world, TBI patients will undoubtedly receive some form of pharmacotherapy with rehabilitation, and GAL (Razadyne) is currently one of the FDA-approved AChEI therapies on the market for the treatment of Alzheimer's disease, of which memory dysfunction is a cardinal symptom.
The results from experiment 1 revealed that daily administration of GAL attenuated injury-induced cognitive impairments in the water maze, but only with the middle dose of 2 mg/kg, compared with the lower and higher doses (1 mg/kg and 3 mg/kg, respectively) or VEH. No motor differences were revealed among the TBI groups, regardless of treatment or dose, as all groups recovered to baseline levels in the beam-balance and beam-walk tasks by the end of the 5 days of testing. Additionally, no differences were observed among the TBI groups in cortical lesion volumes. The lack of cortical sparing coupled with the cognitive improvement in the middle dose GAL-treated group iterates the lack of correlation between histology and behavioral outcome.75
In experiment 2, the TBI groups receiving EE + GAL (2 mg/kg) and EE + VEH performed better than the VEH and GAL-treated TBI groups housed in STD conditions. The cognitive benefits were not a result of drug-related motor impairments or visual disparities, as visible platform and swim speed parameters were comparable among the groups. Moreover, the VEH and GAL groups receiving concomitant enrichment also exhibited smaller cortical lesion volumes relative to the STD-housed groups, regardless of treatment or dose. Lastly, the VEH-treated EE group displayed improved beam-balance and beam-walk performance post-TBI relative to the GAL and VEH-treated STD-housed groups. No benefit over the STD-housed groups was revealed by the EE group receiving concomitant GAL. This latter finding may be construed as a negative interaction between the two therapies, with GAL reducing the efficacy of EE. Indeed, several studies have shown negative interactions with combination therapies.64 However, given that the decrease in behavioral outcome was observed only in gross motor function and not the more sensitive cognitive assay suggests that the effect may be due to behavioral variability versus actual negative synergistic effects.
Contrary to the hypotheses, the combination of GAL and EE did not produce benefits beyond those of the single therapies. The lack of additive effects with the combinational therapy in adult TBI rats is not entirely surprising as several studies from our laboratory have shown similar effects.11,52,64,74,76 Importantly, the combination of therapies did not produce negative effects as has been seen with different treatment paradigms.64 The findings of the current study, and those previously reported may be due to a floor effect. Indeed, closer inspection of Figure 7 shows that the EE groups were performing similarly to the uninjured sham controls by the last day of training. However, these neutral data should not lessen the enthusiasm for combination therapies, as an additive effect has been observed between EE and buspirone in pediatric rats after a CCI injury.63 Additionally, in a non-injury model, combined doses of memantine and GAL enhanced attentional set-shifting performance and reversed deficits in object recognition.77 Further, the combination of GAL and melatonin was reported to demonstrate protective effects in a novel in vitro model of Alzheimer's disease,78 which suggests that in some diseases and with certain therapeutics, additive or synergistic effects can be conferred, just as has been shown in select TBI studies.64
Potential mechanisms for the GAL-induced benefits are varied and include reducing oxidative stress and neuroinflammation, restoring cholinergic integrity, and neuroplasticity. Specifically, GAL downregulates lipid peroxidation and nitrite levels, and increases glutathione and superoxide dismutase,79 which have been reported to correlate with behavioral improvement after TBI.80 Chronic treatment with GAL also reduces cholinergic uptake and vesicular ACh transporter expression.81 These cholinergic alterations have been shown to improve passive avoidance learning and spatial memory in hypoxic rats.82–86 GAL attenuates cognitive deficits and reduces hippocampal cell loss following cerebral ischemia, which can be blocked by administration of the nicotinic antagonist mecamylamine, suggesting that the beneficial effects mediated by GAL may be related to the nAChR.87–89 Specifically, GAL increases synaptic plasticity by elevating the number and binding of nAChRs within the hippocampus, which is associated with improved learning and memory.90–94 Although GAL's activity appears to be much weaker than other clinically available AChEIs, its therapeutic effects on cognitive function in Alzheimer's disease are comparable to the other agents due to its ability to act as an allosterically potentiating ligand for nAChRs.95–97 GAL also appears to stand out more favorably regarding effects on cognitive symptoms, compared with donepezil, which lacks appreciable activity at nAChRs.98–100
Chronic administration of Lu 25–109-T, a partial M1 muscarinic receptor agonist and presynaptic M2 autoreceptor antagonist, attenuates fluid percussion injury-induced reduction of ChAT immunoreactivity in the medial septal nucleus.101 This finding offers another potential mechanism for the GAL-induced benefits observed in the current study. Specifically, the benefits observed may involve muscarinic receptors, whereas donepezil, which is ineffective,25 blocks muscarinic receptor-mediated function.102 Further, reduced binding of presynaptic M2 mAChR autoreceptors in the hippocampus and adjacent cortex has been observed after fluid percussion injury,103 which consistently produces cognitive deficits.104
Similar to GAL, EE also exerts significant motor and cognitive benefits after TBI and the potential mechanisms are also wide ranging.53,54 We have previously shown that EE attenuates ChAT positive cell loss in medial septal cells, while also reducing neuronal damage in the cornu ammonis (CA)1 and CA3 layers of the hippocampus. These neuroprotective effects parallel improved motor and cognitive performance after CCI injury.11 EE promotes plasticity in the hippocampus and frontal cortex, including long-term potentiation, brain-derived neurotrophic factor gene upregulation, enhanced dendritic branching, and stimulation of adult neurogenesis,53,54 while also reducing markers of oxidative stress and inflammation.53,54,105 Any of these EE-induced brain alterations may mediate the benefits conferred by this pre-clinical model of neurorehabilitation.53,54,106
Taken together, the results demonstrate that both EE and GAL enhance cognitive recovery after CCI injury. The narrow dose range of GAL, which also has been reported with donepezil,25 supports the evidence of an inverted U-shaped relationship between ACh levels and performance in a variety of tasks, notably in regard to learning and memory.107,108 The clinical implications of such a narrow therapeutic window is that the potential efficacy of a treatment may be overlooked simply by providing a suboptimal dose. To minimize or prevent all together such occurrences, dose–response profiles should be initiated and doses managed carefully in the clinic. Additionally, future studies should evaluate AChEIs such as GAL in both sexes and with various forms of rehabilitation to make the model even more clinically applicable.
Acknowledgments
This work was supported, in part, by the National Institutes of Health grants NS060005, HD069620, HD069620-S1, NS084967 (AEK), and NS094950 (COB), and the University of Pittsburgh Physicians /UPMC Academic Foundation (COB).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arciniegas D. (2003). The cholinergic hypothesis of cognitive impairment caused by traumatic brain injury. Cur. Psych. Reports 5, 391–399 [DOI] [PubMed] [Google Scholar]
- 2.Dewar D. and Graham D.I. (1996). Depletion of choline acetyltransferase activity but preservation of M1 and M2 muscarinic receptor binding sites in temporal cortex following head injury: a preliminary human postmortem study. J. Neurotrauma 13, 181–187 [DOI] [PubMed] [Google Scholar]
- 3.Dixon C.E., Bao J., Long D.A., and Hayes R.L. (1996). Reduced evoked release of acetylcholine in the rodent hippocampus following traumatic brain injury. Pharmacol. Biochem. Behav. 53, 679–686 [DOI] [PubMed] [Google Scholar]
- 4.Griffin S.L., van Reekum R., and Masanic C. (2003). A review of cholinergic agents in the treatment of neurobehavioral deficits following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 15, 17–26 [DOI] [PubMed] [Google Scholar]
- 5.Murdoch I., Perry E.K., Court J.A., Graham D.I., and Dewar D. (1998). Cortical cholinergic dysfunction after human head injury. J. Neurotrauma 15, 295–305 [DOI] [PubMed] [Google Scholar]
- 6.Kelso M.L., and Oestreich J.H. (2012). Traumatic brain injury: Central and peripheral role of α7 nicotinic acetylcholine receptors. Curr. Drug. Targets 13, 631–636 [DOI] [PubMed] [Google Scholar]
- 7.Dixon C.E., Ma X., and Marion D.W. (1997). Reduced evoked release of acetylcholine in the rodent neocortex following traumatic brain injury. Brain Res. 749, 127–130 [DOI] [PubMed] [Google Scholar]
- 8.Scremin O.U., Li M.G., Roch M., Booth R., and Jenden D.J. (2006). Acetylcholine and choline dynamics provide early and late markers of traumatic brain injury. Brain Res. 1124, 155–166 [DOI] [PubMed] [Google Scholar]
- 9.Saija A., Hayes R.L., Lyeth B.G., Dixon C.E., Yamamoto T., and Robinson S.E. (1988). The effect of concussive head injury on central cholinergic neurons. Brain Res. 452, 303–311 [DOI] [PubMed] [Google Scholar]
- 10.Verbois S.L., Scheff S.W., and Pauly J.R. (2002). Time-dependent changes in rat brain cholinergic receptor expression after experimental brain injury. J. Neurotrauma 19, 1569–1585 [DOI] [PubMed] [Google Scholar]
- 11.Kline A.E., McAloon R.L., Henderson K.A., Bansal U.K., Ganti B.M., Ahmed R.H., and Sozda C.N. (2010). Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin S.S. and Dixon C.E. (2015). Alterations in cholinergic pathways and therapeutic strategies targeting cholinergic system after traumatic brain injury. J. Neurotrauma 32, 1429–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin S.S. and Dixon C.E. (2015). Targeting alpha7 nicotinic acetylcholine receptors: a future neurotprotection from traumatic brain injury. Neural Regen. Res. 10,10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dash P.K., Zhao J., Kobori N., Redell J.B., Hylin M.J., Hood K.N., and Moore A.N. (2016). Activation of alpha7 cholinergic nicotinic receptors reduce blood-brain barrier permeability following experimental traumatic brain injury. Neuroscience 36, 2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber K.B.G, Uteshev V.V., and Pauly J.R. (2016). Targeting the cholinergic system for neuroprotection and/or enhancement of functional recovery following neurotrauma. Cur. Pharm. Des. 22, 2072–2082 [DOI] [PubMed] [Google Scholar]
- 16.Kelso M.L. and Pauly J.R. (2011). Therapeutic targets for neuroprotection and/or enhancement of functional recovery following traumatic brain injury. Prog. Mol. Biol. Transl. Sci. 98, 85–131 [DOI] [PubMed] [Google Scholar]
- 17.Taverni J.P., Seliger G., and Lichtman S.W. (1998). Donepezil medicated memory improvement in traumatic brain injury during post acute rehabilitation. Brain Inj. 12, 77–80 [DOI] [PubMed] [Google Scholar]
- 18.Morey C.E., Cilo M., Berry J., and Cusick C. (2003). The effect of Aricept in persons with persistent memory disorder following traumatic brain injury: a pilot study. Brain Inj. 17, 809–815 [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Plotkin R.C., Wang G., Sandel M.E., and Lee S. (2004). Cholinergic augmentation with donepezil enhances recovery in short-term memory and sustained attention after traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 1050–1055 [DOI] [PubMed] [Google Scholar]
- 20.Khateb A., Ammann J., Annoni J.M., and Diserens K. (2005). Cognition-enhancing effects of donepezil in traumatic brain injury. Eur. Neurol. 54, 39–45 [DOI] [PubMed] [Google Scholar]
- 21.Tenovuo O. (2005). Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury-clinical experience in 111 patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 61–67 [DOI] [PubMed] [Google Scholar]
- 22.Silver J.M., Koumaras B., Chen M., Mirski D., Potkin S.G., Reyes P., and Gunay I. (2006). Effects of rivastigmine on cognitive function in patients with traumatic brain injury. Neurology 67, 748–755 [DOI] [PubMed] [Google Scholar]
- 23.Ballesteros J., Guemes I., Ibarra N., and Quemada J.I. (2008). The effectiveness of donepezil for cognitive rehabilitation after traumatic brain injury: a systematic review. J. Head Trauma Rehabil. 23, 171–180 [DOI] [PubMed] [Google Scholar]
- 24.Yu T.S., Kim A., and Kernie S.G. (2015). Donepezil rescues spatial learning and memory deficits following traumatic brain injury independent of its effects on neurogenesis. PLoS One, 10,e0118793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw K.E., Bondi C.O., Light S.H., Massimino L.A., McAloon R.L., Monaco C.M., and Kline A.E. (2013). Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J. Neurotrauma 30, 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruel-Jungerman E., Lucassen P.J., and Francis F. (2011). Cholinergic influences on cortical development and adult neurogenesis. Behav. Brain. Res. 221(2), 379–388 [DOI] [PubMed] [Google Scholar]
- 27.Narimatsu N., Harada N., Kurihara H., Nakagata N., Sobue K., and Okajima K. (2009). Donepezil improves cognitive function in mice by increasing the production of insulin-like growth factor-I in the hippocampus. J. Pharmacol. Exp. Ther. 330, 2–12 [DOI] [PubMed] [Google Scholar]
- 28.Barnes C.A., Meltzer J., Houston F., Orr G., McGann K., and Wenk G.L. (2000). Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience 99, 17–23 [DOI] [PubMed] [Google Scholar]
- 29.Holschneider D.P., Guo Y., Roch M., Norman K.M., and Scremin O.U. (2011). Acetylcholinesterase inhibition and locomotor function after motor-sensory cortex impact injury. J. Neurotrauma 28, 1909–1919 [DOI] [PubMed] [Google Scholar]
- 30.Scremin O.U., Norman K.M., Roch M., Holschneider D.P., and Scremin A.M. (2012). Acetylcholinesterase inhibition interacts with training to reverse spatial learning deficits after cortical impact injury. J. Neurotrauma 29, 2457–2464 [DOI] [PubMed] [Google Scholar]
- 31.Pike B.R., Hamm R.J., Temple M.D., Buck D.L., and Lyeth B.G. (1997). Effect of tetrahydroaminoacridine, a cholinesterase inhibitor, on cognitive performance following experimental brain injury. J. Neurotrauma 14, 897–905 [DOI] [PubMed] [Google Scholar]
- 32.Colovic M.B., Krstic D.Z., Lazarevic-Pasti T.D., Bondzic A.M., and Vasic V.M. (2013). Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr. Neuropharmacol. 11, 315–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seltzer B. (2005). Donepezil: a review. Expert Opin. Drug Metab. Toxicol. 1, 527–536 [DOI] [PubMed] [Google Scholar]
- 34.Fayuk D., and Yakel J.L. (2004). Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol. Pharmacol. 66, 658–666 [DOI] [PubMed] [Google Scholar]
- 35.Bickel U., Thomsen T., Fischer J. P., Weber W., and Kewitz H. (1991). Galanthamine: pharmacokinetics, tissue distribution and cholinesterase inhibition in brain of mice. Neuropharm. 30, 447–454 [DOI] [PubMed] [Google Scholar]
- 36.Geerts H., Guillaumat P.O., Grantham C., Bode W., Anciaux K., and Sachak S. (2005). Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 1033, 186–193 [DOI] [PubMed] [Google Scholar]
- 37.Reid R.T. and Sabbagh M.N. (2008). Effects of cholinesterase inhibitors on rat nicotinic receptor levels in vivo and in vitro. J. Neural Transm. Vienna 115, 1437–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svoboda Z., Kvetina J., Kunesova G., Herink J., Bajgar J., Bartosova L., and Palicka V. (2006). Effect of galantamine on acetylcholinesterase and butyrylcholinesterase activities in the presence of L-carnitine in rat selected brain and peripheral tissues. Neuroendocrinol. Lett. 27, 183–186 [PubMed] [Google Scholar]
- 39.Arciniegas D., Adler L., Topkoff J., Cawthra E., Filley C.M., and Reite M. (1999). Attention and memory dysfunction after traumatic brain injury: cholinergic mechanisms, sensory gating, and a hypothesis for further investigation. Brain Inj. 13, 1–13 [DOI] [PubMed] [Google Scholar]
- 40.Gorman L.K., Fu K., Hovda D.A., Murray M., and Traystman R.J. (1996). Effects of traumatic brain injury on the cholinergic system in the rat. J. Neurotrauma 13, 457–463 [DOI] [PubMed] [Google Scholar]
- 41.Sarter M., Hasselmo M.E., Bruno J.P., and Givens B. (2005). Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain. Res. Rev. 48, 98–111 [DOI] [PubMed] [Google Scholar]
- 42.McAllister T. W., Zafonte R., Jain S., Flashman L. A., George M. S., Grant G. A., and Stein M. B. (2016). Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 41, 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blesa R., Davidson M., Kurz A., Reichman W., van Baelen B., and Schwalen S. (2003). Galantamine provides sustained benefits in patients with 'advanced moderate' Alzheimer's disease for at least 12 months. Dement. Geriatr. Cogn. Disord. 15, 79–87 [DOI] [PubMed] [Google Scholar]
- 44.Coyle J. and Kershaw P. (2001). Galantamine, a cholinesterase inhibitor that allosterically modulates nicotinic receptors: Effects on the course of Alzheimer's disease. Biol. Psychiatry 49, 289–299 [DOI] [PubMed] [Google Scholar]
- 45.Erkinjuntti T., Kurz A., Gauthier S., Bullock R., Lilienfeld S., and Damaraju C.V. (2002). Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 359, 1283–1290 [DOI] [PubMed] [Google Scholar]
- 46.Mintzer J.E. and Kershaw P. (2003). The efficacy of galantamine in the treatment of Alzheimer's disease: comparison of patients previously treated with acetylcholinesterase inhibitors to patients with no prior exposure. Int. J. Geriatr. Psychiatry 18, 292–297 [DOI] [PubMed] [Google Scholar]
- 47.Wilcock G.K., Lilienfeld S., and Gaens E. (2000). Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 321, 1445–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winblad B., Engedal K., Soininen H., Verhey F., Waldemar G., andWimo A., ; Donepezil Nordic Study Group. (2001). A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57, 489–495 [DOI] [PubMed] [Google Scholar]
- 49.Emre M. (2002). Switching cholinesterase inhibitors in patients with Alzheimer's disease. Int. J. Clin. Pract. Suppl. 127, 64–72 [PubMed] [Google Scholar]
- 50.Sozda C.N., Hoffman A.N., Olsen A.S., Cheng J.P., Zafonte R.D., and Kline A.E. (2010). Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma 27, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng J.P., Shaw K.E., Monaco C.M., Hoffman A.N., Sozda C.N., Olsen A.S., and Kline A.E. (2012). A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J Neurotrauma 29, 2684–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kline A.E., Olsen A.S., Sozda C.N., Hoffman A.N., and Cheng J.P. (2012). Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma 29, 1960–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bondi C.O., Klitsch K.C., Leary J.B., and Kline A.E., 2014. Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J. Neurotrauma 31, 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bondi C.O., Semple B.D., Noble-Haeusslein L.J., Osier N.D., Carlson S.W., Dixon C.E., Giza C.C., and Kline A.E., 2015. Found in translation: understanding the biology and behavior of experimental traumatic brain injury. Neurosci. Biobehav. Rev. 58,123–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamm R.J., Temple M.D., O'Dell D.M., Pike B.R., and Lyeth B.G. (1996). Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma 13, 41–47 [DOI] [PubMed] [Google Scholar]
- 56.Passineau M.J., Green E.J., and Dietrich W.D. (2001). Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 168, 373–384 [DOI] [PubMed] [Google Scholar]
- 57.Hicks R.R., Zhang L., Atkinson A., Stevenon M., Veneracion M., and Seroogy K.B. (2002). Environmental enrichment attenuates cognitive deficits, but does not alter neurotrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience 112, 631–637 [DOI] [PubMed] [Google Scholar]
- 58.Cheng J.P., Shaw K.E., Monaco C.M., Hoffman A.N., Sozda C.N., Olsen A.S., and Kline A.E. (2012). A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma 29, 2684–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman A.N., Malena R.R., Westergom B.P., Luthra P., Cheng J.P., Aslam H.A., Zafonte R.D., and Kline A.E. (2008). Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 431, 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matter A.M., Folweiler K.A., Curatolo L.M., and Kline A.E. (2011). Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 25, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Witt B.W., Ehrenberg K.M., McAloon R.L., Panos A.H., Shaw K.E., Raghavan P.V., Skidmore E.R., and Kline A.E. (2011). Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair 25, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peruzzaro S.T., Gallagher J., Dunkerson J., Fluharty S., Mudd D., Hoane M.R., and Smith J.S. (2013). The impact of enriched environment and transplantation of murine cortical embryonic stem cells on recovery from controlled cortical contusion injury. Restor. Neurol. Neurosci. 31, 431–450 [DOI] [PubMed] [Google Scholar]
- 63.Monaco C.M., Gebhardt K.M., Chlebowski S.M., Shaw K.E., Cheng J.P., Henchir J.J., Zupa M.F., and Kline A.E. (2014). A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 31, 1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kline A.E., Leary J.B., Radabaugh H.L., Cheng J.P., and Bondi C.O. (2016). Combination therapies for neurobehavioral and cognitive recovery after experimental traumatic brain injury: Is more better? Prog. Neurobiol. 142, 45–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maelicke A., Schrattenholz A., Samochocki M., Radina M., and Albuquerque E.X. (2000). Allosterically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer's disease. Behav. Brain Res. 113, 199–206 [DOI] [PubMed] [Google Scholar]
- 66.Wilkinson D.G., Francis P.T., Schwam E., and Payne-Parrish J. (2004). Cholinesterase inhibitors used in the treatment of Alzheimer's disease the relationship between pharmacological effects and clinical efficacy. Drugs Aging 21, 453–478 [DOI] [PubMed] [Google Scholar]
- 67.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 68.Kline A.E., Yu J., Horvath E., Marion D.W., and Dixon C.E. (2001). The selective 5-HT(1A) receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience 106, 547–555 [DOI] [PubMed] [Google Scholar]
- 69.Bondi C.O., Cheng J.P., Tennant H.M., Monaco C.M., and Kline A.E. (2014). Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma 31, 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Phelps T.I., Bondi C.O., Ahmed R.H., Olugbade Y.T., and Kline A.E. (2015). Divergent long-term consequences of chronic treatment with haloperidol, risperidone, and bromocriptine on traumatic brain injury-induced cognitive deficits. J. Neurotrauma 32, 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 72.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., and Kline A.E. (2013). Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olsen A.S., Sozda C.N., Cheng J.P., Hoffman A.N., and Kline A.E. (2012). Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J. Neurotrauma 29, 1898–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kline A.E., Wagner A.K., Westergom B.P., Malena R.R., Zafonte R.D., Olsen A.S., Sozda C.N., Luthra P., Panda M., Cheng J.P., and Aslam H.A., 2007. Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 177, 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyeth B.G., Jenkins L.W., Hamm R.J., Dixon C.E., Phillips L.L., Clifton G.L., Young H.F., and Hayes R.L. (1990). Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 526, 249–258 [DOI] [PubMed] [Google Scholar]
- 76.Leary J.B., Bondi C.O., LaPorte M.J., Carlson L.J., Radabaugh H.L., Cheng J.P., and Kline A.E. (2016). The therapeutic efficacy of environmental enrichment and methylphenidate alone and in combination after controlled cortical impact injury. J. Neurotrauma 2016. May 9; Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nikiforuk A., Potasiewicz A., Kos T., and Popik P. (2016). The combination of memantine and galantamine improves cognition in rats: The synergistic role of the α7 nicotinic acetylcholine and NMDA receptors. Behav Brain Res. 313, 214–218 [DOI] [PubMed] [Google Scholar]
- 78.Buendia I., Parada E., Navarro E., Leon R., Negredo P., Egea J., and Lopez M.G. (2016). Subthreshold concentrations of melatonin and galantamine improves pathological AD-hallmarks in hippocampal organotypic cultures. Mol. Neurobiol. 53, 3338–3348 [DOI] [PubMed] [Google Scholar]
- 79.Kumar A., Prakash A., and Pahwa D. (2011). Galantamine potentiates the protective effect of rofecoxib and caffeic acid against intrahippocampal Kainic acid-induced cognitive dysfunction in rat. Brain Res. Bull. 85, 158–168 [DOI] [PubMed] [Google Scholar]
- 80.Anthonymuthu T.S., Kenny E.M., and Bayır H. (2016). Therapies targeting lipid peroxidation in traumatic brain injury. Brain Res. 1640 (Pt A), 57–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tomassoni D., Catalani A., Cinque C., Di Tullio M.A., Tayebati S.K., Cadoni A., and Amenta F. (2012). Effects of cholinergic enhancing drugs on cholinergic transporters in the brain and peripheral blood lymphocytes of spontaneously hypertensive rats. Curr. Alzheimer Res. 9, 120–127 [DOI] [PubMed] [Google Scholar]
- 82.Dimitrova D.S. and Getova-Spassova D.P. (2006). Effects of galantamine and donepezil on active and passive avoidance tests in rats with induced hypoxia. J. Pharmacol. Sci. 101, 199–204 [DOI] [PubMed] [Google Scholar]
- 83.Muthuraju S., Maiti P., Pati S., Solanki P., Sharma A. K., Singh S.B., and Ilavazhagan G. (2011). Role of cholinergic markers on memory function of rats exposed to hypobaric hypoxia. Eur. J. Pharmacol. 672, 96–105 [DOI] [PubMed] [Google Scholar]
- 84.Muthuraju S., Maiti P., Solanki P., Sharma A.K., Amitabh, Singh S.B., and Ilavazhagan G. (2009). Acetylcholinesterase inhibitors enhance cognitive functions in rats following hypobaric hypoxia. Behav. Brain Res. 203, 1–14 [DOI] [PubMed] [Google Scholar]
- 85.Muthuraju S., Maiti P., Solanki P., Sharma A.K., Pati S., Singh S.B., and Ilavazhagan G. (2011). Possible role of cholinesterase inhibitors on memory consolidation following hypobaric hypoxia of rats. Int. J. Neurosci. 121, 279–288 [DOI] [PubMed] [Google Scholar]
- 86.Muthuraju S., Maiti P., Solanki P., Sharma A.K., Singh S.B., Prasad D., and Ilavazhagan G. (2010). Cholinesterase inhibitors ameliorate spatial learning deficits in rats following hypobaric hypoxia. Exp. Brain Res. 203, 583–592 [DOI] [PubMed] [Google Scholar]
- 87.Iliev A. I., Traykov V.B., Mantchev G.T., Stoykov I., Prodanov D., Yakimova K.S., and Krushkov I.M. (2000). A post-ischaemic single administration of galanthamine, a cholinesterase inhibitor, improves learning ability in rats. J. Pharm. Pharmacol. 52, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 88.Ji X., Li C., Lu Y., Chen Y., and Guo L. (2007). Post-ischemic continuous administration of galantamine attenuates cognitive deficits and hippocampal neurons loss after transient global ischemia in gerbils. Neurosci. Lett. 416, 92–95 [DOI] [PubMed] [Google Scholar]
- 89.Lorrio S., Sobrado M., Arias E., Roda J.M., Garcia A.G., and Lopez M.G. (2007). Galantamine postischemia provides neuroprotection and memory recovery against transient global cerebral ischemia in gerbils. J. Pharmacol. Exp. Ther. 322, 591–599 [DOI] [PubMed] [Google Scholar]
- 90.Barnes C.A., Meltzer J., Houston F., Orr G., McGann K., and Wenk G.L. (2000). Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience 99, 17–23 [DOI] [PubMed] [Google Scholar]
- 91.Benetti F., Mello P.B., Bonini J.S., Monteiro S., Cammarota M., and Izquierdo I. (2009). Early postnatal maternal deprivation in rats induces memory deficits in adult life that can be reversed by donepezil and galantamine. Int. J. Dev. Neurosci. 27, 59–64 [DOI] [PubMed] [Google Scholar]
- 92.de Bruin N., and Pouzet B. (2006). Beneficial effects of galantamine on performance in the object recognition task in Swiss mice: deficits induced by scopolamine and by prolonging the retention interval. Pharmacol. Biochem. Behav. 85, 253–260 [DOI] [PubMed] [Google Scholar]
- 93.Gould T.J. and Feiro O.R. (2005). Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behav. Brain Res. 165, 160–171 [DOI] [PubMed] [Google Scholar]
- 94.Woodruff-Pak D.S., Lander C., and Geerts H. (2002). Nicotinic cholinergic modulation: Galantamine as a prototype. CNS Drug Rev. 8, 405–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raskind M. A. (2003). Update on Alzheimer drugs (galantamine). Neurologist 9, 235–240 [DOI] [PubMed] [Google Scholar]
- 96.Raskind M. A., Peskind E. R., Truyen L., Kershaw P., and Damaraju C.V. (2004). The cognitive benefits of galantamine are sustained for at least 36 months—A long-term extension trial. Arch. Neurol. 61, 252–256 [DOI] [PubMed] [Google Scholar]
- 97.Samochocki M., Hoffle A., Fehrenbacher A., Jostock R., Ludwig J., Christner C., and Maelicke A. (2003). Galantamine is an allosterically potentiating ligand of neuronal nicotinic but not of muscarinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 305, 1024–1036 [DOI] [PubMed] [Google Scholar]
- 98.Farlow M.R. (2001). Pharmacokinetic profiles of current therapies for Alzheimer's disease: implications for switching to galantamine. Clin. Ther. 23 Suppl A, A13–A24 [DOI] [PubMed] [Google Scholar]
- 99.Hwang T.Y., Ahn I.S., Kim S., and Kim D.K. (2016). Efficacy of galantamine on cognition in mild-to-moderate Alzheimer's dementia after failure to respond to donepezil. Psychiatry Investig. 13, 341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maelicke A. (2001). Pharmacokinetic rationale for switching from donepezil to galantamine. Clin. Ther. 23 Suppl A, A8–A12 [DOI] [PubMed] [Google Scholar]
- 101.Pike B.R. and Hamm R.J. (1997). Chronic administration of a partial muscarinic M1 receptor agonist attenuates decreases in forebrain choline acetyltransferase immunoreactivity following experimental brain trauma. Exp. Neurol. 147, 55–65 [DOI] [PubMed] [Google Scholar]
- 102.Ago Y., Koda K., Yano K., Takuma K., and Matsuda T. (2010). Alzheimer's disease drug galantamine, but not donepezil, improves social isolation rearing-induced deficits in prepulse inhibition via muscarinic acetylcholine receptors. Int. J. Neuropsychopharmacol. 13, 58–58 [Google Scholar]
- 103.DeAngelis M.M., Hayes R.L., and Lyeth B.G. (1994). Traumatic brain injury causes a decrease in M2 muscarinic cholinergic receptor binding in the rat brain. Brain Res. 653, 39–44 [DOI] [PubMed] [Google Scholar]
- 104.Thompson H.J., Lifshitz J., Marklund N., Grady M.S., Graham D.I., Hovda D.A., and McIntosh T.K. (2005). Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma 22, 42–75 [DOI] [PubMed] [Google Scholar]
- 105.Briones T.L., Woods J., and Rogozinska M. (2013). Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta. Neuropathol. Commun. 1,57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 106.Garcia A.N., Shah M.A., Dixon C.E., Wagner A.K., and Kline A.E. (2011). Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. P.M. R. 3(6 Suppl 1), S18–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bentley P., Driver J., and Dolan R.J. (2011). Cholinergic modulation of cognition: insights from human pharmacological functional neuroimaging. Prog. Neurobiol. 94, 360–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasselmo M.E. (2006). The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]