Abstract
Photosystem (PS) II centers, which split water into oxygen, protons, and electrons during photosynthesis, require light but are paradoxically inactivated by it. Prolonged light exposure concomitantly decreased both the functional fraction of PSII reaction centers and the integral PSII chlorophyll (Chl) a fluorescence lifetime in leaf segments of Capsicum annuum L. Acceleration of photoinactivation of PSII by a pretreatment with the inhibitors/uncoupler lincomycin, DTT, or nigericin further reduced PSII Chl a fluorescence lifetimes. A global analysis of fluorescence lifetime distributions revealed the presence of at least two distinct populations of photoinactivated PSII centers, one at 1.25 ns, and the other at 0.58 ns. Light treatment first increased the 1.25-ns component, a weak quencher, at the expense of a component at 2.22 ns corresponding to functional PSII centers. The 0.58-ns component, a strong quencher, emerged later than the 1.25-ns component. The strongly quenching PSII reaction centers could serve to avoid further damage to themselves and protect their functional neighbors by acting as strong energy sinks.
Keywords: chlorophyll fluorescence lifetime, excitation quenching, photoinactivation, photoprotection
Oxygenic photosynthetic organisms grow under an ever-changing sunlight environment. Fluctuations of solar energy input can be accommodated by a tradeoff between energy utilization in photosynthetic carbon assimilation and energy dissipation by various photoprotective mechanisms that modulate absorption and dissipation of excess light energy (1–3). Despite the complex suite of strategies for photoprotection, however, photooxidative damage inevitably occurs to photosystem (PS) II, in which P680+ (a special Chl in the PSII reaction center), the strongest biological oxidant, is generated to split water to molecular oxygen, protons, and electrons (4). The consequent loss of PSII photochemical efficiency measured under low light, termed photoinhibition or photoinactivation (5), depends inter alia on the number of absorbed photons during the light exposure (6–8). That is, PSII photoinactivation can occur with a light-dosage-dependent probability under any light environment in nature, ranging from limiting to saturating irradiances. Over a sunny day, the entire population of PSII in a leaf may undergo photoinactivation.
Yet, effects of photoinactivation on PSII efficiency are not always obvious; they are noticeable only when the rate of inactivation exceeds the capacity for rapid and efficient repair of nonfunctional PSII reaction centers by de novo synthesis of D1 protein (psbA gene product in PSII reaction center) (9). The operation of the PSII repair cycle involves coordinated regulation of degradation and synthesis of D1 protein (10). When the supply of newly synthesized D1 protein is insufficient, such as under high light and/or low temperature, the degradation process also slows down (11, 12), resulting in the accumulation of nonfunctional PSII reaction centers in stacked grana domains of the thylakoid membranes of higher plants (13). These nonfunctional PSII centers, capable of light absorption but not of photosynthetic electron transfer, reduce the PSII quantum efficiency of leaves or leaf segments.
An important implication of PSII photoinactivation is that nonfunctional PSII centers, still embracing pigment molecules, can exacerbate photooxidative damage to the thylakoid membranes unless light energy absorbed by the pigments is dissipated safely. Thus, it has been hypothesized that photoinactivated PSII complexes are able to efficiently dissipate excitation energy harmlessly (14), and further, may contribute to photoprotection of their functional neighbors by acting as sinks for excitation energy (13, 15).
Experimental results consistent with this hypothesis have emerged from recent studies using leaves of Capsicum annuum L (16, 17). In leaves of C. annuum in the presence of lincomycin (an inhibitor of chloroplast-encoded protein synthesis), the functional fraction of PSII (f), determined from the oxygen yield per single-turnover flash or from chlorophyll (Chl) a fluorescence, decreased monoexponentially from 1.0 (= the original level) to ≈0.3 under illumination (16). However, the decrease in f thereafter proceeded more slowly so that ≈20% survived further prolonged illumination without being photoinactivated. Based on these observations, it has been suggested that photoinactivated PSII, initially a weak excitation energy quencher, becomes a strong quencher when a substantial number of nonfunctional PSII centers has accumulated (16, 17). However, direct in vivo evidence for weakly and strongly quenching PSII centers is not yet available.
If photoinactivated PSII can quench excitation energy efficiently, one would expect a shortening of the Chl a fluorescence lifetime (τ) in the dark-adapted state after preexposure to light stress. Indeed, several in vitro studies, measuring time-resolved Chl a fluorescence in isolated thylakoid membranes or membrane particles, have indicated a marked decrease in τ upon photoinactivation of PSII (18–21). However, these isolated systems lack the coordinated photoprotective mechanisms that are evident in vivo.
In the present study, we adopted a multifrequency cross-correlation fluorimetric method that has been used successfully to study Chl fluorescence lifetimes under the combined influence of a pH gradient and de-epoxidized xanthophylls in isolated chloroplasts that are competent in charge separation and stabilization (22). We analyzed PSII Chl a fluorescence lifetimes in Capsicum leaves exhibiting a range of PSII functionality to examine the efficiency of excitation energy dissipation by photoinactivated PSII in vivo. Our results clearly show (i) that changes in the integral fluorescence lifetime [Στ, calculated by integrating the function for the lifetime weighted fraction g(τ) from time 0 to ∞, i.e., ∫ g(τ) dτ], measured in the absence of photochemistry, correlate linearly with changes in f, and (ii) that there are at least two distinct populations of photoinactivated PSII, one being a stronger quencher than the other. These results provide direct evidence that photoinactivated PSII dissipates excitation energy in vivo and may play a photoprotective role.
Materials and Methods
Plant Material and Growth Conditions. Plants of C. annuum L. were grown in a potting mixture supplemented by a slow-release fertilizer and watered daily. The growth chamber was maintained at a 24°C/21°C (day/night) regime, the irradiance during the 12-h photoperiod being 250 μmol of photons per m2 per s. Fully expanded leaves were taken from plants at 35–45 d after sowing.
Uptake of Lincomycin, DTT, or Nigericin. Detached leaves were allowed to take up water (control), lincomycin, DTT plus lincomycin, or nigericin plus lincomycin through the cut petiole for 3 h in darkness. Typical average concentrations taken into the leaf tissue were estimated to be 2 mM for lincomycin, 0.6 mM for DTT, and 6 μM for nigericin.
Light Treatment. The adaxial surface of leaf pieces (1.5 cm2), floating on water or a solution (25°C) of lincomycin (1 mM), DTT plus lincomycin (each 1 mM), or nigericin (1 μM) plus lincomycin (1 mM), was illuminated with an HMI Universal Spotlight (HMI575W/GS, Osram, Berlin) at 900 μmol of photons per m2 per s for 1, 3, 5, or 6 h.
PSII Functionality. Leaf pieces were dark-adapted for 30 min before determination of the minimal (Fo) and maximal (Fm) fluorescence yield, corresponding to open and closed PSII reaction centers, respectively. Chl a fluorescence yields were measured by using a Plant Efficiency Analyzer (Hansatech, King's Lynn, U.K.). All fluorescence yields were normalized to the mean Fo value of control leaf pieces before light treatment. The relative content of functional PSII was calculated as 1/Fo – 1/Fm (23, 24), which is directly proportional to the oxygen yield per single-turnover saturating flash in C. annuum (25). The functional fraction of PSII (f) was calculated as the ratio of 1/Fo – 1/Fm for a treated leaf to that for a control, nonilluminated leaf.
PSII Chl a Fluorescence Lifetime Distribution. After a light treatment, leaf pieces were vacuum-infiltrated with 30 μM 3-(3,4-dichlorophenyl)-1,1′-dimethylurea (DCMU), 0.35 M glucose, and 50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.6) in the dark for 20 min. The temperature of the samples was then adjusted to –2°C in the sample holder. During this temperature adjustment, samples were kept in the dark for another 10 min. Fluorescence lifetimes (τ) were then measured at the maximal-fluorescence state (Fm) by using a multifrequency phase fluorimeter (K2–004, ISS Instruments, Urbana, IL) equipped with a laser diode and a red-sensitive micro channel plate detector. Excitation was modulated at 12 logarithmically spaced frequencies ranging from 25 to 200 MHz. The excitation wavelength was 635 nm, and the emission was detected at 689 nm. Reference was taken at 645 nm. The excitation light intensity was attenuated to 140 μmol of photons per m2 per s to avoid photobleaching during the experiment.
All data were globally analyzed by fitting to a continuous Lorentzian distribution model (26, 27). Six lifetime fractional components were found in Capsicum leaf samples when the τ-center values and widths of all components were linked together. The integral of the fractional intensity of all components was normalized to unity, i.e., ∫ g(τ) dτ = 1. The quality of fit was judged by the reduced χ2,
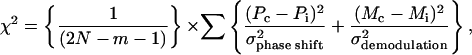 |
where σphase shift (= 0.2°) and σdemodulation (= 0.004) correspond to the standard deviations of phase and modulation measurement, respectively, N is the number of frequency points, m is the number of free fitting parameters, Pc and Pi are calculated and measured values for phase shift, respectively, and Mc and Mi are the same for demodulation (28, 29).
Pigment Analysis. Leaf segments were frozen in liquid nitrogen immediately after the fluorescence lifetime measurements and stored at –80°C until pigment extraction. Samples were ground in 500 μl of acetone/ethyl acetate (3:2), to which 400 μl of water was added. After 5 min of centrifugation at 10,000 × g, the upper layer containing pigments was decanted into a centrifuge tube and centrifuged for another 3 min at 10,000 × g before the injection (10 μl) into the HPLC system, as described in ref. 30.
Results
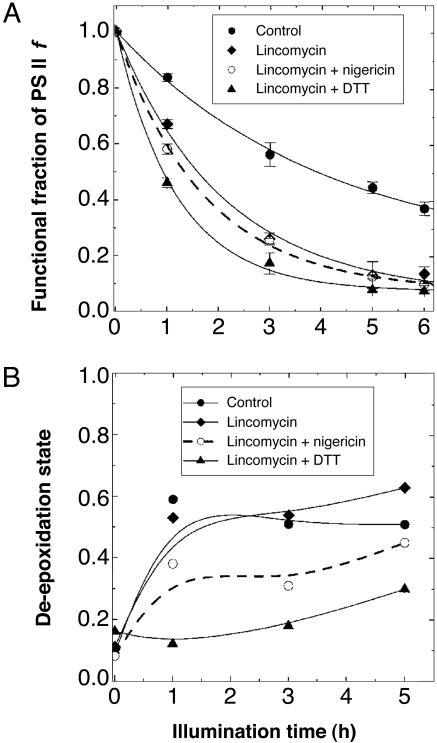
PSII Functionality. After 4 h of light treatment the functional fraction of PSII centers (f) decreased to ≈50% in water-treated leaf discs, in which the PSII repair cycle also occurred (Fig. 1A, control). We used lincomycin in most light treatments, however, to inhibit chloroplast-encoded protein synthesis (particularly the synthesis of the D1 protein) and to observe the enhanced photoinactivation of PSII without the complication of on-going repair (8). Pretreatment with lincomycin alone accelerated the decline in f relative to the control; the rapid decrease in f in the first 3 h was followed by a slower decline (Fig. 1 A, lincomycin). During light treatment, addition of DTT, an inhibitor of the de-epoxidation reactions in the xanthophyll cycle (31), further hastened the decrease, especially in the first hour of the light exposure (Fig. 1 A, lincomycin + DTT). Nigericin, a protonophoric uncoupler that inhibits the establishment of pH gradient (ΔpH) across the thylakoid membrane, had an exacerbating effect similar to that of DTT, albeit to a lesser extent (Fig. 1 A, lincomycin + nigericin). Nigericin, at the nominal concentration used in this study (6 μM), did not completely remove ΔpH in leaf tissues of C. annuum because about half the capacity for photosynthesis was retained (32).
Fig. 1.
The time course of some changes in leaf discs in response to high-light treatment. (A) The time course of decline in the functional fraction (f) of PSII in Capsicum leaf segments during light treatment at 900 μmol of photons per m2 per s and 25°C. Leaf segments were preincubated with water (control), lincomycin, lincomycin plus nigericin, or lincomycin plus DTT. For calculation of f (see Materials and Methods), Chl a fluorescence measurements were performed after 30 min of dark-adaptation, without treating the samples with DCMU. (B) The de-epoxidation state (antheraxanthin + zeaxanthin)/(violaxanthin + antheraxanthin + zeaxanthin) of leaf samples as a function of preillumination time. After the same light treatment at 900 μmol of photons per m2 per s as for A, the samples were vacuum-infiltrated with DCMU and dark-adapted for 30 min for fluorescence lifetime measurement in low light. They were then stored at –80°C until pigment extraction.
Consistent with the previous observations in Capsicum leaves (16), f reached an approximately steady-state level after 5–6 h in the samples treated with lincomycin alone, DTT plus lincomycin, or nigericin plus lincomycin, i.e., in samples in which the operation of the repair cycle and/or photoprotective thermal energy dissipation associated with de-epoxidized xanthophylls and ΔpH were inhibited (or repressed). The functional fraction of PSII measured after 6 h of illumination was 0.14 for lincomycin, 0.08 for DTT plus lincomycin, and 0.10 for nigericin plus lincomycin. Clearly, net photoinactivation of PSII was severe when repair was inhibited.
De-Epoxidation State of the Xanthophyll-Cycle Pigments. Operation of the xanthophyll cycle is an important factor in the dissipation of excitation energy from the light-harvesting antenna (1, 2) and, hence, in determining the extent of net photoinactivation of PSII. Therefore, we examined the de-epoxidation state of the xanthophyll-cycle pigments in the samples used for the PSII f luorescence lifetime measurements (Fig. 1B). The de-epoxidation state was calculated as (antheraxanthin + zeaxanthin)/(violaxanthin + antheraxanthin + zeaxanthin) (33). Substantial amounts of de-epoxidized xanthophylls (50–60%) were retained in the control and the lincomycin-treated samples after the light treatment, even though the samples were kept in the dark for 30 min during the DCMU infiltration and the temperature adjustment before the measurements of fluorescence lifetime. The de-epoxidation state was always somewhat lower in the nigericin plus lincomycin treatment than in the control or the lincomycin treatment alone, presumably because of a partial effect of nigericin on ΔpH. The samples treated with DTT plus lincomycin exhibited no substantial change for 2–3 h, yet a slight increase was observed after prolonged illumination, perhaps due to oxidation of DTT and/or new synthesis of zeaxanthin.
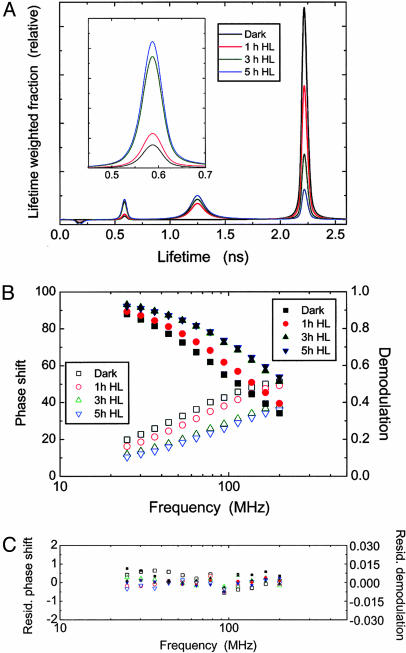
PSII Fluorescence Lifetime. Excitation energy in PSII can be dissipated by competing pathways: photochemical utilization, nonradiative dissipation, or fluorescence emission. If treatments accelerate nonradiative (thermal) dissipation of excitation energy, the population of excited Chl declines faster, and one expects a shorter Chl a fluorescence lifetime. Because the herbicide DCMU blocks forward electron transfer from QA to QB (primary and secondary electron acceptor, respectively) in the PSII reaction center, the fluorescence lifetimes after DCMU infiltration allow us to observe effects of thermal energy dissipation in the absence of concurrent photochemistry (22, 34). Thus, the samples were vacuum-infiltrated with DCMU, after a light treatment, before measurement. Fig. 2A illustrates the changes in the lifetime-weighted components of the PSII Chl a fluorescence lifetime distributions in control samples (i.e., in the absence of inhibitors/uncoupler) as the light treatment progressed. These lifetime distributions were obtained by fitting the phase shift and the demodulation measured at different frequencies (Fig. 2B) to a continuous Lorentzian distribution model (26, 27). Residual phase shift and residual demodulation between experimental values and those fitted by using the model are plotted in Fig. 2C.
Fig. 2.
Changes in the PSII Chl a fluorescence lifetime distributions in the control (water-treated) leaf samples of C. annuum after exposure to high light (900 μmol of photons per m2 per s). Samples were kept in the dark for 30 min during the vacuum-infiltration with 30 μM DCMU (20 min) and the temperature adjustment to –2°C (10 min) before the measurements of fluorescence lifetime. The excitation irradiance (635 nm) was 140 μmol of photons per m2 per s. (A) Lifetime-weighted fractions were derived by fitting the data of PSII fluorescence decay (in B) to a continuous Lorentzian distribution model assuming six components. Lifetime center values and widths of all components are given in Table 1. A minor fraction of the longest lifetime component at 10.8 ns is not shown. Changes in the lifetime-weighted fractional intensity of the component at 0.58 ns are enlarged in Inset. Dark, dark-adapted samples; 1 h HL, 3 h HL, and 5 h HL, samples exposed to high light for 1, 3, and 5 h. (B) Phase shift (open symbols) and demodulation (solid symbols) measured at 12 logarithmically spaced modulation frequencies raging from 25 to 200 MHz. (C) Residual phase shift and residual demodulation between measured and calculated values fitted by using the Lorentzian model.
The fluorescence lifetime center (τ-center) values and widths of all components found by applying a Lorentzian distribution model are summarized in Table 1. The lifetime distribution in a dark-adapted, DCMU-treated leaf segment was dominated by the prominent component centered at around 2.22 ns. Illumination with high light before the DCMU treatment gradually reduced the 2.22-ns component while increasing the amplitudes of the other two major components at 1.25 and 0.58 ns. It should be noted that changes in the lifetime-weighted fraction in Fig. 2 A appear greater for a component with a longer lifetime than for a component with a shorter lifetime. Of the two components, the one centered at 1.25 ns increased earlier than the one at 0.58 ns. Similar transitions of lifetime distribution patterns with progressive photoinactivation were also found in the samples illuminated in the presence of lincomycin, DTT plus lincomycin, or nigericin plus lincomycin (data not shown). However, the decrease in the 2.22-ns component and the increase in the 0.58-ns component were more pronounced in these samples compared with the control shown in Fig. 2, which had been treated with water instead of lincomycin ± DTT or ± nigericin.
Table 1. Fluorescence lifetime τ center value and width of each fractional component (cn) obtained by a Lorentzian distribution model.
| c1 | c2 | c3 | c4 | c5 | c6 | |
|---|---|---|---|---|---|---|
| τ center, ns | 0.01 | 0.18 | 0.58 | 1.25 | 2.22 | 10.83 |
| τ width, ns | 0.03 | 0.03 | 0.03 | 0.14 | 0.03 | 0.03 |
Global χ2 = 1.69; n = 16.
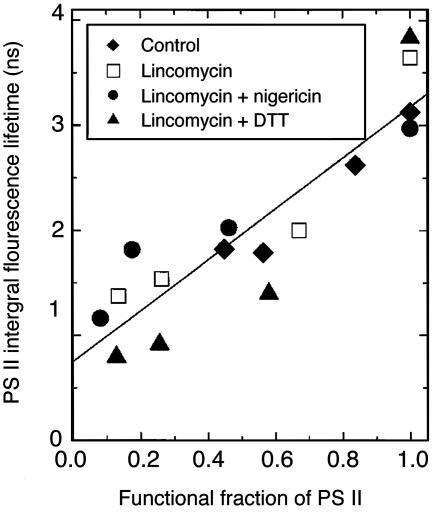
Integration of all lifetime-weighted components gives the integral fluorescence lifetime of PSII [Στ =∫ g(τ) dτ], an average τ of the entire PSII population in a sample. Significantly, a linear relation emerged when Στ was plotted against f for all of the treatments pooled together (Fig. 3). With a decreasing functional fraction of PSII, Στ became shorter, decreasing from ≥3 ns in a nonphotoinhibited state (f = 1) to 1.82 ns (control or water treatment), 1.37 ns (lincomycin), 1.16 ns (nigericin plus lincomycin), or 0.79 ns (DTT plus lincomycin) after 5 h of illumination. The shortest Στ was measured on a DTT plus lincomycin sample with f ≈ 0.1. The results show that excitation energy was dissipated more rapidly as more and more PSII centers were photoinactivated.
Fig. 3.
Correlation of the integral fluorescence lifetime of PSII with the functional fraction of PSII, determined in leaf samples pretreated at 900 μmol of photons per m2 per s for various durations, with or without an inhibitor or an uncoupler. Integral fluorescence lifetime was calculated by integration of the function for the lifetime-weighted fractions g(τ) from time 0 to ∞, i.e., ∫ g(τ) dτ. After illumination, leaf samples were dark-treated for 30 min before measurement of Fo and Fm (used to calculate f). Likewise, the samples used for measurement of the PSII Chl a fluorescence lifetime distribution were dark-treated for 30 min during vacuum-infiltration with DCMU (20 min), followed by temperature adjustment to –2°C in the sample holder (10 min). The excitation irradiance (635 nm) was 140 μmol of photons per m2 per s. Replicate leaf samples were also measured for Fo and Fm, but these were not used for fluorescence lifetime measurements.
Discussion
The Photoinactivation of PSII Centers. Capsicum leaf segments from plants grown under relatively low light (250 μmol of photons per m2 per s) exhibited photoinactivation of PSII on exposure to high light (900 μmol of photons per m2 per s), as revealed by a gradual decrease in the functional fraction of PSII centers f (Fig. 1). The value f, conveniently measured by Chl a fluorescence, is directly proportional to the average yield of oxygen per single-turnover, repetitive flash in Capsicum leaves (16, 25); in turn, the average yield of oxygen per flash represents the number of functional PSII centers in a range of plant species tested (35). Fig. 1 A depicts the acceleration of the decline in f when the repair cycle was inhibited by lincomycin, particularly in the combined presence of DTT. By inhibiting the xanthophyll cycle, and thus the dissipation of excitation energy associated with de-epoxidized xanthophylls (1, 2), DTT led to maximum photoinactivation of PSII when repair was also halted.
Shortening of the Integral Fluorescence Lifetime. In addition to the decline of f in vivo, photoinhibitory treatment resulted in a marked reduction in Στ measured in the absence of nonphotochemical quenching (Fig. 3). The decrease of Στ implies enhanced de-excitation by competing thermal dissipation. Because all samples were pretreated with DCMU before measurements of fluorescence lifetime, ΔpH- or xanthophyll cycle-dependent thermal dissipation in the antenna might have had only a limited contribution to the rapid fluorescence decay in these photoinactivated Capsicum leaves, even though substantial amounts of zeaxanthin and antheraxanthin were retained after light exposure in some samples (Fig. 1B). Consistent with this conclusion, the DTT- and lincomycin-treated samples, despite having the lowest de-epoxidation state, concomitantly exhibited the most drastic reduction in Στ. Importantly, the decrease in Στ was accompanied by a linear decrease in the functional fraction of PSII (Fig. 3). This would be expected if photoinactivated PSII reaction centers could quench excitation energy, thereby shortening the lifetime of Chl a fluorescence.
In the exciton radical pair equilibrium model, decreases in Fm and Fo by nonphotochemical quenching either in the antenna or in the reaction center are linearly related to each other; on the other hand, quenching of Fm but not Fo would indicate an effect on charge separation in PSII (22, 36). Photoinactivation of PSII in our capsicum leaves for 5 h did not decrease Fo (but increased it slightly) while it led to a several-fold decrease in Fm (data not shown), consistent with an inhibitory effect on charge separation in the PSII reaction center.
However, we cannot rule out the possibility that the rapid fluorescence decay in photoinactivated Capsicum leaves (measured in the presence of DCMU) was partly due to quenching in the antenna. Cyclic electron transport via PSI during fluorescence lifetime measurement in the presence of DCMU would give rise to a ΔpH that is required for high-energy-state quenching of excitation energy in the antenna (e.g., see ref. 37). Given that Στ decreased linearly with the extent of photoinactivation, cyclic electron transport measured in the presence of DCMU would be expected to be enhanced as more photoinactivated PSII accumulated, if high-energy-state quenching of excitation energy in the antenna were responsible for shortening the fluorescence lifetime. In photoinhibited Chlamydomonas cells, however, cyclic electron flow via PSI as monitored photoacoustically, although persisting in the presence of DCMU, was no greater than in control cells (38). On the other hand, cyclic electron transport via PSI as monitored photoacoustically in the absence of DCMU was stimulated in photoinhibited sugar maple seedlings as compared with control leaves, although DCMU apparently completely inhibited these photoacoustic signals representing PSI and PSII activities (39). Clearly, more work is needed to define the contribution of cyclic electron transport via PSI to nonphotochemical quenching in the antenna in the presence of DCMU.
A close correlation between Στ and the photochemical efficiency of PSII in dark-adapted leaves (Fv/Fm)[Fv is variable fluorescence (= Fm – Fo)] has been documented previously for cold-hardened snow gum seedlings (27). The parallel decrease in Στ and Fv/Fm observed in winter leaves of snow gums and Australian mistletoes coincided with the sustained de-epoxidation of the xanthophyll cycle and the appearance of a new spectral component in the low-temperature fluorescence emission spectra, associated with formation of stable, energy-dissipating complexes (27, 40, 41). In shade leaves of mistletoes, however, photoinactivation by a short (2 h) exposure to high light intensities at room temperature did not give rise to the new spectral component found in sun leaves during winter (42). Hence, it seems that the mechanism of excitation energy dissipation in leaves photoinactivated at room temperature is not identical with the mechanism of long-term PSII down-regulation in overwintering leaves associated with the fluorescence spectral change, although similar relationships between Στ and PSII activity are manifested. In our Capsicum leaves treated at room temperature, the acceleration of PSII photoinactivation by lincomycin ± DTT or ± nigericin (Fig. 1) with a concomitant decrease in Στ measured in the presence of DCMU (Fig. 3) is probably due to dissipation of excitation energy in photoinactivated PSII reaction centers.
Formation of Weak and Then Strong Quenchers of Excitation Energy Under Prolonged Light Treatment. The component at 2.22 ns (Fig. 2A), representing the majority of PSII centers in a dark-adapted state, could originate from functional, unquenched PSII, whereas the two shorter-lifetime components at 0.58 and 1.25 ns are probably related to components found in isolated thylakoid membranes after photoinhibitory treatment (18, 20, 21). For example, after photoinactivation in aerobic conditions, PSII-enriched membranes from spinach thylakoids showed a large increase in a 0.3-ns component and some increase in a 0.87-ns component at the expense of 1.7- and 3.9-ns components (18). In these PSII-enriched membranes in which no ΔpH could be established and no xanthophyll de-epoxidation could occur, the short fluorescence lifetimes are likely to be associated with reaction-center dissipation rather than antenna quenching of excitation. Similarly, in thylakoids isolated from moderately photoinhibited pea leaves, a 1.5-ns component was increased at the expense of a slow, 2.5-ns component (21). Target analysis of the data suggests that the stable fluorescence quenching induced by strong light is caused by enhanced radiationless recombination to the ground state (21). Therefore, we also reason that our 0.58- and 1.25-ns components are also likely to represent two specific modes of energy dissipation in the reaction center in vivo.
Notably, the increase of the 1.25-ns component preceded that of the 0.58-ns component (Fig. 2 A). Consistent with this sequential formation, the concentration of the 1.25-ns component was relatively steady after the first hour (Fig. 2 A), presumably because this component was converted to the 0.58-ns component at the same time as it was also produced from the 2.22-ns component. Thus, our in vivo data suggest that PSII undergoes a transition from an unquenched to a weakly quenched state and then a strongly quenched state during photoinactivation, as has been proposed (16). The weaker quencher with a fluorescence lifetime centered at 1.25 ns may be related to mechanisms such as quenching by Chl+ or P680+ (43) or cyclic electron transfer within PSII involving cytochrome b559 (or Chlz), β-carotene, and P680 (44–46), which could specifically quench Fm but may have a limited capacity to prevent further photooxidative damage (47). When the situation deteriorates with greater light dosage, and more than half of PSII centers have been photoinactivated, modification of PSII component(s) could also enhance quencher formation (20, 48), which may then give rise to the stronger quencher at 0.58 ns. Another possible mechanism for this strong quenching could be enhanced charge recombination of Q–A and P680+ directly to the ground state (17, 21, 49). Identification of the two quencher populations emerging after the photoinactivation of PSII will require further studies on molecular mechanisms of PSII photoinactivation.
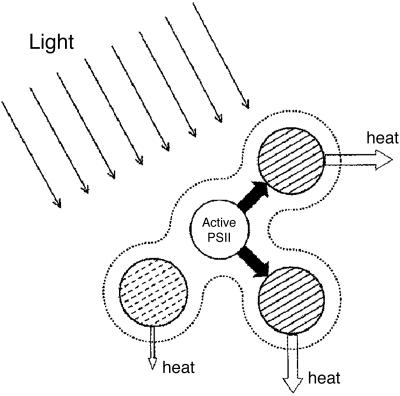
Weakly and strongly quenching PSII reaction centers may prevent the photosynthetic apparatus from irreparable damage, as illustrated in Fig. 4. A weakly quenching PSII reaction center (hatched with dashed lines) is able to dissipate excitation energy to prevent further damage to itself. A strongly quenching PSII reaction center (hatched with solid lines) receives excitation energy from its functional neighbor (nonhatched) and efficiently dissipates it as heat, thereby maintaining a small population of functional PSII. This photoprotective mechanism depends on the connectivity of the antennae (represented by the dotted envelope) that allows the transfer of excitons from a functional PSII to a strong sink.
Fig. 4.
A diagram showing three types of PSII units connected via their antennae (dashed envelope). An active PSII (nonhatched circle), presumed to give Chl a fluorescence lifetime of 2.22 ns when measured in the presence of DCMU, passes its excitation energy to, and is photoprotected by, strongly dissipating, photoinactivated PSII centers (hatched with solid lines) that are presumed to give the fluorescence lifetime of 0.58 ns. An intermediate, weakly dissipating type of photoinactivated PSII (hatched with dashed lines) is presumed to give rise to the fluorescence lifetime of 1.25 ns. When most of PSII centers are photoinactivated, high-energy-state quenching in the antennae that depends on ΔpH is expected to be limited, so it is omitted for simplicity.
Concluding Remarks. The quenched PSII reaction centers found in photoinactivated leaves provide direct evidence that photoinactivated PSII centers are able to dissipate excitation energy in vivo. Photoinactivation of PSII, usually regarded as a purely detrimental process, could confer survival value by sustaining a residual population of functional PSII. In turn, the residual functional PSII could support the subsequent repair of photoinactivated complexes (17).
Acknowledgments
We thank Drs. Marilyn Ball and Barry Pogson for use of their equipment, Dr. Adam Gilmore for advice on fluorescence lifetime analysis, and Profs. Barry Osmond and Jan Anderson for helpful comments on the manuscript. S.M. was supported by the Photobioenergetics Group, Research School of Biological Sciences, Australian National University. W.S.C. thanks the Australian Research Council for funding support (DP0343160).
Author contributions: S.M. and W.S.C. designed research, performed research, analyzed data, and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Chl, chlorophyll; DCMU, 3-(3,4-dichlorophenyl)-1,1′-dimethylurea; ΔpH, pH gradient across the thylakoid membrane; PS, photosystem.
References
- 1.Demmig-Adams, B. & Adams, W. W., III (1992) Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626. [Google Scholar]
- 2.Niyogi, K. K. (2000) Curr. Opin. Plant Biol. 3, 455–460. [DOI] [PubMed] [Google Scholar]
- 3.Öquist, O. & Huner, N. P. A. (2003) Annu. Rev. Plant Biol. 54, 329–355. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, J. M., Park, Y.-I. & Chow, W. S. (1998) Photosynth. Res. 56, 1–13. [Google Scholar]
- 5.Osmond, C. B., Anderson, J. M., Ball, M. C. & Egerton, J. J. G. (1998) in Physiological Plant Ecology, eds. Press, M. C., Scholes, J. D. & Barker, M. G. (Blackwell Scientific, Oxford), pp. 1–24.
- 6.Jones, L. W. & Kok, B. (1966) Plant Physiol. 41, 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park, Y.-I., Chow, W. S. & Anderson, J. M. (1995) Planta 196, 401–411. [Google Scholar]
- 8.Tyystjärvi, E. & Aro, E.-M. (1996) Proc. Natl. Acad. Sci. USA 93, 2213–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aro, E.-M., Virgin, I. & Andersson, B. (1993) Biochim. Biophys. Acta 1144, 113–134. [DOI] [PubMed] [Google Scholar]
- 10.Baena-González, E. & Aro, E.-M. (2002) Philos. Trans. R. Soc. London B 357, 1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rintamäki, E., Kettunen, R. & Aro, E.-M. (1996) J. Biol. Chem. 271, 14870–14875. [DOI] [PubMed] [Google Scholar]
- 12.Salonen, M., Aro, E.-M. & Rintamäki, E. (1998) Photosynth. Res. 58, 143–151. [Google Scholar]
- 13.Anderson, J. M. & Aro, E.-M. (1994) Photosynth. Res. 41, 315–326. [DOI] [PubMed] [Google Scholar]
- 14.Krause, G. H. (1988) Physiol. Plant 74, 566–574. [Google Scholar]
- 15.Öquist, G., Chow, W. S. & Anderson, J. M. (1992) Planta 186, 450–460. [DOI] [PubMed] [Google Scholar]
- 16.Lee, H.-Y., Hong, Y.-N. & Chow, W. S. (2001) Planta 212, 332–342. [DOI] [PubMed] [Google Scholar]
- 17.Chow, W. S., Lee, H.-Y., Park, Y.-I., Park, Y.-M., Hong, Y.-N. & Anderson, J. M. (2002) Philos. Trans. R. Soc. London B 357, 1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vass, I., Gatzen, G. & Holzwarth, A. R. (1993) Biochim. Biophys. Acta 1183, 388–396. [Google Scholar]
- 19.Renger, G., Eckert, H.-J., Bergmann, A., Bernarding, J., Liu, B., Napiwotzki, A, Reifarth, F. & Eichler, H. J. (1995) Aust. J. Plant Physiol. 22, 167–181. [Google Scholar]
- 20.Gilmore, A. M., Hazlett, T. L., Debrunner, P. G. & Govindjee (1996) Photochem. Photobiol. 64, 552–563. [DOI] [PubMed] [Google Scholar]
- 21.Richter, M., Goss, R., Wagner, B. & Holzwarth, A. R. (1998) Biochemistry 38, 12718–12726. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore, A. M., Hazlett, T. L. & Govindjee (1996) Proc. Natl. Acad. Sci. USA 92, 2273–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havaux, M., Strasser, R. J. & Greppin, H. (1991) Photosynth. Res. 27, 41–55. [DOI] [PubMed] [Google Scholar]
- 24.Walters, R. G. & Horton, P. (1993) Photosynth. Res. 36, 119–139. [DOI] [PubMed] [Google Scholar]
- 25.Lee, H.-Y., Chow, W. S. & Hong, Y.-N. (1999) Physiol. Plant. 105, 377–384. [Google Scholar]
- 26.Alcala, J. R., Gratton, E. & Prendergast, F. G. (1987) Biophys. J. 51, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilmore, A. M. & Ball, M. C. (2000) Proc. Natl. Acad. Sci. USA 97, 11098–11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jameson, D. M. & Hazlett, T. L. (1991) in Biophysical and Biochemical Aspects of Fluorescence Spectroscopy, ed. Dewey, T. G. (Plenum, New York), pp. 105–133.
- 29.Lakowicz, J. R. (1999) Principles of Fluorescence Spectroscopy (Kluwer Academic/Plenum, New York), pp. 141–184.
- 30.Pogson, B., McDonald, K. A., Truong, M., Britton, G. & DellaPenna, D. (1996) Plant Cell 8, 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilger, W. & Björkman, O. (1990) Photosynth. Res. 25, 173–185. [DOI] [PubMed] [Google Scholar]
- 32.Lee, H.-Y., Hong, Y.-N. & Chow, W. S. (2002) Funct. Plant Biol. 29, 607–619. [DOI] [PubMed] [Google Scholar]
- 33.Gilmore, A. M. & Yamamoto, H. Y. (1993) Photosynth. Res. 35, 67–78. [DOI] [PubMed] [Google Scholar]
- 34.Gilmore, A. M., Shinkarev, V. P., Hazlett, T. L. & Govindjee (1998) Biochemistry 37, 13582–13593. [DOI] [PubMed] [Google Scholar]
- 35.Chow, W. S., Hope, A. B. & Anderson, J. M. (1989) Biochim. Biophys. Acta 973, 105–108. [Google Scholar]
- 36.Dau, H. (1994) Photochem. Photobiol. 60, 1–23. [Google Scholar]
- 37.Finazzi, G., Johnson, G. N., Dallosto, L., Joliot, P., Wollman, F.-A. & Bassi, R. (2004) Proc. Natl. Acad. Sci. USA 101, 12375–12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canaani, O., Schuster, G. & Ohad, I. (1989) Photosynth. Res. 20, 129–146. [DOI] [PubMed] [Google Scholar]
- 39.Veeranjaneyulu, K., Charland, M. & Leblanc, R. M. (1998) Photosynthetica 35, 177–190. [Google Scholar]
- 40.Matsubara, S., Gilmore, A. M., Ball, M. C., Anderson, J. M. & Osmond, C. B. (2002) Funct. Plant Biol. 29, 1157–1169. [DOI] [PubMed] [Google Scholar]
- 41.Gilmore, A. M., Matsubara, S., Ball, M. C., Barker, D. H. & Itoh, S. (2003) Plant Cell Environ. 26, 1021–1034. [Google Scholar]
- 42.Matsubara, S. (2003) Ph.D. thesis (Australian National University, Canberra).
- 43.Mathis, P. (1981) in Photosynthesis: Proceedings of the 5th International Photosynthesis Congress, ed. Akoyunoglou, G. (Balaban International Science Services, Philadelphia), Vol. 3, pp. 827–837. [Google Scholar]
- 44.Blubaugh, D. J., Atamian, M., Babcock, G. T., Golbeck, J. H. & Cheniae, G. M. (1991) Biochemistry 30, 7586–7597. [DOI] [PubMed] [Google Scholar]
- 45.Hanley, J., Deligiannakis, Y., Pascal, A., Faller, P. & Rutherford, A. W. (1999) Biochemistry 38, 8189–8195. [DOI] [PubMed] [Google Scholar]
- 46.Telfer, A. (2002) Philos. Trans. R. Soc. London B 357, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirilovsky, D., Rutherford, A. W. & Etienne, A.-L. (1994) Biochemistry 33, 3087–3095. [DOI] [PubMed] [Google Scholar]
- 48.Peng, C.-L. & Gilmore, A. M. (2002) Funct. Plant Biol. 29, 1171–1180. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov, A. G., Sane, P., Hurry, V., Król, M., Sveshnikov, D., Huner, N. P. A. & Öquist, G. (2003) Physiol. Plant. 119, 376–383. [Google Scholar]