Abstract
AIM: The main objective was to investigate the physiological effects of ancient wheat whole grain flour diets on the development and progression of type 2 diabetes in Zucker diabetic fatty (ZDF) rats, and specifically to look at the acute glycemic responses. METHODS: An intervention study was conducted, involving 40 ZDF rats consuming one of 5 different diets (emmer, einkorn, spelt, rye and refined wheat) for 9 weeks. Refined wheat flour and whole grain rye flour were included as negative and positive controls, respectively. RESULTS: After 9 weeks of intervention, a downregulation of the hepatic genes PPAR-α, GLUT2, and SREBP-1c was observed in the emmer group compared to the control wheat group. Likewise, expression of hepatic SREBP-2 was lower for emmer, einkorn, and rye compared with the control group. Furthermore, spelt and rye induced a low acute glycemic response. The wheat group had higher HDL- and total cholesterol levels. CONCLUSIONS: Ancient wheat diets caused a downregulation of key regulatory genes involved in glucose and fat metabolism, equivalent to a prevention or delay of diabetes development. Spelt and rye induced a low acute glycemic response compared to wheat.
Keywords: type 2 diabetes, ancient wheat, Zucker diabetic fatty rat, glucose tolerance, metabolism
Abbreviations: BW - body weight; cDNA - complementary deoxyribonukleinsyre; GI - glycemic index; GLUT 2/4 - glucose transporter 2/4; HDL - high density lipoprotein; H NMR - proton nuclear magnetic resonance; HOMA-IR - homeostatic model assessment of insulin resistance; HPRT1 - hypoxanthine phosphoribosyltransferase 1; iAUC - incremental area under the curve; IRS 1/2 - insulin receptor substrate 1/2; Ins - insulin; LDL - low-density lipoprotein; LXR-α - liver X receptor alpha; OGTT - oral glucose tolerance test; PCA - principal component analysis; PCR - polymerase chain reaction; PPARα/γ - peroxisome proliferator activated receptor α/γ; PGC-1α - peroxisome proliferator-activated receptor gamma coactivator 1 alpha; RIA - radioimmunoassay; RNA - ribonucleic acid; SREBP - sterol regulatory element-binding protein; T2D - type 2 diabetes; TG - triglycerides; TMAO - trimethylamine N-oxide; VLDL - very-low-density lipoprotein; ZDF - Zucker diabetic fatty
1. Introduction
Type 2 diabetes (T2D) is a worldwide health problem, and rates are increasing globally. Over the next two decades, the global prevalence is predicted to rise from 382 million people to 592 million [1]. Food products derived from cereal grains like wheat, rice, corn, rye, oat, and barley constitute a major part of the daily diet in numerous countries. Dietary patterns featuring whole grain cereals are associated with improved indices of diabetes risk, including glycemic control, fasting plasma insulin and glucose, and insulin sensitivity [2-4]. Whole grain flour contains endosperm, germ, and bran, in contrast to refined flour from which the germ and bran are removed during the milling process. In contrast to whole grain consumption, consumption of refined flour increases the risk of developing lifestyle diseases because of higher glycemic index, reduced fiber, and lack of nutrient content [5, 6]. Diet, as one aspect of lifestyle, is thought to be one of the modifiable variable risk factors involved in the development of lifestyle diseases including T2D. However, more information is needed as to which components of the diet could be protective for the development and progression of T2D with all its complications [7].
The inclusion of functional food in the diet remains one of the simplest and potentially most attractive options in therapy. Ancient grain is largely unstudied, but could be a rich source of health-promoting substances. Wheat has been used as a food grain by man since the last Stone Age. Einkorn, emmer, and spelt are among the earliest cultivated wheat types, and are referred to as ancient wheat. Today most of the wheat species grown are hybrids which have been created from ancient wheat over the last 100 to 150 years. This "new" wheat has beneficial properties in terms of yield compared with the original ancient wheat. However, these hybrids may lack some of the unique properties and nutrients of the ancient wheats. The beneficial properties of ancient wheat for human health have been ascribed to higher levels of phytochemicals such as phytosterols, phenolic compounds such as ferulic acid and lignans, flavonoids, and carotenoids compared to other wheat species [8-11]. However, the potential health effects of ancient wheat types need to be investigated in more detail.
The aim of the study was to investigate the effects of ancient wheat diets on the development and progression of T2D using a diabetes animal model. We chose the Zucker diabetic fatty (ZDF) rat because this animal progressively develops hyperglycemia and impaired pancreatic beta-cell function with aging, and is thus suitable to test the response to nutritional regimens. The homozygous, recessive ZDF rat is an inbred animal model which in many ways resembles the pathogenesis of human T2D [12]. We studied glycemic control, plasma lipid profile, changes in key regulatory genes involved in glucose and fat metabolism, and finally the 1H nuclear magnetic resonance (NMR) based metabonomic property of the plasma profile. The different cereal types used as whole grain flour were ancient wheat (spelt, emmer, and einkorn) and rye. We used refined wheat flour as a contrast to the ancient wheat flour types. Rye was included because of its well characterized positive effect [13, 14], and refined wheat flour (consisting only of the endosperm) was used as control. Finally, we investigated the acute glycemic responses of the different whole grain flour types in the same animal model, referred to as the glycemic index (GI) study.
2. Materials and methods
2.1 Experimental diets
Five different types of cereals were used: refined wheat (referred to as wheat), spelt, emmer, einkorn, and rye. The flour types were provided by The Bakery Aurion A/S (Hjørring, Denmark). Spelt, emmer, einkorn, and rye were whole grain flour with an extraction rate of 100%, meaning that the grain was milled, but the flour contained bran, germ, and endosperm. The wheat flour consisted of the milled endosperm only.
The flour was analyzed by the Danish Veterinary and Food Administration (Lystrup, Denmark) for fat, protein, carbohydrate, fibers, and energy content (Table 1). All chemical analyses of the flour types were performed on freeze-dried materials. Dry matter was determined by drying to constant weight at 103ºC for 20 hours. Nitrogen was analyzed by DUMAS [15], and protein content was calculated as N x 6.25. Fat was extracted with diethyl ether after hydrogen chloride (HCl) hydrolysis according to the Stoldt procedure [16]. Total energy was calculated as the content of protein, carbohydrate, and fat by using the following conversion factors: 17, 17, and 37, respectively.
Table 1. Energy and crude macronutrient composition of the experimental flour types.
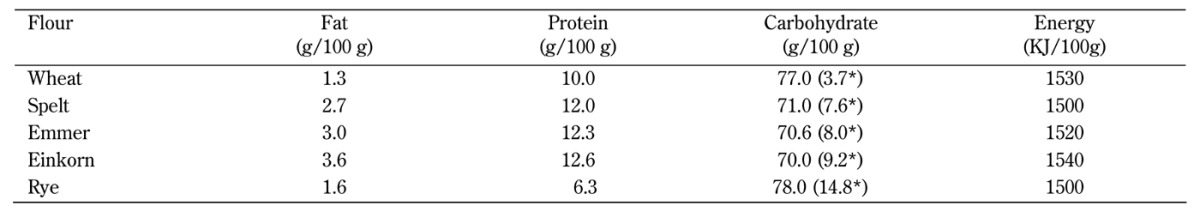
Legend: Values are means. * Fiber content.
The different flour types were mixed with 50% rat chow (Altromin 1324, Altromin GmbH, Lage, Germany), adjusted so that the vitamin and mineral contents were the same as in 100% Altromin 1324, and pelleted afterwards (Brogaarden, Lynge, Denmark).
2.2 Animals
The intervention study included 40 seven-week old male ZDF (Crl-Leprfa) rats (Charles River Laboratories, Sulzfeld, Germany) and 8 seven-week old non-diabetic male Wistar rats (Taconic Denmark Aps, Silkeborg, Denmark). The animals were housed individually in enclosed, ventilated cabinets at a constant temperature of 22°C and relative humidity of 50%, with a 12-h light/dark cycle. The animals were acclimated for 5 days, with free access to tap water and standard chow diet for laboratory rats (Altromin 1324). Prior to experiment, the animals were trained in the use of the restrainer. All experiments were carried out in compliance with the principles of laboratory animal care, and in accordance with the Danish Animal Welfare Council.
3. The intervention study
3.1 Study design
The ZDF rats were randomly divided into 5 groups of 8 rats, each of which was given one of the 5 different diets for 9 weeks. Additionally, 8 Wistar rats received the wheat diet, and were used as a comparison to the diabetic ZDF rats receiving the same diet. During the intervention, the rats had access to water and diet ad libitum.
Body weight, food consumption, and fasting plasma glucose were measured every second week throughout the study period. Plasma glucose was measured after an overnight fast (12 hours) using the OneTouch glucose monitoring apparatus (Precision Xceed, Abbott Laboratories A/S, Denmark). The blood was collected from the tail with the animals placed in a restrainer. Food consumption was monitored by feeding each animal with a specific amount of food in a clean cage. After 24 hours, the remaining food was weighed, and the food intake of each animal was calculated.
After 9 weeks of intervention, the animals underwent an oral glucose tolerance test (OGTT) after a 12-hour fast (water ad libitum). Whole blood glucose was measured using a OneTouch glucose monitoring apparatus. Blood was drawn from the tip of the tail at time points -15, 0, 30, 60, 90, 120, and 180 min. Immediately after the 0-min sample, each animal received a glucose load of 2 g D-glucose/kg body weight (dissolved in 0.9 % saline) by gavage. The trapezoidal method [17] was applied for calculation of the incremental area under the glucose response curve (iAUCglucose).
After 9 weeks of dietary intervention, the animals were anesthetized using pentobarbital (50 mg/kg BW) after an overnight fast, and a blood sample was collected from the retrobulbar plexus. Subsequently, the animals were sacrificed by cervical dislocation. A midline laparotomy was performed and liver tissue was collected. The soleus muscle was taken from the hind leg. Liver and muscle tissue were immediately frozen in liquid nitrogen and transferred to a -80°C freezer for later real-time PCR analysis.
3.2 Biochemical analysis
Before entering the study, baseline blood samples were collected. The procedure was repeated after the intervention period. Blood samples were collected in chilled tubes containing 3 µl heparin/aprotinin mix (7.7 mg/ml aprotinin and 2.300 IU/ml heparin) which were centrifuged immediately (4.000 rpm, 10 minutes, 4ºC); plasma was frozen at -80°C for subsequent analysis. All assays and analyses were carried out according to manufacturer’s instructions.
Plasma glucose levels were analyzed by a glucose oxidase method (GOD-PAP, Roche Diagnostics GmbH, Mannheim, Germany), and plasma insulin was determined using a sensitive rat insulin radioimmunoassay (RIA) kit (Millipore, Billerica, MA, USA). Total plasma triglyceride concentrations were determined by a colorimetric kit (TG GPO-PAP kit, Roche Diagnostics, Boehringer Mannheim, Germany). Total plasma cholesterol and HDL concentrations were determined using the Cholesterol Roche/Hitachi, CHOD-PAP kit (Roche Diagnostics, Boehringer Mannheim, Germany).
3.3 HOMA-IR
The homeostatic model assessment for insulin resistance (HOMA-IR) was used to assess insulin resistance. It was calculated from fasting insulin and glucose concentrations using the formula developed by Matthews et al. [18]. This model has proven valid in rats [19].
3.4 RNA isolation and real-time quantitative PCR
Total RNA was extracted from liver and muscle tissue by QIAcube (QIAgen, Copenhagen, Denmark) using tissue-specific RNeasy minikits (QIAgen, Copenhagen, Denmark). The procedures were carried out according to the manufacturer's instructions. RNA quantity and quality was verified by absorbance measurements at 260 nm and 280 nm (NanoDrop ND-8000 UV Spectrophotometer, NanoDrop Technologies, Wilmington, DE, USA). Furthermore, gel electrophoresis was used to examine the 18S and 28S ribosomal band on a 1% non-denaturing agarose gel stained with SYBR green. cDNA was made from RNA using the iScrip cDNA synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol.
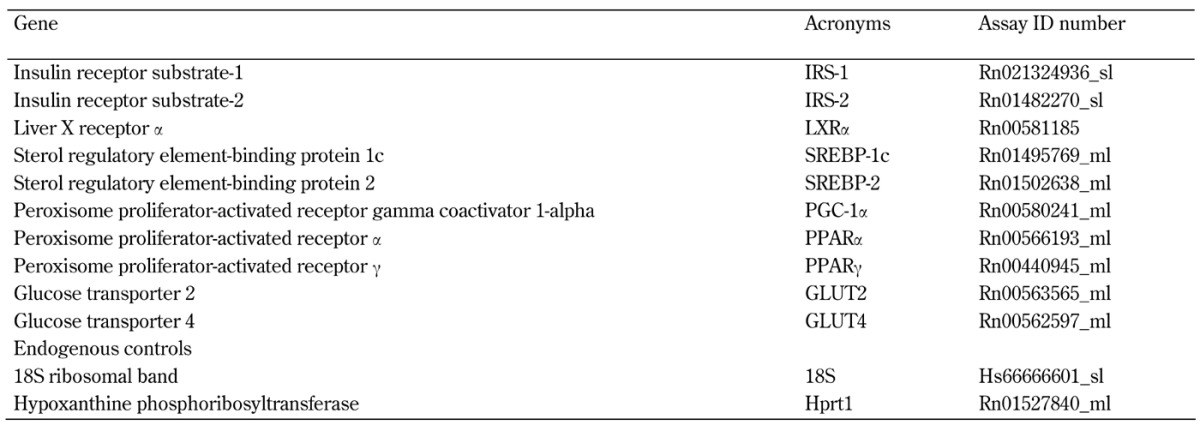
Real-time PCR was performed using TagMan assay (Applied Biosystems, Foster City, CA, USA), which consists of predesigned probes and primers (Table A1, in the Appendix). The following genes were examined:
Table A1. Genes, acronyms, and assay ID numbers in real-time PCR performed using TagMan assays. Predesigned probes and primers were obtained from Applied Biosystems.
- IRS1
- IRS-2
- LXR-α
- SREBP-1c
- SREBP-2
- PGC-1α
- PPARα
- PPARγ
- GLUT2
- GLUT4
IRS1, IRS-2, LXR-α, SREBP-1c, SREBP-2, PGC-1α, PPARα, PPARγ, and GLUT2 were examined from liver tissue, IRS-1, GLUT4, PPARα, and PPARγ from muscle tissue. Real-time PCR assay was performed using the ABI 7500 FAST machine (ABI, Foster City, CA, USA). Real-time PCR was carried out in reaction mixtures consisting of 5 µl 2x TagMan FAST Universal Master Mix (Applied Biosystems, Foster City, CA, USA), 0.5 µl 20x TagMan assay mix (Applied Biosystems, Foster City, CA, USA), 3.5 µl AcuGENE H2O, and 1 µl cDNA diluted 1:1000 (18S), 1:10 (IRS1, IRS-2, LXR-α, SREBP-1c, SREBP-2, PGC-1α, PPARα, GLUT2, GLUT4 and HPRT-1), and undiluted (PPARγ).
Thermocycling was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using the following protocol: 95ºC for 20 seconds followed by 40 cycles of 95ºC for 3 seconds and 60º for 30 seconds. PCR efficiency of each assay was tested 3 times on liver and muscle cDNA using standard curve. Efficiencies of 80-100% and correlation coefficients of 0.995 or better were accepted. Variation of the efficiency of 5% between analyses of the same gene was accepted. Target gene expression levels were normalized to the levels of two housekeeping genes (18S and HPRT1) and related to a calibrator (pool of liver or muscle cDNA). For the calibrator, Ct-value differences of up to 1 between plates for a given gene were accepted. The expression levels of the different target genes were calculated using the 2-∆∆Ct method [20]. Analyses were carried out in duplicates for both liver and muscle tissue, but in triplicates for the calibrator.
3.5 Metabonomics
Plasma from the animals was used in a 1H NMR-based metabonomic investigation to measure the plasma metabolome in the rats. The 1H NMR measurements were performed on a Bruker Avance III 600 spectrometer, which was operated at a 1H frequency of 600.13 MHz, and equipped with a 5 mm 1H TXI probe (Bruker BioSpin, Rheinstetten, Germany).
Plasma samples (400 µl) were mixed with 200 µl D2O. One-dimensional spectra were then created using the Carr-Purcell-Meiboom-Gill method. The spectra were delay-added to attenuate broad signals from high-molecular weight components. Subsequently, the spectra were aligned to alfa-glucose which has a signal from CH1 at 5.23 ppm. After the alignment, the spectra were subdivided into 0.0037 ppm integral regions and integrated. The interval from 4.5 ppm to 4.8 ppm was excluded because it contained the water resonance. The intervals 1.16-1.20 ppm and 3.62-3.68 ppm were also removed because they contained signals from ethanol, and finally region 1.11-1.24 ppm was left out as it included aprotinin resonances. Principal component analysis (PCA), which is an unsupervised method, was performed on all the preprocessed 1H NMR spectra to illustrate differences in the metabolic profile.
4. The Glycemic Index (GI) Study
The GI experiment included 12 seven-week old male ZDF rats (Charles River, Germany), which had the same living conditions as previously described for the intervention study, but their diet was standard chow (Altromin 1324). The study was designed to evaluate the acute glycemic response to each of the five cereal types (wheat, spelt, emmer, einkorn, rye). This was a crossover study, i.e. each rat underwent an experiment with one of the 5 cereal types at 5 different times. The order in which the animals received the different flour tablets was randomized, but all the flour types were tested at the same time points. There was a wash-out period of 7 days between each experiment. The response from wheat was used as a control. In order to compare the acute responses from week to week, no adjustments were made as the animals grew older and became more diabetic.
Each experiment was performed after 12 hours of fasting. Plasma glucose was measured with an OneTouch glucose monitoring apparatus using blood from the tail. Plasma glucose was measured at -15, 0, 30, 60, 90, 150, 210, 300, and 390 minutes. The 0-min point was set immediately before the rat was given a 6.0 g tablet consisting solely of one of the different flour types. The animals ate the tablet within the first 10 min or sooner.
5. Statistics
Data analysis in the Intervention Study was performed using GraphPad Software (GraphPad Prism version 4.0, San Diego, California, USA), and data were expressed as mean ± SEM. Data were tested for normal distribution and equal variances across groups using Q-Q-plots and Levene's test, respectively. One-way ANOVA followed by Bonferroni's multiple comparisons test was used to compare differences in baseline means between the groups. Body weight and food intake were analyzed by a two-way ANOVA followed by post hoc Bonferroni's multiple comparisons. The significance between baseline and week 9 values within each group was determined by using a paired Student’s t-test. Differences between the control wheat group and the other groups were tested by unpaired t-test. P < 0.05 was considered significant. Data points from the 1H NMR spectra were analyzed by Simca-P+ 12.0.1. (Umetrics, Malmö, Sweden), and principal component analysis (PCA) was used to group data in an unbiased way. By identifying groups of data, PCA inherently reduces the dimensionality of a data set, and it allows multidimensional data vectors to be projected onto a hyperplane of lower dimensions.
Data collected from the Wistar group were not used in any of the statistical calculations, except for the data from the 1H NMR. Unpaired t-test was used to test differences among the iAUC from the wheat control group and the different grain types in the GI study.
6. Results
The following subsections present the results of the Intervention Study. Subsection 6.11 is dedicated to the results of the GI Study.
6.1 Body weight
At week 0, there were no differences in body weight between the groups, but a gradual increase was observed during the first 8 weeks. At week 6, the emmer group had a lower weight than the rye group (p < 0.05), and the difference in bodyweight between the two groups was more pronounced at week 8 and 9 (p < 0.01). At week 9, the wheat group had a significantly lower bodyweight than the rye group (p < 0.05).
6.2 Food intake
The 24-hour food consumption for groups on rye, einkorn, emmer, spelt, and wheat nutrition was measured in the weeks when the rats were not weighed. After 7 and 9 weeks respectively, the rats on rye diet ate less than those in the einkorn group (p < 0 .01). At week 9, the einkorn group had a higher intake than the spelt group (p < 0.05), and the emmer group likewise had a higher intake than the rye group (p < 0.05).
6.3 Fasting glucose
At baseline, the mean fasting glucose of all ZDF rats was 6.8 ± 0.3 mmol/l; there were no differences between groups. During the intervention period, fasting glucose levels rose in all ZDF rats. After intervention, all ZDF groups had significant within-group differences between week 0 and week 9 (Figure 1A).
Figure 1.
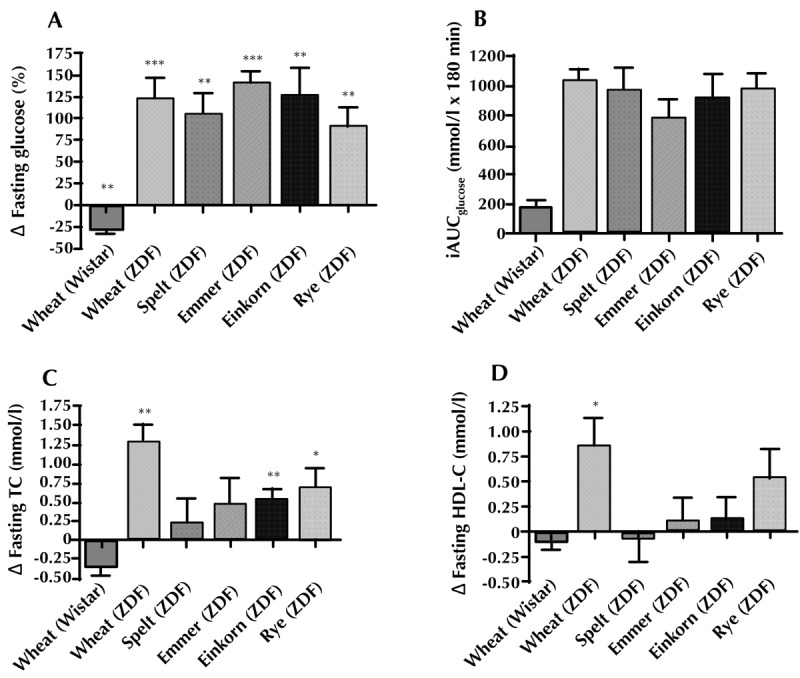
Percentage changes (Δ) in fasting glucose differences (A), area under the glucose response curve (iAUCglucose) during an oral glucose tolerance test (B), changes (Δ) in fasting plasma concentration of total cholesterol (C), and HDL-cholesterol (D). Data are presented as mean (± SEM). All statistical calculations are within-group differences. * p < 0.05, **p < 0.01, ***p < 0.001. Abbreviations: HDL-C – high-density lipoprotein cholesterol, iAUC – incremental area under the curve, TC – total cholesterol, ZDF – Zucker diabetes fatty.
6.4 Insulin
No significant difference was observed in baseline insulin levels. After 9 weeks of intervention, all ZDF groups showed a decline in insulin levels. However, this decline was significant in the emmer (p < 0.05) and einkorn (p < 0.05) groups only, with a percentage decline of 51 ± 19% and 33 ± 13%, respectively.
6.5 Insulin sensitivity
Insulin sensitivity was estimated using the HOMA-IR model. No significant differences were found between the ZDF groups.
6.6 Glucose tolerance
To assess the impact of the different diets on glucose uptake, we performed an OGTT after intervention. No significant differences were observed between any of the ZDF intervention groups regarding iAUCglucose. However, the emmer group had the lowest response with a mean of 787 ± 129 iAUCglucose mmol/l x 180 min (Figure 1B).
6.7 Plasma lipids
At baseline, the five ZDF groups had similar levels of total cholesterol, HDL-cholesterol, and triglycerides. An increase in total cholesterol was found in the groups wheat (p < 0.01), einkorn (p < 0.01), and rye (p < 0.05) (Figure 1C). No difference was observed between week 0 and week 9 regarding changes in triglyceride levels. There was an increase in HDL cholesterol (p < 0.05) in the ZDF wheat group only (Figure 1D).
6.8 Hepatic gene expression
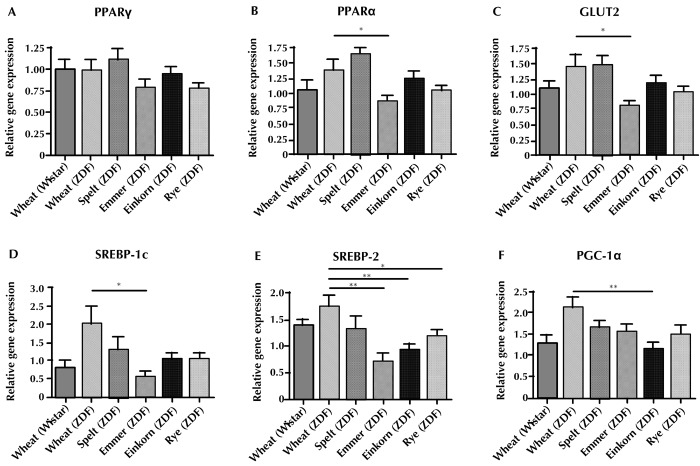
The results of real-time PCR gene expression assays for the liver tissue sampled at the end of the intervention are shown in Figure 2. The investigated genes are PPARγ, PPARα, GLUT-2, SREPB-1c, SREBP-2, and PGC-1α. We also looked at LXR-α, IRS-1, and IRS-2, but no differences were found between the ZDF groups (Table A2). Wistar rats on wheat diet were used for comparison of the responses between diabetic and normal animals. The gene expression of PPAR-α, GLUT2, and SREBP-1c was lower in the emmer group compared with the wheat group (p < 0.05) (Figures 2B-D). SREBP-2 was downregulated in the rye (p < 0.05), emmer (p < 0.01), and einkorn (p < 0.01) group compared with wheat (Figure 2E). Regarding the expression of PGC-1α, a downregulation was found in the einkorn group compared with the wheat group (p < 0.01) (Figure 2F).
Figure 2. Effects of wheat, spelt, emmer, einkorn, and rye diets on gene expression in liver tissue of ZDF and Wistar rats.
The diagrams show the relative expressions of the genes PPARγ (A), PPARα (B), GLUT-2 (C), SREBP-1c (D), SREBP-2 (E), PGA-1α (F). Quantitative RT-PCR was performed for selected genes and normalized to the geometric mean of the two endogenous controls (18S and Hprt1). Data are shown as mean (± SEM). Wheat was used as control. * p < 0.05, ** p < 0.01. Abbreviations: GLUT 2 – glucose transporter 2, PPAR – peroxisome proliferator activated receptor, PGC-1α – peroxisome proliferator-activated receptor gamma coactivator 1 alpha, SREBP-1c/2 – sterol regulatory element-binding protein 1c/2, ZDF – Zucker fatty diabetic.
Table A2. Relative gene expression: Effects of wheat, spelt, emmer, einkorn and rye diets on gene expression in liver and muscle tissue of ZDF and Wistar rats.
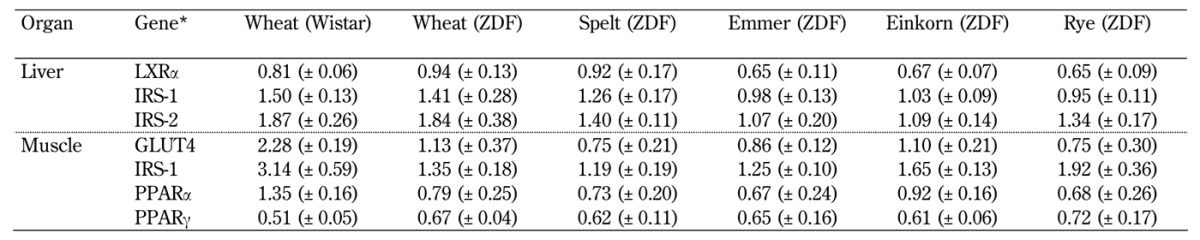
Legend: Data are shown as mean (±SEM). Wheat (ZDF) was used as control. * Quantitative RT-PCR was performed for selected genes and normalized to the geometric mean of the two endogenous controls (18S and Hprt1).
6.9 Muscle gene expression
We investigated the expression of PPARγ, PPARα, IRS1, and GLUT4 in muscle tissue. No significant differences were found between the five ZDF groups (Table A2, in the Appendix).
6.10 Metabonomics
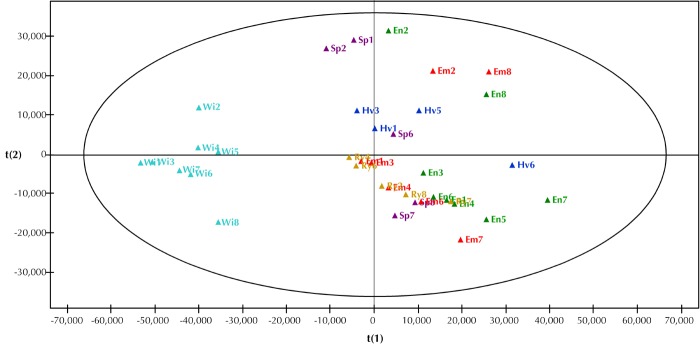
1H NMR spectra of plasma taken from the ZDF rats after the intervention period showed a distinct separation between the Wistar group and the 5 ZDF groups. The 6 groups are distinguished by different colors (Figure 3). The ZDF rats are scattered in the right part of the plot, whereas the Wistar rats are clustered together in the left part of the plot, apart from the other rats. The loading plot (not shown) revealed the most distinct metabolic changes responsible for the separation of the diabetic from the Wistar rats. The ZDF rats had decreased levels of lactate (δ1.33) and choline (δ3.22), but elevated levels of VLDL/LDL (δ1.3), lipids (δ0.89), TMAO (δ3.26), amino acids (δ3.77), and glucose (δ3.40-3.91).
Figure 3. Score scatter plot based on 1H NMR spectra of plasma samples from the 6 different groups.
The plots represent the results for emmer (Em, red), einkorn (En, green), wheat (Hv, blue), rye (Ry, brown), spelt (Sp, purple), and Wistar (Wi, turquois). The spectrum regions (variables) responsible for the discrimination of diabetic rats from normal rats are lower levels of lactate and choline combined with elevated levels of VLDL/LDL, lipids, amino acids, and glucose. Component 1 (x-axis) 49.76% and component 2 (y-axis) 14.16%.
6.11 The Glycemic Index Study
Figure 4 shows the incremental area under the glucose response curve (iAUCglucose) from the crossover study with the five different flour types. The analysis revealed that wheat had the largest iAUCglucose (mean 4134 ± 118 mmol/l x 390 min) and rye the smallest (mean 2973 ± 264 mmol/l x 390 min). Rye and spelt had significantly lower iAUCglucose than wheat (p < 0.001 and p < 0.01, respectively).
Figure 4. The iAUC glucose from the GI study.
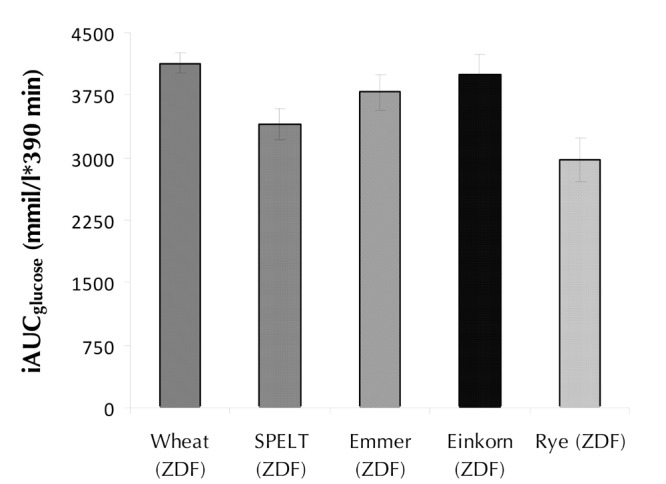
The diagram shows the glycemic response to each of the five cereal types. Wheat, spelt, emmer, einkorn, and rye were measured over a 6.5-hour period. Data are presented as mean (± SEM). **p < 0.01. ***p < 0.001.Wheat was used as control.
7. Discussion
In the present study, we demonstrated differential effects on lipid metabolism and liver gene expression levels as a result of ancient grain diets in a diabetic animal model. We found emmer, einkorn, and rye to induce a downregulation of hepatic gene expression. Diabetic rats consuming normal wheat diet had the most severe form of T2D with an increase in both total cholesterol and HDL cholesterol. By comparing the glycemic responses of the different grain types, we found rye and spelt to induce the lowest glycemic response compared with wheat.
Considering food consumption and weight, all rats slowly increased their food intake as they gained weight. Within the last week of intervention, the bodyweight reached a plateau or even started to decline in all the ZDF groups, although the food intake continued to increase. This was expectable due to severe glucosuria as diabetes progressed in the ZDF rats.
Interestingly, the rats eating rye were the heaviest, but had the lowest food intake of all the ZDF rats. Rye has a large content of dietary fibers (Table 1), which results in a larger satiety and slower emptying of the intestine, hence a lower food intake. This may be due to the soluble fibers in rye and their ability to increase viscosity in the gut, thereby slowing down gastric emptying, digestion, and absorption [21]. Whole grain products would be expected to result in a higher satiety than refined products [22]. In the GI study, we observed that both emmer and einkorn had similar iAUCglucose responses, although higher fiber content was found in both flours. We would have expected all ZDF groups (emmer, einkorn, spelt, and rye) in the intervention to produce a lower acute glycemic response than the refined wheat [23], but this was not the case.
The intervention was initiated when the rats were 7 weeks old. At this point, the ZDF rats already had very elevated levels of insulin (as much as 5 times that of the Wistar rats). As time went by, the diabetic condition of the animals became more severe, and eventually the insulin levels in the blood reached a maximum, thereby initiating a decrease in insulin levels. The exact time at which the animals reached their maximum insulin levels in the study period is unknown, since insulin levels were only measured at the end and at the beginning of the study period. During the study period, the insulin levels declined despite continued increase in plasma glucose levels, meaning that the diabetes had progressed in the animals. Impaired insulin action in suppressing hepatic glucose production and glycogenolysis caused an increase in hepatic glucose production [24].
The overall levels of total cholesterol were increased after the intervention period in all 5 ZDF groups, but only the wheat, einkorn, and rye group had a significant inter-group increase. The pattern is similar to the rise in HDL cholesterol, where only the wheat group showed a significant inter-group difference. The plasma lipid profile in animals is not applicable to humans, because the predominant plasma cholesterol in rats is HDL and not LDL as in humans. The rat has low levels of VLDL cholesterol and very low levels of LDL cholesterol [25], and provides very little information on potential effects on HDL [26]. The long fasting period of 12 hours did not cause a change in TG levels as a result of the intervention.
We investigated relative gene expression levels in the two most important insulin-sensitive tissues, namely liver and muscle, to identify potential actions of the different diets on key regulatory genes. The liver plays a major role in the regulation of glucose metabolism; the glycogenic and gluconeogenic pathways, which are major regulators of blood glucose levels, are unique to the liver [24]. GLUT2 gene expression in the liver was found to be significantly elevated in the control wheat group compared with the emmer group. The high GLUT2 expression level might be caused by a hepatic overproduction of glucose. Hence, elevated hepatic gluconeogenesis caused elevated glucose production resulting in a higher level of GLUT2 [27, 28].
SREBP-1c, an insulin-activated isoform of the sterol-regulatory element-binding family of transcription factors, has been identified as the principal insulin-responsive mediator of increased hepatic lipogenic enzyme gene expression in rodents fed high-carbohydrate diets. It regulates both hepatic gene expression of lipogenic enzymes and triglyceride deposition in the liver [29]. SREBP-1c can thereby be considered to be a factor that is partly responsible for insulin resistance. This corroborates our study, with the most insulin-resistant group being the wheat group in which a significant within-group increase in both total and HDL-cholesterol, and a significant higher gene expression of both SREBP-1c (compared with emmer) and SREBP-2 (compared with emmer, einkorn, and rye) occurred.
Overexpression of SREBP-1c in the liver is observed in insulin-resistant animals such as the ZDF rat, where insulin signaling is impaired [30]. A high expression of SREBP-1c induces a high GLUT2 expression. The emmer group had the lowest SREPB-1c expression and also the lowest GLUT2 expression. PPARα is involved in lipid metabolism (fatty acid uptake and oxidation), whereas PPARγ is involved in glucose metabolism in the liver. Reverse cholesterol transport has been demonstrated to be activated by PPARα [31]. The emmer group had a significantly lower expression of PPARα compared to wheat. In the liver, PGC-1α promotes activation of gluconeogenesis and fatty acid oxidation through its association with nuclear hormone receptors like PPARα. Our results in the ZDF wheat group corroborate an upregulation of PGC-1α as previously demonstrated [32].
Metabonomic analysis is a novel approach used for rapid identification of metabolic changes in biological systems. To our surprise, the different grain diets did not result in any detectable changes in the plasma metabolome between the ZDF groups when using 1H NMR. This might be due to the small number of animals in each group and the variations within the ZDF groups. Also, the 1H NMR spectra had an increased glucose resonance, dominating the variation of the dataset. When 1H NMR is used to study diabetes, the glucose resonance can conceal more subtle metabolic changes which are caused by the intervention [33]. A limitation of the 1H NMR spectra was that we experienced a huge amount of peak-overlap. This can be reduced by increasing the magnetic fields and pulse-sequence development [33]; however, this was not possible in our study.
A comparison between ZDF and Wistar rats using 1H NMR spectra confirms our previous knowledge regarding fasting plasma glucose levels, levels of total cholesterol, insulin, HDL cholesterol, and triglycerides: the ZDF rat is both hyperglycemic and hyperlipidemic. Both Wistar and ZDF wheat groups had wheat as their diet during the intervention study, but the huge difference is that the Wistar rat is not diabetic. This causes the Wistar group to differ from the ZDF groups in a way confirmed by other studies comparing a diabetic rat with a non-diabetic rat [34, 35].
In order to measure the acute glycemic response to the different grain types in the diabetic rats we conducted the randomized GI cross-over study. Six gram of each flour type used in the intervention study was given to ensure that the rats ate everything quickly. The amount of 6 g was selected on the basis of several test-runs, where different doses were examined. All the experiments were performed in exactly the same way to make the results comparable. The blood glucose response curve was monitored for as long as 6.5 hours. However, the curve did not reach baseline, despite this long monitoring period.
The rye and spelt diets induced a lower glycemic response than wheat. It was thus expected that the intake of these diets first of all would give a lower overall plasma glucose concentration [36]. Rye has previously been found to elicit a low postprandial insulin response and prolonged low glucose profiles in humans [14]. Therefore, we chose to use rye as a positive control. The second control was wheat flour consisting only of the endosperm. As expected, the ZDF rats on wheat diet had the most severe diabetes, and were therefore most insulin-resistant. Surprisingly, emmer and einkorn did not induce a lower acute glycemic response than wheat, despite both emmer and einkorn being used as whole grain flour.
In the intervention study, we used a dose of 50% flour in combination with standard chow. However, this dose might not have been high enough to detect significant differences between the grain types. We used whole grain flour with no intact grains, but earlier studies reported using less milled fractions of grain to induce a better glycemic control [37]. The grains had been processed during milling, grinding, and flaking, and although these treatments may reduce the content of phytochemicals, their bioavailability is often increased [38].
Whole grains generally contain combinations of phytochemicals depending on the type of cereal, location within the grain, and how the grain has been processed. The outer structures of grain, in particular the pericarp seed coat and aleurone layers, contain higher levels of phytochemicals like phenolic compounds, phytosterols, tocols, betaine, and folate than the germ and endosperm [39, 40]. Other major phytochemicals occurring in whole grain, including various carotenoids, notably α- and β-carotene, lutein, β-cryptoxanthin, and zeaxanthin, may play a role in the protection against diabetes. All of these phytochemicals are located in the bran and germ fractions [41].
To our knowledge, this is the first study to compare the impact of ancient wheat types (emmer, einkorn, and spelt) with refined wheat (as negative control) and rye (as positive control). The results of plasma measurements were compared with those of gene expression to provide information about the animals’ different diabetic conditions. A weakness of the study is the limited number of animals in each group. One might speculate that a higher number would amplify potential differences between the experimental diets. Also, the pelleting process of the flour may have diluted the effects of the different diets, and thus may hide minor differences. Before each experiment, the animals fasted for 12 hours which might have been too long a fasting period, blurring the results from the intervention. An intervention period of 9 weeks could also cause this diabetic animal model to develop a severe diabetes status contributing to masking the effects of the intervention.
8. Conclusions
In conclusion, the development and progression of diabetes in the ZDF rats may be less pronounced for the group fed with ancient grains (spelt, emmer, einkorn) and the rye group compared with the wheat group after 9 weeks of intervention because of a downregulation of key regulatory genes in the liver. However, no uniform conclusion can be drawn from the results of the intervention. Thus, further experiments are needed on this issue to confirm or deny possible beneficial effects of ancient wheat types.
Funding: The study was supported by the Aarhus University Research Foundation (E-2009-FLS-5-27).
Conflict of interests: The authors report no competing financial interests.
Warning: The results of this study do not support any nutritional recommendation for human consumption of the wheat variants studied in this project.
Appendix
Table A3. Actual values for fasting glucose, total cholesterol, and HDL-cholesterol from the start and end of the intervention period.
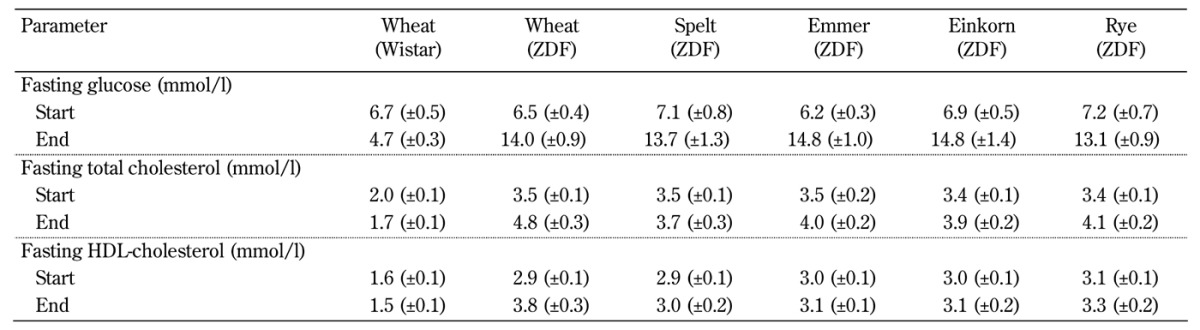
Legend: Data are shown as mean (± SEM).
Acknowledgments
We thank The Bakery Aurion A/S (Hjørring Denmark) for sponsoring the flour used in the study. Moreover, we thank Tove Skrumsager, Dorthe Rasmussen, Kirsten Eriksen, and Dorthe Rasmussen for excellent technical assistance. Thank you also to Ditte B. Ditlev and Nina Eggers for their support with the metabonomics analysis. Finally, we thank Susanne Rasmussen Molboe at the Danish Veterinary and Food Administration for performing the macronutrient and dietary fiber analyses of the flour.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Pereira MA, Jacobs DR Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75(7):848–855. doi: 10.1093/ajcn/75.5.848. [DOI] [PubMed] [Google Scholar]
- 3.Venn BJ, Mann JI. Cereal grains, legumes and diabetes. Eur J Clin Nutr. 2004;58:1443–1461. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- 4.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. Plos Med. 2007;4:e261. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [DOI] [PubMed] [Google Scholar]
- 6.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 7.Frolich W, Aman P, Tetens I. Whole grain foods and health - a Scandinavian perspective. Food Nutr Res. 2013;57:18503. doi: 10.3402/fnr.v57i0.18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shewry PR. Wheat. J Exp Bot. 2009;60:1537–1553. doi: 10.1093/jxb/erp058. [DOI] [PubMed] [Google Scholar]
- 9.Charmet G. Wheat domestication: lessons for the future. C R Biol. 2011;334:212–220. doi: 10.1016/j.crvi.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Giambanelli E, Ferioli F, Kocaoglu B, Jorjadze M, Alexieva I, Darbinyan N, D'Antuono LF. A comparative study of bioactive compounds in primitive wheat populations from Italy, Turkey, Georgia, Bulgaria and Armenia. J Sci Food Agric. 2013;93(14):3490–3501. doi: 10.1002/jsfa.6326. [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo A, Brandolini A. Nutritional properties of einkorn wheat (Triticum monococcum L.) J Sci Food Agric. 2014;15:601–612. doi: 10.1002/jsfa.6382. [DOI] [PubMed] [Google Scholar]
- 12.Shiota M, Printz RL. Diabetes in Zucker diabetic fatty rat. Methods Mol Biol. 2012;933:103–123. doi: 10.1007/978-1-62703-068-7_8. [DOI] [PubMed] [Google Scholar]
- 13.Magnusdottir OK, Landberg R, Gunnarsdottir I, Cloetens L, Akesson B, Rosqvist F, Schwab U, Herzig KH, Hukkanen J, Savolainen MJ. et al. Whole grain rye intake, reflected by a biomarker, is associated with favorable blood lipid outcomes in subjects with the metabolic syndrome - a randomized study. Plos One. 2014;9(10):e110827. doi: 10.1371/journal.pone.0110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen LA, Silva LO, Andersson UK, Holm C, Ostman EM, Bjorck IM. Endosperm and whole grain rye breads are characterized by low post-prandial insulin response and a beneficial blood glucose profile. Nutr J. 2009;25:8–42. doi: 10.1186/1475-2891-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen B. Determination of Nitrogen as elementary-N, an alternative to Kjeldahl. Acta Agric Scand. 1989;39:113–118. [Google Scholar]
- 16.Stoldt W. Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln. Fette Seifen. 1952;54:206–207. [Google Scholar]
- 17.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;27:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Ibrugger S, Vigsnaes LK, Blennow A, Skuflic D, Raben A, Lauritzen L, Kristensen M. Second meal effect on appetite and fermentation of wholegrain rye foods. Appetite. 2014;80:248–256. doi: 10.1016/j.appet.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Kristensen M, Jensen MG, Riboldi G, Petronio M, Bugel S, Toubro S, Tetens I, Astrup A. Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite. 2010;54(1):163–169. doi: 10.1016/j.appet.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Bornet FR, Jardy-Gennetier AE, Jacquet N, Stowell J. Glycaemic response to foods: impact on satiety and long-term weight regulation. Appetite. 2007;49:535–553. doi: 10.1016/j.appet.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6(1):87–97. [PubMed] [Google Scholar]
- 25.Terpstra AH, Sanchez-Muniz FJ, West CE, Woodward CJ. The density profile and cholesterol concentration of serum lipoproteins in domestic and laboratory animals. Comp Biochem Physiol B. 1982;71:669–673. doi: 10.1016/0305-0491(82)90479-5. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Muniz FJ, Garcia-Linares MC, Garcia-Arias MT, Bastida S, Viejo J. Fat and protein from olive oil-fried sardines interact to normalize serum lipoproteins and reduce liver lipids in hypercholesterolemic rats. J Nutr. 2003;133:2302–2308. doi: 10.1093/jn/133.7.2302. [DOI] [PubMed] [Google Scholar]
- 27.Slieker LJ, Sundell KL, Heath WF, Osborne HE, Bue J, Manetta J, Sportsman JR. Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (Avy/a) Diabetes. 1992;41:187–193. doi: 10.2337/diab.41.2.187. [DOI] [PubMed] [Google Scholar]
- 28.Marks J, Carvou NJ, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol. 2003;15:137–145. doi: 10.1113/jphysiol.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yahagi N, Shimano H, Hasty AH, Matsuzaka T, Ide T, Yoshikawa T, Amemiya-Kudo M, Tomita S, Okazaki H, Tamura Y. et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277(22):19353–19357. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, Ogawa W, Teshigawara K, Inoue H, Miyake K, Sakaue H, Kasuga M. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signaling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51(6):1672–1680. doi: 10.2337/diabetes.51.6.1672. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe J, Tamasawa N, Yamashita M, Matsuki K, Murakami H, Matsui J, Sugimoto K, Yasujima M, Suda T. Effects of combined PPARgamma and PPARalpha agonist therapy on reverse cholesterol transport in the Zucker diabetic fatty rat. Diabetes Obes Metab. 2008;10(9):772–779. doi: 10.1111/j.1463-1326.2007.00810.x. [DOI] [PubMed] [Google Scholar]
- 32.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10(5):530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 33.Maher AD, Lindon JC, Nicholson JK. (1)H NMR-based metabonomics for investigating diabetes. Future Med Chem. 2009;1:737–747. doi: 10.4155/fmc.09.54. [DOI] [PubMed] [Google Scholar]
- 34.Diao C, Zhao L, Guan M, Zheng Y, Chen M, Yang Y, Lin L, Chen W, Gao H. Systemic and characteristic metabolites in the serum of streptozotocin-induced diabetic rats at different stages as revealed by a (1)H-NMR based metabonomic approach. Mol Biosyst. 2014;10(3):686–693. doi: 10.1039/c3mb70609e. [DOI] [PubMed] [Google Scholar]
- 35.Zhao L, Liu X, Xie L, Gao H, Lin D. 1H NMR-based metabonomic analysis of metabolic changes in streptozotocin-induced diabetic rats. Anal Sci. 2010;26:1277–1282. doi: 10.2116/analsci.26.1277. [DOI] [PubMed] [Google Scholar]
- 36.Henry CJ, Lightowler HJ, Tydeman EA, Skeath R. Use of low-glycaemic index bread to reduce 24-h blood glucose: implications for dietary advice to non-diabetic and diabetic subjects. Int J Food Sci Nutr. 2006;57:273–278. doi: 10.1080/09637480600931626. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DJ, Wesson V, Wolever TM, Jenkins AL, Kalmusky J, Guidici S, Csima A, Josse RG, Wong GS. Wholemeal versus wholegrain breads: proportion of whole or cracked grain and the glycaemic response. BMJ. 1988;297(6654):958–960. doi: 10.1136/bmj.297.6654.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slavin JL, Jacobs D, Marquart L. Grain processing and nutrition. Crit Rev Biotechnol. 2001;21:49–66. doi: 10.1080/20013891081683. [DOI] [PubMed] [Google Scholar]
- 39.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 40.Belobrajdic DP, Bird AR. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr J. 2013;12:62. doi: 10.1186/1475-2891-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adom KK, Sorrells ME, Liu RH. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J Agric Food Chem. 2005;23:2297–2306. doi: 10.1021/jf048456d. [DOI] [PubMed] [Google Scholar]