Abstract
OBJECTIVES: Type 2 diabetes (T2D) may be caused by elevated oxidative stress, inflammation, and hyperglycemia. The phytochemicals in several herbal medicines are reported to effectively improve diabetes and to ameliorate diabetic complications. The aim of the present study was to determine the effects of cinnamon, cardamom, saffron, and ginger as supplementary remedies in T2D. METHODS: This randomized controlled, clinical trial included 204 T2D patients. The participants were randomly assigned to four intervention groups receiving 3 glasses of black tea and either 3 g cardamom, or cinnamon, or ginger, or 1 g saffron and one control group which consumed only 3 tea glasses without any herbal medicine for 8 weeks. Markers of inflammation, oxidative stress, fasting blood sugar, lipid profile, and anthropometric measures were evaluated at baseline and after 8 weeks of intervention. RESULTS: After 8 weeks of intervention, cinnamon, cardamom, ginger, and saffron consumption had significant effects on total cholesterol, LDL, and HDL levels (p < 0.05) compared with controls. However, the herbal products did not have significant effects on measures of glycemic control, anthropometry, inflammation, and oxidative stress. In within-group comparisons only, cinnamon intake significantly decreased fasting blood sugar (FBS). CONCLUSIONS: The herbal remedies examined had significantly beneficial effects on cholesterol, but not on measures of glycemic control, oxidative stress, and inflammation. Based on the contradictory results reported in the literature, the effects of herbal medicine in diabetic patients should undergo further detailed investigation.
Keywords: type 2 diabetes, herbal medicine, cholesterol, glycemic control, oxidative stress, inflammation
Abbreviations: ACC – acetyl-CoA carbohydrate; AMP – adenosine monophosphate; AMPK – AMP-activated protein kinases; apoB – apolipoprotein B; BMI – body mass index; COX-2 – cyclooxygenase-2; DPPH – 2,2-diphenyl-1-picrylhydrazyl; ELISA – enzyme-linked immune sorbent assay; FBS – fasting blood sugar; HbA1c – hemoglobin A1c ; HDL – high-density lipoprotein; hs-CRP – high-sensitivity C-reactive protein; IL – interleukin; IPAQ – international physical activity questionnaire; Kcal/d – kilocalorie per day; LDL – low-density lipoprotein; LDLc – low-density lipoprotein cholesterol; MANCOVA – multivariate analysis of covariance; MAPKS – mitogen-activated protein kinases; MDA – malondyaldehyde; MET – metabolic equivalent of task; MET-h/wk – MET hours per week; mRNA – messenger ribonucleic acid; NO – nitric oxide; OHA – oral hypoglycemic agent; ROS – reactive oxygen species; SE – standard error; SPSS – software package used for statistical analysis; TC – total cholesterol; T2D – type 2 diabetes; TG – triglyceride; TNF-α – tumor necrosis factors α
1. Introduction
Type 2 diabetes (T2D) is the fourth cause of death in developed countries [1] and affects 6% of the world’s adult population [2]. In Iranian adults, the prevalence is 11.4% aged 25-70 years [3]. Diabetes is characterized by elevated oxidative stress and inflammation that may eventually lead to insulin resistance [4].
Lifestyle modification, in particular dietary management, may be useful in controlling diabetes risk factors. Recently, herbal medicines have become more respected as supplementary medicine since they have phytochemicals with potential antioxidative effects that may improve the regulation of glycemic metabolism [5].
Cinnamon's major components include cinnamaldehyde, cinnomic acid, eugenol, coumarin, and procyanidins that have antioxidant effects elucidated by scavenging reactive oxygen species (ROS) and free-radicals, suppressing lipid peroxidation, and reducing malondialdehyde (MDA) [6, 7]. Cinnamon has anti-inflammatory compounds that can reduce inflammatory mediators such as prostaglandin-E2, interleukin (IL) 6, and nitric oxide (NO) production [8]. Contradictory evidence exists regarding the effectiveness of herbal remedies. On the one hand, Khan et al. indicated that herbal remedies are effective in reducing fasting blood sugar (FBS), total cholesterol (TC), LDL cholesterol (LDLc), and triglyceride (TG) levels in subjects with type 2 diabetes [9], but in another study, beneficial effects of herbal remedies on FBS, TC, and TG could not be shown [10].
Cardamom, another herbal medicine, contains essential lipids such as sterol and phenolic acids. These lipids have antioxidative potential that might increase antioxidant enzyme activities such as superoxide dismutase, catalase, and glutathione peroxidase [11, 12]. Studies suggest that cardamom exerts anti-inflammatory effects and can significantly reduce NO production by macrophages [13]. However, there is only limited evidence about the biological role of cardamom ingredients in human glucose and lipid metabolism.
Saffron is a carotenoid-rich spice that consists of crocetin and crocetin glycosides. It is used in the treatment of different complications of diabetes [14]. In an in vitro study, crocetin decreased the levels of ROS, free radical-mediated lipid peroxidation, and MDA, and increased radical scavenging activity [15]. Furthermore, saffron has been reported to possess anti-inflammatory effects exerted by the lavonoids, tannins, saponins, and crocins it contains [14]. The herb improves the lipid profile and increases glucose uptake by an insulin-dependent pathway that stimulates phosphorylation of AMP-activated protein kinases (AMPK), acetyl-CoA carbohydrate (ACC), and mitogen-activated protein kinases (MAPKS). Co-treatment of saffron and insulin has been shown to improve insulin sensitivity [16].
New evidence suggests that ginger, of which the root is used as spice and medicine, has antioxidative effects by virtue of its components gingerols, shogaols, paradols, and zingiberene [17]. It seems that these components scavenge free radicals, hydroxyl, and superoxide radicals, protect cell membrane lipids from oxidation, and inhibit ROS and NO production [18]. A recent study has revealed that ginger suppresses the synthesis of prostaglandin and leukotriene [19]. Furthermore, the root has been reported to reduce inflammatory cytokines and arachidonate-5-lipoxygenase, and to inhibit the expression of inflammatory response genes [19, 20]. Ginger is also considered to reduce blood sugar and to improve lipid profiles by increasing the activity of antioxidant enzymes [21].
Although beneficial effects of these herbal medicines have been indicated in several studies, to our knowledge there is no study that has compared the effects of them on blood sugar, lipid profiles, oxidative stress, and inflammation among diabetic patients. Therefore, in this study we intended to compare the beneficial effects of cinnamon, cardamom, saffron, and ginger consumption on markers of fasting blood sugar, lipid profile, oxidative stress, and inflammatory mediators in type 2 diabetes patients.
2. Methods
2.1 Patients
This is a parallel, randomized, single-blind, placebo-controlled clinical trial, conducted among 208 subjects with T2D (fasting blood glucose ≥126 mg/dl) for eight weeks from October 1, 2012 through September 1, 2013. Subjects with diabetes were recruited from the Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. Inclusion criteria were subjects with T2D, aged ≥30 years, overweight (BMI ≥25 kg/m2), not on insulin therapy, and not taking medications except for oral hypoglycemic agents (OHAs), namely metformin or glibenclamide. Participants were excluded from the study if they planned to get pregnant, needed to start insulin therapy during intervention, or consumed special spices (cinnamon, cardamom, ginger, or saffron) during the running period.
All patients signed informed written consent, and the Ethics Committee of the university approved the study. The trial was registered by the Iranian Registry of Clinical Trials; website at http://www.irct.ir (IRCT201206185062N5). We estimated that at least 200 subjects should participate in the study to calculate between-group differences regarding oxidative stress and inflammation. Considering dropouts, we included 280 subjects in this study.
2.2 Study design
Before intervention, all participants were included in a three-week run-in period to match their tea consumption pattern because tea was the major carrier of the herbal remedies in the study. The randomization was stratified by the permuted block method.
The patients were randomly assigned to four intervention groups, receiving either 3 g cardamom, or 3 g cinnamon, or 3 g ginger, or 1 g saffron, and one control group. All participants used this dose of herbal medicine in combination with three glasses of black tea (golestan tea bags, Golestan Inc., Tehran, Iran). Individuals in the control group consumed three glasses of tea without any spices. We used raw, dried powder from cardamom (cardamomum) small seed pods, ginger (Zingiberaceae) rhizome of the Zingiber officinale plant, cinnamon (Cinnamomum verum) sticks (inner bark of tree), and saffron (saffron crocus) stigmas of the Crocus sativus flower. All herbs used in the present study were approved by the Ministry of Health (License No.16/13777).
Three grams herbal medicines were given to participants in packed plastics containing cardamom, ginger, or cinnamon, or 1 g of saffron. We filled these packs with purified herbal products without any extra materials. The participants were interviewed once a week to assess their compliance; at these occasions, they received 7 packages of herbs for consumption the following week. They were also asked to return the empty packages on their next visit.
The participants were trained to stew herbs with black tea in the kettle for 10 minutes in a traditional way. Compliance was determined by performing dietary records and phone interviews. All patients were asked to continue their usual diets without any changes; all were requested to complete the 24-hour food record form every two weeks at the duration of intervention to make sure that the results were not influenced by dietary intakes. In order to gather information about their level of physical activity, validated short IPAQ questionnaires were used [22]. These questionnaires were completed by trained nutritionists.
2.3 Assessment of biochemical indicators
For biochemical measurements, 15 ml venous blood samples were gathered after 12-hour overnight fasting. Blood samples were centrifuged immediately, and serum samples were separated and stored at -70°C until analysis. Glucose levels were determined by the hexokinase method (Gluco-quant, Roche Diagnostics, Mannheim, Germany). HbA1c was assessed by immunoturbidimetric determination (RocheTina-quant II, Roche Diagnostics, Mannheim, Germany). Serum concentrations of triacylglycerol and total cholesterol were measured by Peridochrom Triglyceride GPO-PAP Kit and CHOD-PAP kit, respectively (Roche Diagnostics, Mannheim, Germany). Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol were measured enzymatically (Wako Chemicals, Neuss, Germany). To assess inflammation and oxidative stress, we quantified serum hs-CRP and F2-Isoprostane levels, respectively, using commercially available ELISA kits. Inter- and intra-assay coefficients of variations were determined for hs-CRP < 9% and < 7.8% and for F2-Isoprostane 5% and 7%, respectively.
2.4 Assessment of other variables
Demographic characteristics and anthropometric measures were determined through pretested questionnaires. Weight was evaluated without shoes and minimally clothed to the nearest 100 g using a digital scale. Height was measured without shoes in a standing position using a tape meter to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meter. Waist circumference was measured at the level of the umbilicus with a flexible tape to the nearest millimeter.
2.5 Statistical methods
Statistical analysis was performed using SPSS version 16 (spssInc, Chicago, IL, USA). Quantitative data are shown as mean ± SE. One-way multivariate analysis of variance (or covariance as appropriate) (MANCOVA), followed by Dunnett's post hoc pairwise comparisons test was applied. Within-group comparisons were performed using paired samples t-test. Normality distribution of data was evaluated using Kolmogorov-Smirnov test. For non-normal data log-transformation was conducted.
3. Results
From 208 participants included in the study, four patients were excluded due to a lack of blood sample at the end of the intervention. No adverse reactions caused by the use of the herbal medicines were reported during the study period. Baseline characteristics of all study participants are presented in Table 1. The participants in the saffron group were significantly older and more obese than those in the other groups. No significant differences were present between the five groups regarding other baseline characteristics. Table 1 also shows the levels of physical activity. Again, no significant differences in the physical activity levels (MET-h/wk) were observed across the five groups.
Table 1. Baseline characteristics of diabetes patients in the five groups, cinnamon, cardamom, saffron, ginger, and control.
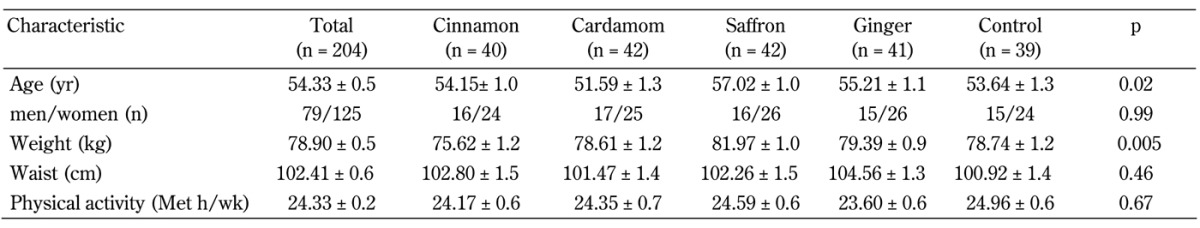
Legend: Data are mean ± SE. P-value by comparisons of between-group differences using MANCOVA.
Dietary intake was determined based on five 24-hour records during the intervention (Table 2). No significant difference in dietary intakes of energy, macro-, and micronutrients between the five groups was observed, demonstrating good compliance of the participants to the study procedures.
Table 2. Dietary consumption of the participants during intervention in the five groups, cinnamon, cardamom, saffron, ginger, and control.
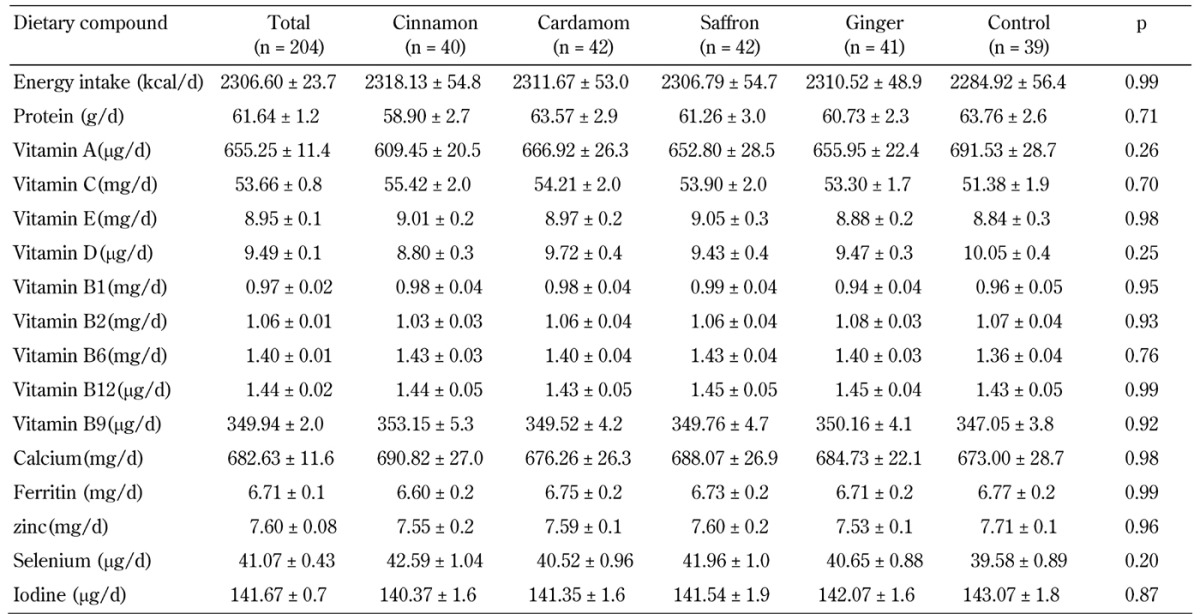
Legend: Data are mean ± SE. P-value by comparisons of between-group differences using MANCOVA.
The effects of herbal medicines on anthropometric measures, FBS, insulin, HbA1c, lipid profiles, F2-isoprostane, and hs-CRP concentrations are shown in Table 3. In all analyses, age and weight were controlled because there was a significant difference in these measures between the groups.
Table 3. Anthropometric biomarkers of the type 2 diabetes patients before and after 8 weeks of intervention in the 5 groups, cinnamon, cardamom, saffron, ginger, and control.
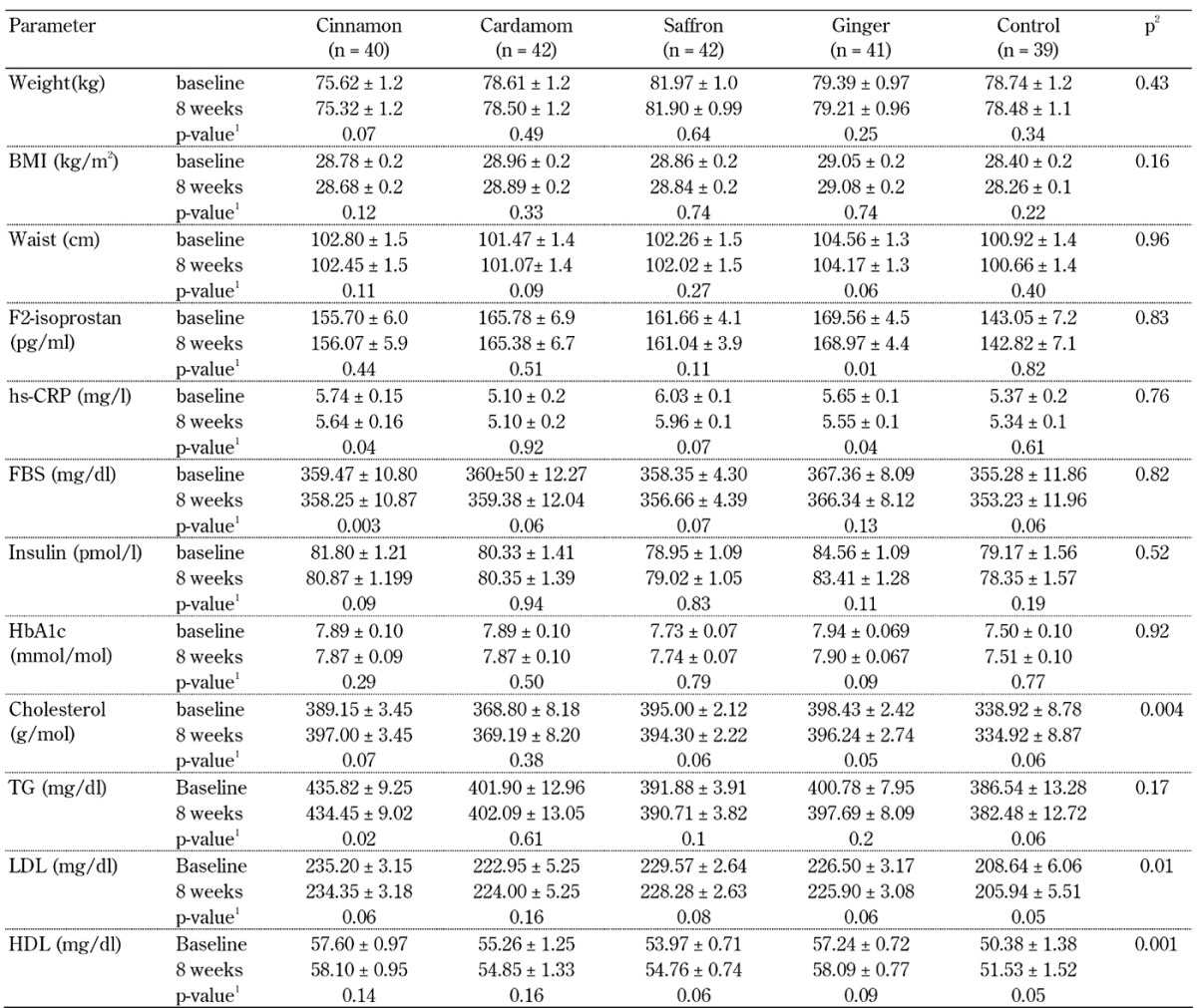
Legend: Data are mean ± SE. 1 P-value by comparisons of within-group differences between baseline and after 8 weeks using Student's paired t-test. 2 P-value by comparisons of between-group differences using MANCOVA.
After 8 weeks of intervention, cinnamon, cardamom, ginger, and saffron consumption had significant effects on total cholesterol, LDL, and HDL levels (p < 0.05) compared with the control consumption. However, the herbal products did not have significant effects on measures of glycemic control, anthropometry, inflammation, and oxidative stress. There was no antidiabetic effect observed by comparison between the groups of cinnamon, cardamom, ginger, saffron, and control.
As a marginalia, but worth noting, in within-group comparisons, cinnamon intake significantly decreased fasting blood sugar (359.47 ± 10.80 vs. 358.25 ± 10.87, p < 0.01) and hs-CRP levels (5.74 ± 0.15 vs. 5.64 ± 0.16, p < 0.05), and ginger consumption significantly decreased F2-isoprostane concentration (169.56 ± 4.5 vs. 168.97 ± 4.4, p < 0.01) and hs-CRP levels (5.65 ± 0.1 vs. 5.55 ± 0.1, p = 0.04). Other intervention groups showed no significant differences in FBS, insulin, HbA1c, and anthropometric measures, similarly to the inter-group comparisons.
4. Discussion
In the current study, we found that cinnamon, cardamom, ginger, and saffron had significant effect on total cholesterol, LDL, and HDL levels, but no significant effects on FBS, insulin, HbA1c, serum F2-isoprostane, hs-CRP concentrations, and anthropometric measures. In within-group comparisons only, there was a significant effect of cinnamon consumption to reduce FBS, and ginger intake to reduce F2-isoprostane concentrations; also cinnamon and ginger intake significantly decreased hs-CRP level.
Type 2 diabetes is characterized by abnormalities in carbohydrate, lipid, and lipoprotein metabolism, which may cause hyperglycemia and many complications such hyperlipidemia and hyperinsulinemia [23]. We found no significant effects of the herbal medicines on FBS concentrations. Another human study with 25 type 2 diabetes patients also did not find a significant effect on FBS and insulin concentration after the administration of 1.5 g cinnamon for 6 weeks [6]. In contrast to these findings, Soni et al. reported that cinnamon is an effective means of decreasing blood glucose in diabetic patients [24]. The inconsistent picture regarding the antidiabetic effectiveness of herbal medicines draws through the entire literature, with either positive or negative results in human and animal studies. Notable examples are an animal study, where cardamom and ginger decreased serum glucose [25], and a human study with 20 major depressive disorder patients who did not show a significant effect on FBS after consumption of 15 mg saffron twice daily [26].
In the present study, the herbal products had significant effect only on total cholesterol, LDL, and HDL levels in diabetic patients after eight weeks of consumption. Some reports have indicated cinnamon, saffron, and ginger to be effective in reducing TC, LDLc, and TG levels in subjects with type 2 diabetes by different mechanisms such as improved insulin sensitivity and increasing the activity of antioxidant enzymes [9, 16, 21].
Oxidative stress results from an imbalance in the production of reactive oxygen species (ROS) and the cell's own antioxidant defense; it may contribute to numerous diseases [25]. At the end of study, there was no significant difference between all groups regarding hs-CRP reduction. In within-group comparisons only, cinnamon and ginger intake may have caused a significant reduction in hs-CRP concentrations. Antioxidant activity of cinnamon by scavenging of free radicals and inhibition of 5-lipoxygenase enzyme is reported in different studies [26-28]. Human studies showed that cinnamon can reduce lipid peroxidation and increase total antioxidant power in healthy subjects [29, 30]. Other studies have supported the antioxidant effects of cinnamon in humans. In a cross-sectional study by Ranjbar et al., cinnamon could increase total antioxidant capacity and decrease lipid peroxidation in normal subjects [31].
Emerging evidence has indicated that ginger has a strong antioxidant effectiveness both in vivo and in vitro by the prevention of ROS production and cyclooxygenase 2 (COX-2) expression [32, 33]. In an experimental study, ginger significantly attenuated lipid peroxidation and raised the levels of antioxidant enzymes. This effect of ginger is similar to that of ascorbic acid [34, 35]. Antioxidative effects of saffron were shown in in vitro studies [30, 31]. Assimopoulou et al. has indicated that the antioxidant capacity of saffron is due to its ability of donating a hydrogen atom to the 2,2-diphenyl-1-picrylhydra-zyl (DPPH) radical [36]. In human subjects, 50 mg of saffron dissolved in 100 ml milk was administered twice a day to patients with coronary artery disease, and this caused a reduction in lipoprotein oxidation [37]. A human study revealed that after the administration of 3 g cardamom powder, the total antioxidant status was significantly increased by 90% at the end of the 3-month study [38]. In our study, we did not find any significant effect of saffron and cardamom on serum hs-CRP concentrations, adding again to the inconsistent picture of the effectiveness of herbal medicines. However, reports on the bioavailability of cardamom ingredients are limited. Therefore, further studies are needed to assess the effect of cardamom or its ingredients on human health.
Inflammatory mediators are strongly recognized as diabetes risk factors; elevated inflammatory cytokines may contribute to insulin resistance [7, 39]. In the present study, F2-isoprostane concentrations were not significantly reduced in diabetic patients after eight weeks of cinnamon, cardamom, ginger, and saffron consumption (only for ginger in within-group comparisons). In the early 1980s, it was reported for the first time that ginger has anti-inflammatory properties because it can inhibit prostaglandin synthesis [40]. In a human study, ginger could reduce inflammation through suppression of NO, inflammatory cytokines, and the enzyme prostaglandin synthase [41]. Thomson et al. confirmed the inhibitory action of ginger on prostaglandin by daily administration of ginger (500 mg/kg) to rats for 4 weeks; they reported that ginger significantly reduced serum prostaglandin-E2 [42]. A retrospective human survey suggested that a daily dose of 1 g ginger or higher is more effective than lower doses [43]. Thus, the picture of herbal medicine seems to be inconsistence also regarding anti-inflammatory effectiveness.
In an in vitro study, cardamom has exerted significantly anti-inflammatory properties through reducing NO production [13]. Ahmad et al. showed cardamom to significantly inhibit tumor necrosis factor α (TNF-α) production and suppress COX-2 expression [44]. Saffron has potentially bioactive components such as flavonoids, tannins, anthocyanin, alkaloids, and saponins, which may have contributed to the anti-inflammatory effects seen in mice [45].
Although we could not find a significant effect of cinnamon consumption on the level of inflammation, other authors have found beneficial anti-inflammatory effects of this herb. In an animal study, oral treatment with cinnamon inhibited the postprandial overproduction of apoB48-containing lipoproteins and inflammation in rats and hamsters [45]. Another study demonstrated that cinnamon may significantly decrease mRNA expression of inflammatory factors, including IL1, IL6, and TNF-α [46].
In this study, we did not find any significant effect of herbal medicines on anthropometric measures. Another study found positive effects of 3 g cinnamon per day on the body weight of diabetic patients [23]. Ginger supplementation and progressive resistance training were also shown to decrease waist circumference significantly in obese men [47].
This study had several strengths and limitations. The sample size was relatively large, the study design was prospective, and the study participants were almost completely followed up. The control group provided us the opportunity to determine whether our findings were due to actual treatment or usual diet. The statistical analysis was controlled for potential confounders, including dietary patterns through five 24-hour food records and physical activity by questionnaire. Before the intervention, a 3-week run-in period was established to ensure that the patients’ tea consumption patterns were identical. The limitations refer to the food records which were not representative of usual intake of micro- and macronutrients, and may have caused under- or oversupply.
5. Conclusions
We tested the effects of herbal medicines, including cardamom, cinnamon, ginger, and saffron, on anthropometric measures and diabetes biomarkers. We found that the herbs could significantly change lipid profiles, including total cholesterol, LDL, and HDL levels, but they could not reduce FBS, insulin, HbA1c, serum F2-isoprostane, hs-CRP concentrations, or change anthropometric measures. FBS was significantly reduced in the cinnamon group, F2-isoprostane was reduced in the ginger group, and hs-CRP in the cinnamon and ginger group determined by within-group comparisons only, which may be regarded as a marginalia.
This study could thus not confirm positive effects of herbal medicines on measures of glycemic control, inflammation, oxidative stress, and anthropometry. It adds to the inconsistent picture that lances the literature, with positive and negative findings in animal and human studies. Therefore, further investigation of the matter with longer duration, various doses, and different age ranges are necessary to shed more light on the therapeutic effects of herbal medicines in diabetic patients.
Disclosures: None of the authors reported any personal or financial conflict of interests.
Acknowledgments
The authors would like to express their gratitude to the participants in the present study. This study was supported by financial grants from the Iran National Science Foundation and the Isfahan University of Medical Sciences.
References
- 1.Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease. 2009. chapter 1; p. 30. [Google Scholar]
- 2.Meetoo D, Mcgovern P, Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16(16):1002–1007. doi: 10.12968/bjon.2007.16.16.27079. [DOI] [PubMed] [Google Scholar]
- 3.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Noshad S, Asgari F, Mousavizadeh M, Rafei A, Khajeh E. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005-2011. Diabetes Res Clin Pract. 2014;103(2):319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27(2):362–366. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Zhang M, Wang W, Grimsgaard S. Chinese herbal medicines for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2004;3:CD003642. doi: 10.1002/14651858.CD003642.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136(4):977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 7.Qin B, Panickar KS, Anderson RA. Cinnamon: potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J Diabetes Sci Technol. 2010;4(3):685–693. doi: 10.1177/193229681000400324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tung YT, Chua MT, Wang SY, Chang ST. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour Technol. 2008;99(9):3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 10.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in Non-insulin-dependent type 2 diabetes. Diabetes Care. 2007;30(9):2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 11.Dhuley J. Anti-oxidant effects of cinnamon (Cinnamomum verum) bark and greater cardamom (Amomum subulatum) seeds in rats fed high fat diet. Indian J Exp Biol. 1999;37(4):238–242. [PubMed] [Google Scholar]
- 12.Jamal A, Javed K, Aslam M, Jafri M. Gastroprotective effect of cardamom, Elettaria cardamomum Maton. fruits in rats. J Ethnopharmacol. 2006;103(2):149–153. doi: 10.1016/j.jep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Majdalawieh AF, Carr RI. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum) J Med Food. 2010;13(2):371–381. doi: 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- 14.Ochiai T, Shimeno H, Mishima K-i, Iwasaki K, Fujiwara M, Tanaka H, Shoyama Y, Toda A, Eyanagi R, Soeda S. Protective effects of carotenoids from saffron on neuronal injury in vitro and in vivo. Biochim Biophys Acta. 2007;1770(4):578–584. doi: 10.1016/j.bbagen.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107(1):25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Kang C, Lee H, Jung ES, Seyedian R, Jo M, Kim J, Kim JS, Kim E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chem. 2012;135(4):2350–2358. doi: 10.1016/j.foodchem.2012.06.092. [DOI] [PubMed] [Google Scholar]
- 17.Butt MS, Sultan MT. Ginger and its health claims: molecular aspects. Crit Rev Food Sci Nutr. 2011;51(5):383–393. doi: 10.1080/10408391003624848. [DOI] [PubMed] [Google Scholar]
- 18.Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN. Comparative antioxidant and anti-inflammatory effects of (6)-gingerol,(8)-gingerol,(10)-gingerol and (6)-shogaol. J Ethnopharmacol. 2010;127(2):515–520. doi: 10.1016/j.jep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Grzanna R, Lindmark L, Frondoza CG. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8(2):125–132. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 20.Shimoda H, Shan SJ, Tanaka J, Seki A, Seo J-W, Kasajima N, Tamura S, Ke Y, Murakami N. Anti-inflammatory properties of red ginger (Zingiber officinale var. Rubra) extract and suppression of nitric oxide production by its constituents. J Med Food. 2010;13(1):156–162. doi: 10.1089/jmf.2009.1084. [DOI] [PubMed] [Google Scholar]
- 21.Vijaya Durga P, Kumaraswamy B, Dhanaraju M, Ramachandran S. Antihyperglycemic, hypolipidemic and antioxident effect of aqueous extract of coriander sativum (seed) and ginger officinale (rhizome) combination in streptozotocin induced diabetes mellitus rats. Int J Biol Pharm Res. 2013;4(12):872–877. [Google Scholar]
- 22.Craig C, Marshall A, Sjöström M, Bauman A, Booth M, Ainsworth B, Pratt M, Ekelund U, Yngve A, Sallis J. et al. International Physical Activity Questionnaire (IPAQ): 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Vafa M, Mohammadi F, Shidfar F, Sormaghi MS, Heidari I, Golestan B, Amiri F. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3(8):531–536. [PMC free article] [PubMed] [Google Scholar]
- 24.Soni R, Bhatnagar V. Effect of cinnamon (Cinnamomum Cassia) intervention on blood glucose of middle aged adult male with non-insulin dependent diabetes mellitus (NIDDM) Ethno Med. 2009;3(2):141–144. [Google Scholar]
- 25.Bandara T, Uluwaduge I, Jansz ER. Bioactivity of cinnamon with special emphasis on diabetes mellitus: a review. Int J Food Sci Nutr. 2012;63(3):380–386. doi: 10.3109/09637486.2011.627849. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, Schoene NW, Graves DJ. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52(1):65–70. doi: 10.1021/jf034916b. [DOI] [PubMed] [Google Scholar]
- 27.Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J Agric Food Chem. 2009;57(5):1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- 28.Roussel AM, Hininger I, Benaraba R, Ziegenfuss TN, Anderson RA. Antioxidant effects of a cinnamon extract in people with impaired fasting glucose that are overweight or obese. J Am Coll Nutr. 2009;28(1):16–21. doi: 10.1080/07315724.2009.10719756. [DOI] [PubMed] [Google Scholar]
- 29.Ranjbar A, Khorami S, Safarabadi M, Shahmoradi A, Malekirad AA, Vakilian K, Mandegary A, Abdollahi M. Antioxidant activity of Iranian Echium amoenum Fisch and CA Mey flower decoction in humans: a cross-sectional before/after clinical trial. Evid Based Complement Alternat Med. 2006;3(4):469–473. doi: 10.1093/ecam/nel031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 2009;8(1):2–10. doi: 10.2174/187152809787582561. [DOI] [PubMed] [Google Scholar]
- 31.Ranjbar A, Ghasmeinezhad S, Zamani H, Malekirad AA, Baiaty A, Mohammadirad A, Abdollahi M. Antioxidative stress potential of Cinnamomum zeylanicum in humans: a comparative cross-sectional clinical study. Therapy. 2006;3(1):113–117. [Google Scholar]
- 32.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 33.Ghasemzadeh A, Jaafar HZ, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15(6):4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed RS, Seth V, Banerjee B. Influence of dietary ginger (Zingiber officinales Rosc) on antioxidant defense system in rat: comparison with ascorbic acid. Indian J Exp Biol. 2000;38(6):604–606. [PubMed] [Google Scholar]
- 35.Afshari AT, Shirpoor A, Farshid A, Saadatian R, Rasmi Y, Saboory E, Ilkhanizadeh B, Allameh A. The effect of ginger on diabetic nephropathy, plasma antioxidant capacity and lipid peroxidation in rats. Food Chem. 2007;101(1):148–153. [Google Scholar]
- 36.Assimopoulou A, Sinakos Z, Papageorgiou V. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Bordia A. Antioxidant property of Saffron in man. Indian J Med Sci. 1998;52(5):205–207. [PubMed] [Google Scholar]
- 38.Verma S, Jain V, Katewa S. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of Cardamom (Elettaria cardamomum) Indian J Biochem Biophys. 2009;46(6):503–506. [PubMed] [Google Scholar]
- 39.O'Keefe JH, Gheewala NM, O'Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51(3):249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Kiuchi F, Shibuya M, Sankawa U. Inhibitors of prostaglandin biosynthesis from ginger. Chem Pharm Bull. 1982;30(2):754–757. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- 41.Phan PV, Sohrabi A, Polotsky A, Hungerford DS, Lindmark L, Frondoza CG. Ginger extract components suppress induction of chemokine expression in human synoviocytes. J Altern Complement Med. 2005;11(1):149–154. doi: 10.1089/acm.2005.11.149. [DOI] [PubMed] [Google Scholar]
- 42.Thomson M, Al-Qattan K, Al-Sawan S, Alnaqeeb M, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. 2002;67(6):475–478. doi: 10.1054/plef.2002.0441. [DOI] [PubMed] [Google Scholar]
- 43.Bliddal H, Rosetzsky A, Schlichting P, Weidner M, Andersen L, Ibfelt HH, Christensen K, Jensen O, Barslev J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis and Cartilage. 2000;8(1):9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad S, Israf DA, Lajis NH, Shaari K, Mohamed H, Wahab AA, Ariffin KT, Hoo WY, Aziz NA, Kadir AA. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur J Pharmacol. 2006;538(1):188–194. doi: 10.1016/j.ejphar.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC pharmacology. 2002;2(1):7–14. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, Darwiche G, Almer LO. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89(3):815–821. doi: 10.3945/ajcn.2008.26807. [DOI] [PubMed] [Google Scholar]
- 47.Atashak S, Peeri M, Azarbayjani MA, Stannard SR, Haghighi MM. Obesity-related cardiovascular risk factors after long-term resistance training and ginger supplementation. J Sports Sci Med. 2011;10(4):685–691. [PMC free article] [PubMed] [Google Scholar]


