Abstract
Prior studies indicate that anxiety disorders are associated with heightened sensitivity to uncertain threat (U-threat). Individual differences in reactivity to U-threat have been measured in the laboratory with two methodologies - startle eyeblink potentiation and functional magnetic resonance imaging (fMRI). While startle and fMRI are purported to relate to each other, very little research exists on whether individual differences in one measure are associated with individual differences in another and thus, whether startle and fMRI capture shared mechanisms. Therefore, the current study was designed to investigate if and where in the brain measures of startle potentiation and fMRI blood-oxygen-level dependent (BOLD) signal correlate during response to U-threat across two independent samples. Participants in both studies completed two threat anticipation tasks – once during collection of startle potentiation and once during fMRI. In Study 1 (n = 43), the startle and fMRI tasks both used electric shock as the threat. As an extension, in Study 2 (n = 38), the startle task used electric shock but the fMRI task used aversive images. Despite these methodological differences, greater startle potentiation to U-threat was associated with greater dorsal anterior cingulate (dACC), caudate, and orbitofrontal cortex (OFC) reactivity to U-threat in both samples. The findings suggest that startle and fMRI measures of responding to U-threat overlap, and points towards an integrated brain-behavior profile of aberrant U-threat responding.
Keywords: unpredictable threat, anxiety, startle potentiation, fMRI
Introduction
Exaggerated responding to the anticipation of uncertain aversive events is a core feature of several anxiety disorders. For example, social anxiety disorder (SAD) and panic disorder (PD) are characterized by exaggerated anticipatory anxiety to the possibility of negative social evaluation and uncertain onset of panic attacks, respectively (APA, 2013). Moreover, elevated self-reported intolerance of uncertainty has been evidenced in SAD, PD, posttraumatic stress disorder (PTSD), and generalized anxiety disorder (GAD) (see Carleton, 2016a). Heightened reactivity to the anticipation of uncertain threat (U-threat) may therefore contribute to the pathogenesis of multiple internalizing disorders and has been proposed as a lower-order, fundamental individual difference factor underlying anxiety and neuroticism (Carleton, 2016b; Hong & Cheung, 2015). Reactivity to U-threat therefore reflects a potential transdiagnostic treatment target (Grupe & Nitschke; Barlow, 2000; Grillon et al., 2002) and identifying biobehavioral correlates of heightened reactivity to U-threat is critical in order to develop valid assays for treatment of this aberrant affective response tendency.
Electromyography (EMG) of the eyeblink startle reflex is one of the primary methods that are used to assess reactivity to U-threat. The eyeblink startle reflex is a rapid contraction of the orbicularis oculi muscle around the eye to a brief, unexpected, intense stimulus (e.g., short burst of white noise), and is reliably potentiated during aversive motivational states (e.g., threat) and attenuated during appetitive motivational states (e.g., pleasure) (Bradley, Cuthbert, & Lang, 1999; Lang, 1995). Relative to healthy controls, heightened potentiation of the startle response during the anticipation of U-threat has been evidenced in PD, SAD, specific phobia, and PTSD (Gorka et al., in press; Grillon et al., 2008, 2009; Shankman et al., 2013; Lieberman et al., in press). Of note is that all but one of these studies (Shankman et al., 2013) found that psychopathology was only related to exaggerated U-threat responding, and not predictable threat (P-threat) responding, suggesting specificity to U-threat. The distinction between U-threat and P-threat is also supported by animal (Davis, 1998; Davis et al., 2010) and pharmacological challenge (Grillon et al., 2006) studies, and the fact that startle potentiation to U-threat, but not P-threat, is associated with familial risk for anxiety disorders (Nelson et al., 2013). Taken together, heightened startle potentiation to U-threat is a potential psychophysiological indicator of anxiety-based psychopathology (Gorka et al., in press).
Studies have also used functional magnetic resonance imaging (fMRI) to identify the neural correlates of heightened reactivity to U-threat. Converging evidence suggests that there is a specific frontolimbic circuit, referred to as the anticipatory anxiety network (AAN), which is engaged during the processing of U-threat and may contribute to aberrant responding in anxiety psychopathology (Bach & Dolan, 2012; Grupe & Nitschke, 2013). The AAN consists of affect-generating limbic regions, such as the amygdala, anterior insula (aINS), and bed nucleus of the stria terminalis (BNST), which have bidirectional connections with affect-modulating prefrontal regions, such as the prefrontal cortex, orbitofrontal cortex, and dorsal anterior cingulate cortex (dACC; Aupperle, Melrose, Stein, & Paulus, 2012; Grupe & Nitschke, 2013; Shankman et al., 2014; Walker & Davis, 1997). Several regions within the AAN have been implicated in anxiety psychopathology. In particular, hyperactivity of the aINS has been evidenced in various anxiety disorders (e.g., PD, SAD, GAD) during the anticipation of U-threat (Gorka et al., 2014; Simmons et al., 2011). The aINS has also been associated with interoceptive awareness during the anticipation of aversive events (Craig et al., 2009; Critchley et al., 2004; Khalsa et al., 2009), which is noteworthy given that heightened interoceptive awareness is involved in the pathogenesis of PD and SAD (Stevens et al., 2011; Ehlers & Bruer, 1992).
Hyperactivity of the dACC has also been observed in anxiety disorders during the anticipation of U-threat (Lieberman et al., in press; Straube, Mentzel & Miltner, 2007). Akin to the aINS, the dACC has been associated with interoceptive awareness and the generation and modulation of affective and physiological responding (Etkin, Egner & Kalisch, 2011; Milad & Rauch, 2007). Although the amygdala is another region involved in responding to threatening stimuli (Carleton et al., 2016b, Öhman, 2005; Thayer et al., 2012), few studies have found that amygdala reactivity differentiates anxiety disorder patients from controls during U-threat (e.g., Straube et al., 2007).
Taken together, anxiety disorders are associated with heightened startle potentiation to U-threat and hyperactivity of the aINS and dACC. These behavioral and neural responses to U-threat may therefore represent a transdiagnostic, anxiety disorder brain-behavior profile. However, the fMRI and startle literatures have been conducted in parallel and although it is often assumed that neural and startle reactivity to U-threat are related, very little research exists on whether individual differences in startle and fMRI response to U-threat converge. In other words, it is currently unclear whether individuals who display heightened startle potentiation to U-threat also exhibit hyperactivity of the aINS and dACC during the anticipation of U-threat, as expected. The question of convergence is further complicated by the fact that startle potentiation is often thought to be mediated by the amygdala, not the aINS or dACC (Davis et al., 2010; Lang & Davis, 2006), though the amygdala has dense projects to many AAN nodes including the aINS and dACC (Ochsner, Silvers, & Buhle, 2012).
The issue of multimethod convergence is particularly relevant to the National Institute on Mental Health’s (NIMH) Research Domain Criteria (RDoC) Initiative. The RDoC Initiative seeks to reconceptualize psychopathology using a research framework based on transdiagnostic, multilayered, dimensional constructs that reflect core mechanisms of psychopathology (Sanislow et al., 2010; Cuthbert & Kozak, 2013). U-threat responding is a key construct in the RDoC framework and the RDoC Initiative implies that there is convergence across multiple units of analysis of a given construct (e.g., genes, circuits, physiology, behavior) (see Shankman & Gorka, 2015 for further discussion). Thus, startle indicators of reactivity to U-threat should converge with fMRI indicators of reactivity to U-threat. In order to establish this relation, it is necessary that studies assess whether individual difference in startle potentiation during U-threat meaningfully predict fMRI blood oxygenation level-dependent (BOLD) signal during U-threat.
There is only one study addressing the literature gap by linking panic disorder symptoms with neural and startle responses to U-threat. In Lieberman et al. (2016), our lab demonstrated that panic symptoms were positively associated with startle potentiation to U-threat and dACC activation to U-threat. Moreover, within this sample, startle and dACC activation to U-threat was significantly correlated in a post hoc region-of-interest analysis. These findings suggest that startle potentiation to U-threat correlates with dACC reactivity to U-threat but because the Lieberman et al. analysis was post hoc, no other regions of the AAN were examined and it is presently unknown whether startle potentiation to U-threat relates to reactivity within any other AAN regions such as the aINS or amygdala.
Other studies have used startle potentiation as an index of aversive responding within fMRI paradigms, but have not looked at the correlations between the two measures (Eippert et al., 2007; van Well et al., 2012). Some studies have also collected simultaneous startle potentiation and fMRI to assess their associations yet none have examined this question during U-threat explicitly. For example, across several startle tasks, Neuner and colleagues (2010) found that non affect modulated startle (i.e., baseline startle) was correlated with activity in the somatosensory cortices, insula, thalamus, temporal pole, middle cingulate cortex, and cerebellum. Meanwhile, during an affective picture viewing task in healthy adults, Anders et al. (2004) found that greater startle changes to both positive and negative stimuli were associated with greater amygdala activity. These studies highlight that individual differences in startle potentiation relate to individual differences in specific neural structures; however, there is a need to directly examine the correspondence between startle and fMRI during the anticipation of U-threat given its relevance to psychopathology.
The current study was designed to examine whether individual differences in startle potentiation to U-threat were associated with neural reactivity to U-threat across two samples. In both studies, EMG startle responding to U-threat was assessed during modified versions of a well-validated threat-of-shock paradigm. In Study 1, fMRI BOLD response was assessed during an analogous threat-of-shock paradigm. In Study 2, however, fMRI BOLD response was assessed during the anticipation of uncertain aversive images, allowing us to examine whether startle and fMRI reactivity to U-threat converge even if the aversive stimulus differs between the two tasks. Study 2 therefore addresses whether there is an association between brain and behavioral (i.e., startle) responses to U-threat that generalizes across types of threat/stimulus. Based on the existing literature, we hypothesized that greater startle potentiation to U- threat would be associated with greater dACC, aINS, and amygdala activation to U-threat.
Method
Participants
Participants for Studies 1 and 2 were recruited from the community as a part of larger investigations on affective and physiological abnormalities related to internalizing disorders. Participants were recruited via advertisements posted in the Chicago community, local psychiatric clinics, and nearby college campuses. A variety of advertisements were used to target different populations in an effort to enroll a diverse, internalizing disorder patient sample. Examining the impact of DSM diagnoses on the association between startle and fMRI measures of reactivity to U-threat is not an aim of the current study. However, exaggerated reactivity to U-threat has been observed across internalizing diagnoses (Carleton 2016b, Gorka et al., in press) and having individuals with a range of internalizing symptoms provides variability in individual differences to U-threat reactivity (Grillon et al., 2013; Shankman et al., 2013) and thus, variability in our startle and fMRI measures. Variability in responding is necessary to accurately detect statistical relationships making the current sample well-suited for the present aims.
Both studies took place at the University of Illinois-Chicago and were approved by the university Institutional Review Board. All participants provided written informed consent after review of the respective study protocols. Participants in each study completed a set of laboratory tasks and battery of questionnaires. All participants received cash as payment for participation.
Study 1
In line with the aims of the larger study, participants were included if they either (1) were seeking and would merit treatment with pharmacotherapy (selective serotonin reuptake inhibitors/SSRIs) or cognitive behavioral therapy (CBT) for their anxiety or depressive symptoms (i.e., patients); or (2) had no lifetime history of psychopathology (i.e., healthy controls). Participants were required to be between the ages of 18 and 65 years. Exclusion criteria included an inability to provide consent and read and write in English, a major active medical or neurological problem that could impact psychophysiological and brain function, lifetime history of mania or psychosis, any contraindication to receiving SSRIs, being already engaged in any form of psychiatric treatment, history of traumatic brain injury, left handedness, and being pregnant. All data used in the current study was assessed prior to treatment (i.e., baseline assessment) and none of the participants were taking psychoactive medications or engaged in psychotherapy at study entry. A total of 48 individuals met inclusionary criteria; however, 5 were excluded due to missing/poor quality raw startle data (n = 4), or poor quality fMRI data (i.e., excessive motion; n = 1). The final sample included 43 individuals, the demographics for which are listed in Table 1.
Table 1.
Participant demographics and clinical characteristics
|
Sample 1 (n=43) |
Sample 2 (n=38) |
|
|---|---|---|
| Demographics | Mean (SD) or % | Mean (SD) or % |
| Age (years) | 25.2 (7.6)a | 31.2 (11.9)a |
| Sex (% female) | 68.2%a | 70.7%a |
| Race (% Caucasian) | 61.9%a | 52.5%a |
| Clinical Variables | ||
| Current Major Depressive Disorder | 36.6%a | 53.7%b |
| Current Anxiety Disorder | 58.1%a | 31.7%b |
| Current Comorbid Anxiety Disorders | 27.9%a | 0.0%b |
| Current Comorbid Depression and Anxiety | 44.2%a | 31.6%a |
| Lifetime Major Depressive Disorder | 51.2%a | 53.7%a |
| Lifetime Anxiety Disorder | 62.8%a | 31.7%b |
| Lifetime Alcohol Use Disorder | 9.3%a | 26.3%b |
| Lifetime Substance Use Disorder | 11.6%a | 31.6%b |
| Taking Psychiatric Medication | 0.0%a | 10.5%b |
| Startle Variables | ||
| Startle Potentiation to U-Threat | 12.9 (22.1)a | 18.7 (19.4)a |
| Startle Potentiation to P-Threat | 16.4 (28.0)a | 19.0 (17.3)a |
|
IAPS fMRI Image Ratings Arousal (Mean; Range) |
||
| U-threat Negative Images | - | 4.9 (1.9); 1.0 – 8.9 |
| U-threat Neutral Images | - | 2.7 (1.7); 1.0 – 7.4 |
| P-threat Negative Images | - | 4.7 (1.9); 1.0 – 8.9 |
| P-threat Neutral Images | - | 2.8 (1.6); 1.0 – 7.2 |
| Valence (Mean; Range) | ||
| U-threat Negative Images | - | 3.3 (1.4); 1.2 – 8.5 |
| U-threat Neutral Images | - | 5.8 (0.9); 4.0 – 8.1 |
| P-threat Negative Images | - | 3.3 (1.4); 1.0 – 8.4 |
| P-threat Neutral Images | - | 5.7 (0.9); 4.1 – 7.7 |
Note. Means (and standard deviations) or percentages with different subscripts across rows were significantly different in pairwise comparisons (p < .05, chi-square test for categorical variables and Tukey’s honestly significant difference test for continuous variables). IAPS = International Affective Picture System.
Study 2
As part of the aims for Shankman et al. (2013), participants in Study 2 were in one of three groups: (1) current PD with comorbid MDD (n = 13), (2) current MDD-only (n = 9), or (3) no lifetime history of any psychopathology (n = 19). Current and lifetime diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996). For the larger study, individuals were excluded if they had a lifetime diagnosis of a psychotic disorder, bipolar disorder, or dementia, were unable to read or write in English, had a history of head trauma with loss of consciousness, or were left handed. A total of 39 individuals met inclusionary criteria; however, 1 was excluded due to missing fMRI data. The final sample included 38 individuals, the demographics for which are also listed in Table 1.
Procedure
For both studies, modified versions of the NPU-threat tasks (Schmitz & Grillon, 2012) were used to assess neural and startle reactivity to U-threat during fMRI and startle data collection, respectively. The startle and fMRI tasks were completed on separate days. Participants in both studies always completed the startle task first due to study constraints.
Prior to each shock task, a shock work-up procedure was completed in which participants received increasing levels of shock intensity until they reached a level that they described as “highly annoying but not painful.” Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman and Harris, 1987; max shock = 5mA). For Study 1 and 2, the startle task shock electrodes were placed on participants’ left wrist. For Study 1, the fMRI shock electrodes were placed on participants’ left foot in order to minimize movement and potential scan artifacts. After shock electrode placement, to prevent early exaggerated startle responding, participants completed a 2.5 min baseline habituation task during which 9 acoustic startle probes were presented.
Startle Threat Tasks and Data Collection
The startle tasks included three within-subjects conditions - no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informed participants of the current threat condition and each condition lasted for 90 s. In Study 1, the cue was a 6 s countdown, displayed five times within each condition, and in Study 2 the cue was an 8 s geometric shape (blue circle for N, red square for P, and green star for U; not counterbalanced), presented four times within each condition. Interstimulus intervals (ISIs; i.e., times between cues) ranged from 7 to 17 s during which only the text describing the condition was on the screen.
During N, no shocks were delivered. During P, participants received a shock at the end of each cue. During U, shocks were administered at random (i.e., any time). Of note, shocks were not administered during every UCue and UISI as to ensure that the U condition was fully unpredictable. In Study 1, participants received 20 shocks (10 each during P and U) and 48 startle probes (16 each during N, P, and U). In Study 2, participants received 12 shocks (6 during P and 6 during U) and 72 startle probes (24 during N, 24 during P, and 24 during U).
In Study 1, EMG startle data was acquired and presented using BioSemi Active Two system (BioSemi, Amsterdam, The Netherlands) and Presentation (Albany, CA), respectively. In Study 2, EMG startle data were acquired and presented using Neuroscan 4.4 (Compumedics, Charlotte, NC) and PSYLAB (Contact Precision Instruments, London, UK), respectively. For both studies, acoustic startle probes were 40 ms duration, 103 dB bursts of white noise with near instantaneous rise time presented binaurally through headphones, and electric shocks lasted 400 ms.
Startle responses were recorded from two 4 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye in Study 1 and right eye in Study 2. The ground electrode was located at the frontal pole (Fpz) of an electroencephalography cap that participants were wearing as part of the larger studies. One startle electrode was placed 1 cm below the pupil and the other was placed 1 cm lateral of that electrode. Data for Study 1 were collected using a bandpass filter of DC-500 Hz at a sampling rate of 2000 Hz, and for Study 2 using a bandpass filter of DC-200 Hz at a sampling rate of 1000 Hz.
fMRI Threat Task and Data Collection
Study 1
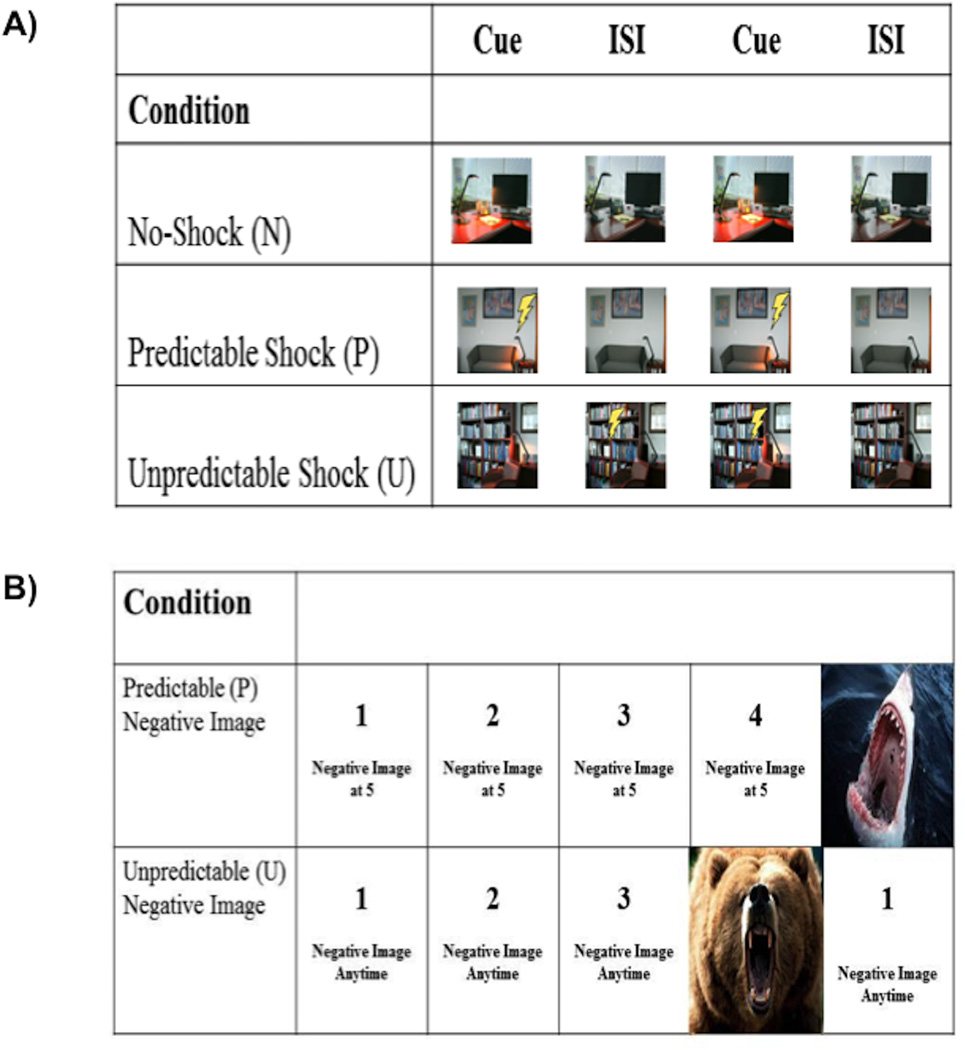
The fMRI threat task was designed to be analogous to the startle threat task. There were three within-subjects conditions – safe, predictable, and unpredictable (see Figure 1). Rather than text at the bottom of the screen indicating the condition, three discrete images of different rooms were used to signal each condition (counterbalanced across participants). Within each room image, a lamp, which turned on and off, was the cue. In N, participants never received a shock. In P, participants received a shock when the lamp was turned on. In U, participants were shocked at random (though not during every trial). Each condition lasted 6 s, during which the lamp was turned on once, for a duration of 2 s. The task consisted of 90 trials, with 30 trials in each condition. Light onset occurred at either 0 s (for 1/3rd of trials), 2 s (for 1/3rd of trials), or 4 s (for 1/3rd of trials) across all conditions. A fixation cross was presented in between trials, ranging from 1 to 8 s (M = 4.3 s).
Fig 1.
Illustration of the fMRI tasks administered in Study 1 (A) and Study 2 (B).
Functional MRI data was collected using a 3T GE magnetic resonance scanner at the University of Illinois at Chicago Medical Center. Functional images were acquired using a gradient-echo echo-planar images (2s TR, 25ms TE, 82° flip, 64×64 matix, 200 mm FOV, 3mm slice thickness, 0mm gap, with 40 axial slices).
Study 2
The fMRI threat sensitivity task was modified to test the convergence between reactivity to electric shock and negative aversive images (i.e., two different forms of threat). Participants viewed a series of count-ups (CU; e.g., 1-2-3-4) that ended with the presentation of a negative (Neg) or neutral (Neut) image selected from the International Affective Picture System (IAPS; Lang et al., 2008; see Figure 1). The timing of the presentation of each image was either predictable (P; i.e., it was known when the image would appear) or unpredictable (U; i.e., it was unknown when the image would appear). Therefore, the task included two within-subjects factors: timing (P vs. U) and picture valence (Neg vs. Neut). For each trial, text initially appeared at the bottom of the screen for 2 s indicating the condition including image valence and P or U timing (i.e., P-Neut, P-Neg, U-Neut, or U-Neg). Next, the CU was presented for 4 to 11 s and at the end of the CU the image appeared for 1–5 s. In the P condition, text at the bottom of the screen told participants when the CU would end and the valence of the image that would appear (e.g., “Neutral image at 5”). In the U condition, text at the bottom of the screen indicated the valence of the image but did not specify when the image would appear (e.g., “Negative image at anytime”). For each condition, trials were presented during 42 s blocks during which the CU was presented four times. Each condition block (P-Neut, P-Neg, U-Neut, U-Neg) was presented four times, counterbalanced across two runs. In-between blocks, a fixation cross was presented for 10 s.
After completing the task, participants were shown each IAPS image again in a random order and provided valence and arousal ratings on a 1 to 9 scale (valence: very unpleasant to very pleasant; arousal: not at all arousing to very arousing). Mean valence and arousal ratings, as well as score ranges, for the current sample are presented in Table 1. A list of the individual IAPS images used in the task and the normative valence and arousal ratings are included in Supplemental Methods. It is necessary to highlight that some participants rated the negative and neutral stimuli as highly positive and consequently, the range of scores for negative and neutral images does not match the range of scores from the normative IAPS data. However, the mean valence and arousal ratings are comparable across the datasets suggesting that on average, the current sample responded as intended.
Functional MRI was performed on a 3.0 Tesla GE MR 750 scanner (General Electric Healthcare; Waukesha, WI) using an 8-channel phased-array radio frequency head coil. A standard T2-sensitive gradient-echo echoplanar imaging (EPI) sequence was used (2s TR; 22.2ms TE; 90° flip; 64×64 matrix; 22cm FOV; 44 axial slices; 3.44 × 3.44 × 3.0 mm voxels; 336 volumes).
Data Processing and Analyses
Startle blinks were scored according to published guidelines (Blumenthal et al., 2005), and in an identical manner across the two studies. Data processing included applying a 28 Hz high-pass filter, rectifying, and then smoothing using a 40 Hz low-pass filter. Blink response was defined as the peak amplitude of EMG activity within the 20–150 ms period following startle probe onset relative to baseline. Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as non responses if EMG activity during the 20–150 ms post stimulus timeframe did not produce a blink peak that was visually differentiated from baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Blink amplitude values were used in all analyses. Consistent with prior studies (e.g., Gorka et al., 2013, 2016, in press), we created startle potentiation scores for the P- and U-threat conditions to account for baseline individual differences in startle amplitude. Specifically, for P-threat, we subtracted startle amplitude during NCue from PCue. For U-threat, we subtracted average startle amplitude during NCue and NISI from average startle amplitude during UCD and UISI because both phases of the U conditions (and N conditions) had the same meaning during the task. Startle potentiation variables were normally distributed within each sample (all skew values < 1.2).
Like the startle data, fMRI data across the two studies were processed using identical steps implemented in Statistical Parametric Mapping software (SPM8, Wellcome Department of Imaging NeuroScience, London, UK). Images were spatially realigned to correct for head motion, warped to standardized Montreal Neurological Institute (MNI) space using the participants’ mean functional image, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 kernel to minimize noise and residual differences in gyral anatomy. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Condition effects were modeled with event-related regressors representing the occurrence of each anticipation phase (variable durations). Effects were estimated at each voxel and for each participant.
The two fMRI tasks differed in their design but both included anticipation phases for temporally uncertain threat. Therefore, to be consistent across the two studies, individual contrast maps (statistical parametric maps) for U-threat anticipation > fixation were created for each subject. It is necessary to highlight that anticipation phases of both fMRI tasks are most analogous to the startle potentiation scores as they were collected during the anticipation of shock (Study 1) or pictures (Study 2), not during the actual receipt of shock/picture. For Study 1, at the first level, U-threat anticipation, P-threat anticipation, and no-threat anticipation were modeled separately. For Study 2, at the first level, U-threat anticipation, P-threat anticipation, U-neutral anticipation, and P-neutral anticipation were modeled separately. Parameter estimates were included as regressors in all first levels models to account for motion.
To test our hypotheses, for both studies, contrast maps for U-threat anticipation > fixation and P-threat anticipation > fixation were entered into second-level, one-sample t-tests in SPM. This approach allowed for an examination of the main effects of the task, independent of startle potentiation. Next, individual U-threat startle potentiation values were entered as a regressor-of-interest for each U-threat model (Study 1 and Study 2). We considered activations that survived p < 0.001 (uncorrected), with a cluster extent threshold of greater than 20 contiguous voxels (volume > 160mm3), as significant. This threshold has been used by others (Banks et al., 2007; Labuschagne et al., 2010) but is considered lenient and we intentionally chosen to focus on replication rather than adopt conservative statistical threshold cutoffs.
We next extracted BOLD signal responses (i.e., parameter estimates, β weights [arbitrary units]) from 10mm (radius) spheres surrounding significant peak activations across models. Then, to test the relative specificity of the findings to U-threat, we explored whether the associations between U-threat startle and peak BOLD signal responses remained significant when controlling for startle potentiation to P-threat using partial correlation analyses. We also tested whether any of the U-threat findings were also observed during P-threat.
Results
Sample Descriptives
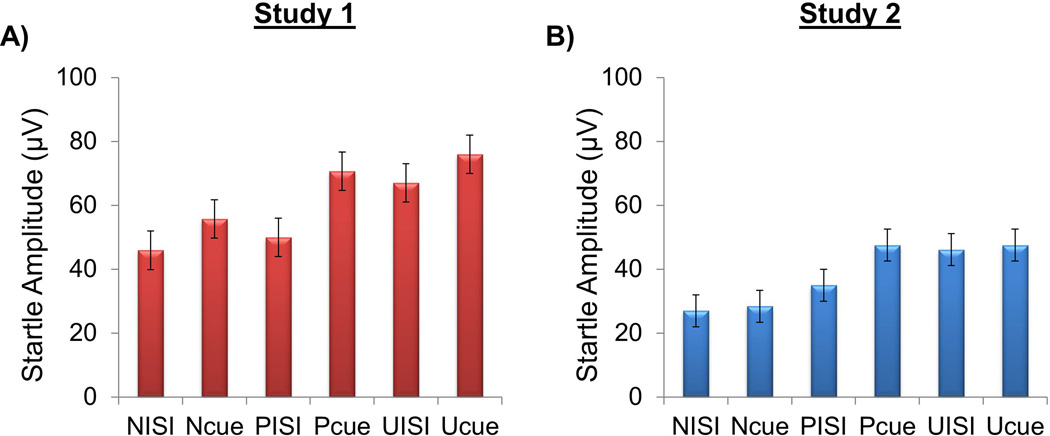
Descriptive information regarding participants is presented in Table 1. Mean startle amplitude for all task conditions is presented in Figure 2. The two samples were comparable in age, sex, and race/ethnicity. Despite the fact that both studies were designed to recruit individuals with a range of internalizing symptoms, there were important clinical differences between samples. For example, Study 1 included a greater proportion of individuals with current anxiety disorders whereas Study 2 included a greater proportion of individuals with depression. Study 2 also included a greater proportion of individuals with lifetime alcohol and substance use disorders relative to Study 1. Lastly, the two samples differed on use of psychotropic medication such that no individual in Study 1 was taking medication at the time of evaluation but a small percentage of individuals in Study 2 (10.5%) were taking medications.
Fig 2.
Mean startle amplitude for each of the startle task conditions in Study 1 (A) and Study 2 (B). N = no-shock; P = predictable shock; U = unpredictable shock; Cue = numeric countdown in Study 1 and geometric shape in Study 2; ISI = interstimulus interval. Bars reflect standard error.
Main Effects of fMRI Threat Tasks
Neural activation elicited by U- and P-threat for both tasks is presented in Table 2. Despite the fact that the two tasks used different forms of threat, activation patterns were remarkably consistent. For example, individuals exhibited robust occipital lobe, insula and hippocampus activation during U-threat in both tasks. As for differences, individuals in Study 1, but not Study 2, notably demonstrated right amygdala activation during U-threat.
Table 2.
Main effect of the threat task, whole-brain corrected, for Study 1 and Study 2.
| Region | MNI Coordinates | Volume (mm3) |
Z-score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| U-Threat > Fixation | |||||
| Study 1 | |||||
| Occipital Lobe | −12 | −104 | 6 | 144640 | 7.56 |
| L Orbitofrontal Cortex | −40 | 48 | −8 | 7776 | 4.88 |
| R Orbitofrontal Cortex | 46 | 46 | −6 | 3344 | 4.84 |
| R Middle Frontal Gyrus | 52 | 30 | 36 | 3816 | 4.08 |
| L Hippocampus | −22 | −26 | −8 | 728 | 3.77 |
| R Hippocampus | 22 | −26 | −6 | 664 | 3.43 |
| R Insula | 52 | 12 | 0 | 4272 | 3.22 |
| L Middle Frontal Gyrus | −50 | 4 | 50 | 208 | 3.10 |
| R Inferior Parietal Lobe | 58 | −44 | 50 | 232 | 3.10 |
| R Amygdala | 34 | −2 | −16 | 848 | 3.09 |
| Study 2 | |||||
| Occipital Lobe | −12 | −96 | −10 | 143392 | 5.82 |
| L Hippocampus | −24 | −28 | −4 | 6048 | 5.82 |
| R Hippocampus | 24 | −28 | −2 | 9304 | 5.77 |
| R Orbitofrontal Cortex | 46 | 34 | −16 | 329680 | 5.16 |
| L Orbitofrontal Cortex | −48 | 36 | −16 | 319280 | 5.05 |
| R Superior Frontal Gyrus | 12 | 42 | 54 | 2160 | 3.50 |
| R Insula | 40 | 24 | −2 | 800 | 3.49 |
| L Superior Frontal Gyrus | −10 | 60 | 28 | 1560 | 3.45 |
| R Inferior Temporal Lobe | 54 | 6 | −44 | 208 | 3.34 |
| P-Threat > Fixation | |||||
| Study 1 | |||||
| Occipital Lobe | 28 | −94 | 20 | 127064 | 6.16 |
| L Middle Frontal Gyrus | −48 | 36 | 28 | 9776 | 4.50 |
| L Insula | −46 | 14 | 2 | 9624 | 3.88 |
| R Insula | 40 | 24 | 0 | 4360 | 3.75 |
| L Hippocampus | −20 | −30 | −6 | 1344 | 3.73 |
| L Superior Frontal Gyrus | −12 | 6 | 72 | 800 | 3.49 |
| R Hippocampus | 22 | −26 | −4 | 832 | 3.44 |
| R Middle Frontal Gyrus | 38 | 54 | 24 | 1240 | 3.25 |
| Study 2 | |||||
| R Insula | 36 | 22 | −18 | 28720 | 4.78 |
| R Thalamus | 6 | −10 | 18 | 85232 | 4.23 |
| R Superior Frontal Gyrus | 6 | 30 | 54 | 3744 | 4.03 |
| R Occipital Lobe | 40 | −68 | −8 | 3488 | 3.48 |
| L Insula | −28 | 20 | −20 | 184 | 3.41 |
| L Orbitofrontal Cortex | −46 | 36 | −18 | 320 | 3.35 |
| R Superior Frontal Gyrus | 18 | 62 | 14 | 384 | 3.20 |
| R Inferior Parietal Lobe | 54 | −52 | 50 | 800 | 3.20 |
| R Superior Temporal Gyrus | 58 | −48 | 20 | 312 | 3.18 |
Note. Reporting of all significant clusters at p < 0.001, uncorrected, with a cluster extent threshold of k (number of contiguous voxels) >20. All associations are positive. L =Left; R =Right; MNI = Montreal Neurologic Institute.
Whole Brain Association between Startle and fMRI Measures
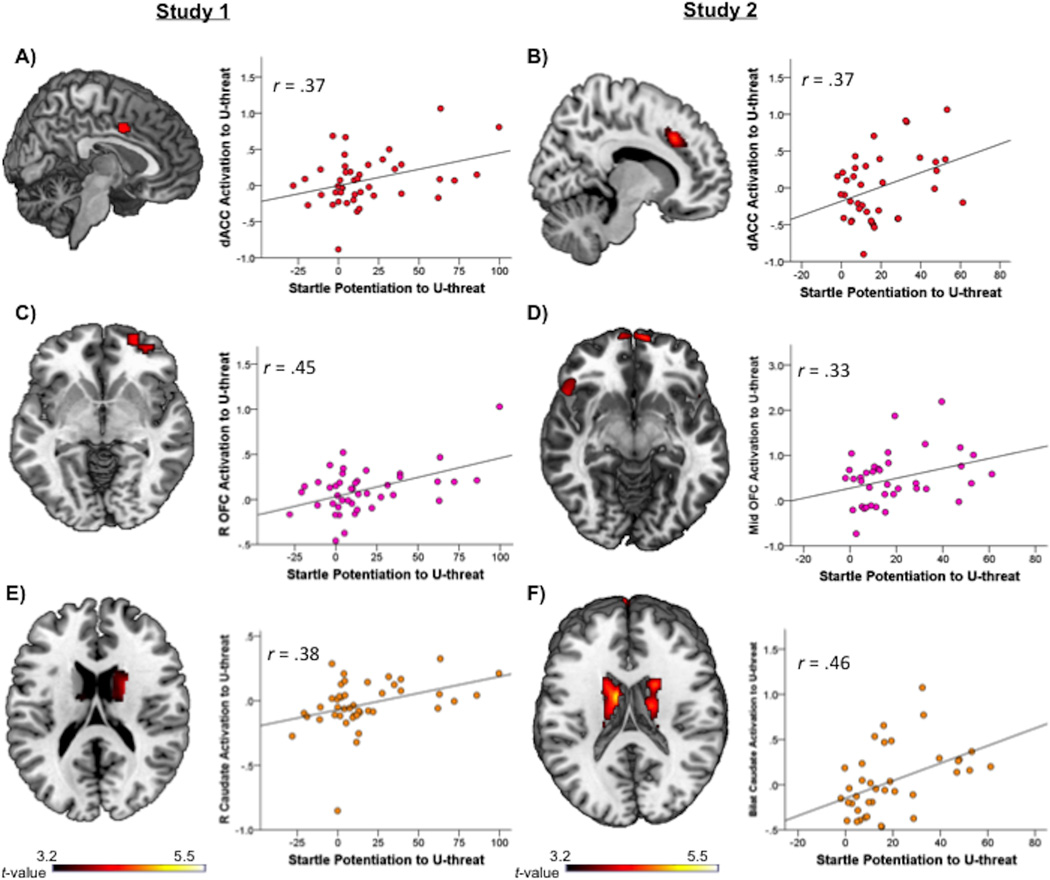
Significant correlations between U-threat startle and U-threat neural activation for Study 1 and Study 2 are presented in Table 3. In Study 1, greater startle potentiation to U-threat was associated with greater dACC, right caudate, and right orbitofrontal cortex (OFC) activation (Figure 3). Importantly, these correlations were replicated in Study 2 such that greater startle potentiation to U-threat was associated with greater dACC, bilateral caudate, and mid OFC activation (Figure 3). In addition to the consistency of location, in both studies, the magnitude of the correlations between startle potentiation and neural activation were consistent (rs ranging from 0.33 to 0.46; see Figure 3).
Table 3.
Neural regions during U-threat that significantly correlated with startle potentiation to U-threat
| Region | MNI Coordinates | Volume (mm3) |
Z-score | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Study 1: Positive Correlations | |||||
| L Occipital Lobe | −34 | −90 | 24 | 170 | 3.94 |
| R Middle Temporal Gyrus | 36 | −64 | 16 | 158 | 3.80 |
| R Occipital Lobe | 26 | −80 | 20 | 126 | 3.68 |
| R Orbitofrontal Cortex | 32 | 48 | −14 | 72 | 3.58 |
| L Precentral Gyrus | −40 | −14 | 30 | 108 | 3.57 |
| R Precentral Gyrus | 40 | −10 | 30 | 63 | 3.49 |
| R Precuneus | 18 | −60 | 56 | 136 | 3.47 |
| R Superior Frontal Gyrus | 20 | 66 | 2 | 57 | 3.46 |
| Dorsal Anterior Cingulate Cortex | 2 | 4 | 40 | 66 | 3.37 |
| R Caudate | 4 | 16 | −4 | 22 | 3.25 |
| Study 1: Negative Correlations | |||||
| No significant clusters | |||||
| Study 2: Positive Correlations | |||||
| L Inferior Parietal Lobe | −34 | −38 | 46 | 917 | 4.71 |
| L Middle Frontal Gyrus | −28 | 44 | 26 | 2196 | 4.63 |
| L Fusiform Gyrus | −36 | −16 | −28 | 41 | 4.25 |
| R Postcentral Gyrus | 56 | −30 | 40 | 466 | 4.11 |
| R Fusiform Gyrus | 42 | −12 | −22 | 306 | 4.09 |
| R Superior Parietal Lobe | 30 | −54 | 64 | 265 | 3.98 |
| L Superior Parietal Lobe | −28 | −64 | 64 | 418 | 3.92 |
| R Caudate | 18 | −6 | 26 | 121 | 3.86 |
| R Superior Frontal Gyrus | 30 | 50 | 20 | 89 | 3.86 |
| L Caudate | −10 | −2 | 16 | 98 | 3.72 |
| Inferior Frontal Gyrus | 52 | 16 | −4 | 114 | 3.66 |
| Supp. Motor Area | 0 | 10 | 60 | 78 | 3.62 |
| Orbitofrontal Cortex | −8 | 64 | −10 | 283 | 3.60 |
| R Middle Frontal Gyrus | 48 | 10 | 44 | 111 | 3.46 |
| R Precuneus | 6 | −64 | 40 | 59 | 3.35 |
| Dorsal Anterior Cingulate Cortex | −6 | 20 | 40 | 110 | 3.34 |
| L Medial Frontal Gyrus | −14 | 36 | 32 | 29 | 3.33 |
| Study 2: Negative Correlations | |||||
| No significant clusters | |||||
Note. Reporting of all significant clusters at p < 0.001, uncorrected, with a cluster extent threshold of k (number of contiguous voxels) >20. L =Left; R =Right; MNI = Montreal Neurologic Institute.
Fig. 3.
Associations between startle potentiation to U-threat and neural activation to U-threat. All brains display voxel-wise statistical t-maps on canonical brains reflecting significant correlations at p < 0.001, uncorrected, and a cluster extent threshold greater than 20 contiguous voxels. Scatter plots reflect the relation between extracted BOLD parameter estimates and startle potentiation to U-threat. A) and B) depict significant startle and dACC correlations for Study 1 and Study 2. C) and D) depict significant startle and OFC correlations for Study 1 and Study 2. E) and F) depict significant startle and caudate correlations for Study 1 and Study 2. U-threat = unpredictable threat; dACC = dorsal anterior cingulate cortex; OFC = orbitofrontal cortex.
Post Hoc Analyses
In order to test whether the above U-threat findings were better accounted for by broad threat responding we conducted a series of follow up analyses. First, we conducted partial correlations between startle to U-threat and neural activation during U-threat while controlling for startle to P-threat. For Study 1, results indicated that the U-threat startle and dACC (r = 0.31), right caudate (r = 0.32) and right OFC (r = 0.43) correlations remained significant. For Study 2, the U-threat startle and dACC (r = 0.36) and bilateral caudate (r = 0.34) remained significant; however, the U-threat startle and OFC correlation became non significant (r = 0.18) suggesting that the OFC finding was not specific to U-threat (i.e., independent of the effects of general threat responding). Second, we confirmed that startle to P-threat was not correlated with dACC, OFC, or caudate activation during U-threat (all ps > 0.15) or P-threat (all ps > 0.18).
Given that both samples included many individuals with DSM internalizing disorders, we also explored whether the associations between startle and neural responding during U-threat differed as a function of current MDD and/or anxiety disorder diagnoses using a series of hierarchical linear regression analyses. Two diagnostic variables were created – one reflecting current MDD (yes/no) and the other reflecting any current anxiety disorder (yes/no; PD, SAD, GAD, PTSD, or specific phobia). For each model, startle potentiation to U-threat, MDD status and anxiety disorder status were entered in Step 1. The two-way interactions between startle and depression, and startle and anxiety, were entered in Step 2 and the three-way interaction was entered in Step 3. Neural activation (dACC, OFC or caudate) was specified as the dependent variable. Across all models there were no two-way or three-way interactions indicating that the present startle-brain correlations did not differ between those with and without internalizing disorders (all ps > 0.55).
Discussion
Separate studies have shown that individuals with anxiety disorders display heightened startle potentiation (e.g., Shankman et al., 2013) and hyperactivity of the aINS and dACC during the anticipation of U-threat (e.g., Simmons et al., 2011); however, it has been unclear whether measures of startle potentiation and fMRI BOLD signal during U-threat are correlated and thus reflect similar individual differences. To address this important gap in the literature, the current study investigated if and where in the brain startle potentiation and fMRI BOLD signal response were correlated during U-threat anticipation tasks in two independent samples. Results revealed that across all subjects, regardless of DSM diagnoses, individuals with greater startle potentiation to U-threat exhibited greater dACC, caudate and OFC activation to U-threat. These results were notably replicated in two samples using varying tasks, different forms of threat, and participants with differing types of psychopathology. The consistency of the findings is striking and strongly supports that startle and fMRI during U-threat capture overlapping individual differences.
Consistent with our hypotheses, startle and dACC reactivity to U-threat were positively correlated, which fits with prior studies and extant theory. As previously noted, various anxiety disorders have been associated with heightened startle potentiation to U-threat (Grillon et al., 2008, 2009; Lieberman et al., in press), and in separate studies, hyperactivity of the dACC during the anticipation of U-threat (Straube, Mentzel & Miltner, 2007); indirectly suggesting that individual differences in startle and dACC activity are related. In more direct support, our lab has recently shown that within individuals with a range of panic symptoms, startle potentiation to U-threat was positively associated with dACC reactivity to U-threat (Lieberman et al., in press). The current study corroborates this prior finding and extends the work by demonstrating the consistency of startle-dACC correlations across samples and varying threat tasks.
The dACC is a particularly interesting site of convergence given that there are several putative roles that the dACC may play in exaggerated responding to U-threat. First, the dACC has been implicated in the psychophysiological expression of aversive responding to threatening stimuli (Milad & Quirk, 2012; Hartley et al., 2011; Milad et al., 2007) and is speculated to play a modulatory role in startle reactivity and/or other autonomic defensive responses (e.g., skin conductance and heart rate) to U-threat. That is, individuals with greater dACC reactivity to aversive stimuli also appear to exhibit heightened defensive responding and relatedly, exaggerated anticipatory anxiety to U-threat. In addition to anxiety expression, the dACC has been implicated in threat appraisal, or the evaluation of the degree of danger associated with a stimulus or situation (Etkin, Egner & Kalisch, 2011; Maier et al., 2012). Greater dACC reactivity could therefore reflect exaggerated appraisal of U-threat, which may prime the defensive motivational system and potentiate the startle reflex.
Although not originally hypothesized as a site of overlap, current findings suggest that OFC reactivity to U-threat is also positively correlated with startle. The OFC is part of the AAN (Grupe & Nitschke, 2013) and implicated in responding to U-threat. OFC hyperactivity is also linked to several forms of psychopathology including obsessive-compulsive disorder (OCD) (Chamberlain et al., 2008; Evans, Lewis & Iobst, 2004; Milad & Rauch, 2007), which is characterized by high intolerance of uncertainty and maladaptive defensive responding (Chamberlain et al., 2007; Evans, Lewis & Iobst, 2004; Tolin et al., 2003). The broader literature indicates that the OFC contributes to the regulation of emotional responding to aversive stimuli and appraisals of threat salience (Holtz et al., 2012; Milad & Rauch, 2007), similar to the dACC. Interestingly, though, whereas the dACC finding was relatively specific to U-threat, results suggest that OFC reactivity may have been more reflective of individual differences in general threat responding.
In addition to the dACC and OFC, results indicate that caudate and startle reactivity to U-threat are also related. The caudate is often activated in tasks involving conscious threat appraisals (see Mechias et al., 2010 for a review) and is posited to mediate emotional arousal (Colibazzi et al., 2010). Relatedly, there have been a few studies noting increased caudate activity during threat anticipation (e.g., Choi et al., 2012) and one study which found that caudate activation was positively correlated with heart-rate reactivity to social threat (Wager et al., 2009). Therefore, although not frequently considered part of the AAN or anxiety-related networks, the caudate has a clear role in threat responding and reactivity. In addition, the caudate is known to regulate the feedback loop between the OFC and thalamus (Hansen et al., 2002; Milad & Rauch, 2007) and may therefore be related to OFC reactivity to threat. Indeed, like the OFC, caudate hyperactivity is commonly implicated in the pathophysiology of OCD and it has been speculated that exaggerated caudate reactivity is related to maladaptive thinking styles during aversiveness (Milad & Rauch, 2012; Tolin et al., 2003). Together, this literature implies that the dACC, OFC and caudate are all regions implicated in threat responding and based on the present results, may be particularly important for reactivity to U-threat.
Although the current findings fit with the existing literature, we did not find in either study that startle reactivity to U-threat was correlated with amygdala or aINS reactivity, as we initially hypothesized. We did, however, find that that across all participants both threat tasks robustly elicited the aINS, and in Study 1 (the threat-of-shock task) participants also displayed right amygdala activation. These findings suggest that the aINS and amygdala are indeed engaged by U-threat but that activation in these regions does not necessarily correlate with the magnitude of startle potentiation to U-threat. The aINS and amygdala null findings have potentially important implications as to date much of the anxiety disorder literature has been focused on individual differences in aINS and amygdala reactivity (e.g., Gorka et al., 2014; Klumpp, Angstadt & Phan, 2012; Stein et al., 2007). The current findings suggest that although these are two important threat-related regions, we may also consider further investigating the dACC, OFC and caudate as potential anxiety disorder treatment targets, especially for those who display an exaggerated psychophysiological reactivity to U-threat.
The convergence of fMRI and startle during U-threat is important from a methodological standpoint but also has broader implications. First, the study supports one of the basic premises of the NIMH RDoC Initiative which is that two ‘units of analysis’ (i.e., the columns in the RDoC matrix) within the same construct/domain (i.e., potential threat) are related. This relation was not moderated by internalizing psychopathology suggesting that psychophysiology relates to neural functioning similarly across individuals. Second, given that heightened startle potentiation to U-threat, and hyperactivity of the dACC, caudate and OFC have each been implicated in various anxiety disorders, the pattern of responding identified in the present study may point towards a biobehavioral profile of aberrant affective responding that contributes to the pathogenesis of anxiety psychopathology. Future studies should continue to explore this brain-behavior profile as a transdiganostic, anxiety prevention and/or intervention target.
The present study had many strengths but also some important limitations. First, fMRI and startle data was not collected simultaneously. Doing so may have revealed greater overlap between startle and neural reactivity to U-threat and this is an important avenue for future work. Second, both studies recruited individuals with and without psychopathology. Although this approach allowed for the necessary variability in psychophysiological responding, it is unclear whether the current findings would generalize to other populations (e.g., primary externalizing disorder patients). Third, both study sample sizes were relatively small and although the present results were replicated in a second study, the analyses were likely underpowered to detect associations with regions that did not have robust signals. Lastly, a subset of participants in Study 2 reported that the negative (and neutral) images presented during the fMRI task were positive in valence. It is unclear whether this reflects an error in self-report or if some participants actually appraised the negative stimuli as pleasant. Although the findings replicate across the two studies, it is possible that the Study 2 fMRI task did not robustly elicit aversive responding across all individuals and it is therefore necessary that future studies attempt to corroborate the current findings.
In sum, the current study suggests that two separate measures of sensitivity to U-threat, across two units of analysis, correlate such that individuals with greater startle potentiation exhibit greater dACC, caudate, and OFC activation. This study is one of the first in a series of investigations that are needed to fully understand the extent to which different psychophysiological methodologies converge in assessing core constructs as the field moves towards a more dimensional, multi-measure conceptualization of normal and abnormal affective processes.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01MH101497 [PI: Phan] and R21MH080689 [PI: Shankman]) and the Brain and Behavior Research Foundation (PI: Shankman). The funding sources had no role in the present manuscript.
Footnotes
All authors declare no conflicts of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: A response-related fMRI study. Human brain mapping. 2004;23(4):200–209. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Allard CB, Grimes EM, Simmons AN, Flagan T, Behrooznia M, … Stein MB. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Archives of General Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- Bach DR, Dolan RJ. Knowing how much you don’t know: a neural organization of uncertainty estimates. Nature Reviews Neuroscience. 2012;13(8):572–586. doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmet AH, editors. Startle modification: Implication for neuroscience, cognitive science, and clinical science. New York: Cambridge University Press; 1999. pp. 157–193. [Google Scholar]
- Carleton RN. Into the Unknown: A review and synthesis of contemporary models involving uncertainty. Journal of Anxiety Disorders. 2016a;39:30–43. doi: 10.1016/j.janxdis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Carleton RN. Fear of the unknown: One fear to rule them all? Journal of Anxiety Disorders. 2016b;41:5–21. doi: 10.1016/j.janxdis.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Fineberg NA, Menzies LA, Blackwell AD, Bullmore ET, Chir B, Sahakian BJ. Impaired cognitive flexibility and motor inhibition in unaffected first degree relatives of patients with obsessive-compulsive disorder. American Journal of Psychiatry. 2007;164(2):335–338. doi: 10.1176/appi.ajp.164.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Menzies L, Hampshire A, Suckling J, Fineberg NA, del Campo N, Robbins TW. Orbitofrontal dysfunction in patients with obsessive compulsive disorder and their unaffected relatives. Science. 2008;321:421–422. doi: 10.1126/science.1154433. [DOI] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threatmonitoring and cognition. Neuroimage. 2012;59:1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi T, Posner J, Wang Z, Gorman D, Gerber A, Yu S, Peterson BS. Neural systems subserving valence and arousal during the experience of induced emotions. Emotion. 2010;10(3):377. doi: 10.1037/a0018484. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? the anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122(3):928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28(5):409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Breuer P. Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology. 1992;101(3):371. doi: 10.1037//0021-843x.101.3.371. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and sessive-compulsive disorder. Brain and cognition. 2004;55(1):220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press; 1996. [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, Shankman SA. Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biology of Mood & Anxiety Disorders. 2014;4(1):9. doi: 10.1186/2045-5380-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Sarapas C, Campbell M, Lewis GF, Bishop JR, Shankman SA. Relation between respiratory sinus arrythymia and startle response during predictable and unpredictable threat. Journal of psychophysiology. 2013;27(2):95–104. doi: 10.1027/0269-8803/a000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug and Alcohol Dependence. 2016;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL. Startle Potentiation to Uncertain Threat as a Marker of Fear-based Psychopathology: An Examination across Multiple Internalizing Disorders. Journal of Abnormal Psychology. doi: 10.1037/abn0000233. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165(7):898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses; evidence for enhanced anxious anticipation. PloS One. 2013;8(8):e70969. doi: 10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated a new classification framework for research on mental disorders. American Journal of Psychiatry. 2013;167(7):748–751. [Google Scholar]
- Hansen ES, Hasselbalch S, Law I, Bolwig TG. The caudate nucleus in obsessive compulsive disorder. Reduced metabolism following treatment with paroxetine: a PET study. International Journal of Neuropsychopharmacology. 2002;5(1):1–10. doi: 10.1017/S1461145701002681. [DOI] [PubMed] [Google Scholar]
- Holtz K, Pané-Farré CA, Wendt J, Lotze M, Hamm AO. Brain activation during anticipation of interoceptive threat. Neuroimage. 2012;61(4):857–865. doi: 10.1016/j.neuroimage.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Hong RY, Cheung MWL. The structure of cognitive vulnerabilities to depression and anxiety: Evidence for a common core etiologic process based on a meta-analytic review. Clinical Psychological Science. 2015;3:892–912. [Google Scholar]
- Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nature neuroscience. 2009;12(12):1494–1496. doi: 10.1038/nn.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Progress in Brain Research. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lieberman L, Gorka SM, Shankman SA, Phan KL. Impact of Panic on Psychophysiological and Neural Reactivity to Unpredictable Threat in Depression and Anxiety. Clinical Psychological Science. doi: 10.1177/2167702616666507. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier ME, Di Pellegrino G. Impaired conflict adaptation in an emotional taskcontext following rostral anterior cingulate cortex lesions in humans. Journal of Cognitive Neuroscience. 2012;24(10):2070–2079. doi: 10.1162/jocn_a_00266. [DOI] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage. 2010;49(2):1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007;1121(1):546–561. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico striatal pathways. Trends in cognitive sciences. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122(3):662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner I, Stöcker T, Kellermann T, Ermer V, Wegener HP, Eickhoff SB, Shah NJ. Electrophysiology meets fMRI: Neural correlates of the startle reflex assessed by simultaneous EMG-fMRI data acquisition. Human Brain Mapping. 2010;31(11):1675–1685. doi: 10.1002/hbm.20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a ynthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Science. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Perception & Psychophysics. 1987;42(3):257–268. doi: 10.3758/bf03203077. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of Abnormal Psychology. 2010;119(4):631. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM. Psychopathology research in the RDoC era: Unanswered questions and the importance of the psychophysiological unit of analysis. International Journal of Psychophysiology. 2015 doi: 10.1016/j.ijpsycho.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, Gorka SM. A psychophysiological investigation of threat and reward sensitivityin individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport. 2014;25(8):596. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32(11):1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Nesse RM. Threat detection, precautionary responses, and anxiety disorders. Neuroscience and Biobehavioral Reviews. 2011;35:1075–1079. doi: 10.1016/j.neubiorev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Stevens S, Gerlach AL, Cludius B, Silkens A, Craske MG, Hermann C. Heartbeat perception in social anxiety before and during speech anticipation. Behaviour research and therapy. 2011;49(2):138–143. doi: 10.1016/j.brat.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. Journal of Anxiety Disorders. 2003;17(2):233–242. doi: 10.1016/s0887-6185(02)00182-2. [DOI] [PubMed] [Google Scholar]
- van Well S, Visser RM, Scholte HS, Kindt M. Neural substrates of individual differences in human fear learning: evidence from concurrent fMRI, fear-potentiated startle, and US-expectancy data. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(3):499–512. doi: 10.3758/s13415-012-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF. Brain mediators of cardiovascular responses to social threat: part I: Reciprocal dorsal and ventral sub-regions of the medial prefrontal cortex and heart-rate reactivity. Neuroimage. 2009;47(3):821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.