Abstract
Background
Some patients with acute or acute-on-chronic hepatic failure die before a suitable human liver allograft becomes available. Encouraging results have been achieved in such patients by the transplantation of human hepatocyte progenitor cells from fetal liver tissue. The aim of the study was to explore survival of hepatocytes from genetically-engineered pigs after direct injection into the spleen and other selected sites in immunosuppressed baboons to monitor the immune response and the metabolic function and survival of the transplanted hepatocytes.
Methods
Baboons (n=3) were recipients of GTKO/hCD46 pig hepatocytes. All three baboons received anti-thymocyte globulin (ATG) induction and tapering methylprednisolone. Baboon 1 received maintenance immunosuppressive therapy with tacrolimus and rapamycin. Baboons 2 and 3 received an anti-CD40mAb/rapamycin-based regimen that prevents sensitization to pig solid organ grafts. The baboons were euthanized 4 or 5 weeks after hepatocyte transplantation. The baboon immune response was monitored by measurement of anti-nonGal IgM and IgG antibodies (by flow cytometry) and CFSE-mixed lymphocyte reaction. Monitoring for hepatocyte survival and function was by (i) real-time PCR detection of porcine DNA, (ii) real-time PCR for porcine gene expression, and (iii) pig serum albumin levels (by ELISA). The sites of hepatocyte injection were examined microscopically.
Results
Detection of porcine DNA and porcine gene expression was minimal at all sites of hepatocyte injection. Serum levels of porcine albumen were very low – 500–1,000-fold lower than in baboons with orthotopic pig liver grafts, and approximately 5,000-fold lower than in healthy pigs. No hepatocytes or infiltrating immune cells were seen at any of the injection sites. Two baboons (Baboons 1 and 3) demonstrated a significant increase in anti-pig IgM and an even greater increase in IgG, indicating sensitization to pig antigens.
Discussion and Conclusions
As a result of this disappointing experience, the following points need to be considered. (i) Were the isolated pig hepatocytes functionally viable? (ii) Are pig hepatocytes more immunogenic than pig hearts, kidneys, artery patch grafts, or islets? (iii) Does injection of pig cells (antigens) into the spleen and/or lymph nodes stimulate a greater immune response than when pig tissues are grafted at other sites? (iv) Did the presence of the recipient’s intact liver prevent survival and proliferation of pig hepatocytes? (v) Is pig CD47-primate SIRP-α compatibility essential? In conclusion, the transplantation of genetically-engineered pig hepatocytes into multiple sites in immunosuppressed baboons was associated with very early graft failure. Considerable further study is required before clinical trials should be undertaken.
Keywords: Baboon, Hepatocytes, Pig, Xenotransplantation
INTRODUCTION
Some patients with acute or acute-on-chronic hepatic failure will die before a suitable human liver allograft becomes available. Encouraging results have been achieved in such patients by the transplantation of human hepatocyte progenitor cells from fetal liver tissue into the spleen (1). These cells have contributed towards the maintenance of life until a liver allograft has become available.
Human fetal tissue-derived hepatocytes may not be available in the numbers required to treat a large number of patients. Hepatocytes from genetically-engineered pigs are an alternative source that could be unlimited. There is considerable evidence from the literature that hepatocytes from one species function in another (2). Progress in the field of both hepatocyte allotransplantation and xenotransplantation has recently been reviewed (3–8). Hepatocytes from wild-type pigs have been reported to be resistant to complement-mediated injury (9), and capable of producing pig proteins when transplanted into immunosuppressed monkeys. The genetic engineering of these pigs renders their cells no more immunogenic than their human counterparts (10,11). The present study was aimed to test the hypothesis that genetically-engineered pig hepatocytes would function in baboons and would be relatively non-immunogenic.
Based on preliminary clinical experience of fetal hepatocyte allotransplantation (1), the spleen was selected as the primary site for transplantation. Additional sites included (i) lymph nodes (based on encouraging results in rodent models from our center and others (12–15), (ii) the subcapsular space of the kidney (16), and (iii) the subcutaneous fat of the abdominal wall (17).
The aim of the study was to explore survival of hepatocytes from genetically-engineered pigs after transplantation into the spleen (via direct injection into the splenic pulp) and other selected sites of immunosuppressed baboons to monitor the immune response and the metabolic function and survival of the transplanted hepatocytes.
MATERIALS AND METHODS
Animals
Baboons (Papio species, n=3; Division of Animal Resources, Oklahoma University Health Sciences Center, Oklahoma City, OK), 3–4 years-old, weighing 7–9kg and of known AB blood type, were recipients of pig hepatocytes (n=3). The baboons were housed in an environmentally-controlled room to which they were acclimated for at least one month before any experimental procedures. They had free access to food and water, and were provided with social enrichment.
α1,3-galactosyltransferase gene-knockout pigs that expressed high levels of the human complement-regulatory protein CD46 (GTKO/hCD46 pigs) of blood group O (nonA), weighing 8–14kg (Revivicor, Blacksburg, VA), generated by nuclear transfer/embryo transfer from modified fibroblasts from Large White/Landrace/Duroc cross-breed pigs (10,18–20), served as sources of hepatocytes. All pigs were tested to confirm (i) absence of galactose-α1,3-galactose (Gal) expression and (ii) expression of hCD46 (10). GTKO/hCD46 pig cells have been demonstrated to provide considerable protection against the primate humoral immune response (10).
All animal care was in accordance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication No. 86-23, revised 1985). Protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
In addition, for preliminary studies, portions of liver were obtained from deceased wild-type (genetically-unmodified) pigs through a local slaughterhouse, and used to establish the hepatocyte isolation method.
Excision of GTKO/hCD46 pig livers
Under full anesthesia, a midline abdominal incision was made in the pig to expose the liver. The pig was fully heparinized (300 IU/kg), and the hepatic artery (through the abdominal aorta) and portal vein were flushed with cold (4°C) Dulbecco’s PBS (without calcium and magnesium, but including 1mM Na-EDTA and antibiotic-antimycotic) [Thermo Fisher Scientific]. A hepatectomy was carried out.
Pig hepatocyte isolation
A five-step perfusion of the pig liver was performed with modifications adapted from Gerlach et al. (21–24). The liver was perfused ex vivo for 10min at 37°C with 1L DPBS (without calcium and magnesium, but including 2g/L sodium bicarbonate, 2g/L glucose, and antibiotic-antimycotic) (100units/mL penicillin, 100µg/mL streptomycin, 0.25µg/mL amphotericin B), followed by perfusion at 37°C with 1L of William’s Medium E, including antibiotic-antimycotic [all Thermo Fisher Scientific]. Digestion was initiated by recirculating the perfusate at 37°C with 1L of William’s Medium E, including 0.1% collagenase-IV (Gibco/Thermo Fisher Scientific) and antibiotic-antimycotic. The digestion medium was recirculated for 40min until the liver capsule showed visible signs of digestion. A final perfusion was performed with 1L cold (4°C) William’s Medium E, including antibiotic-antimycotic. The liver capsule was slit with a scalpel, and cells were flushed out with cold William’s Medium E, including antibiotic-antimycotic. Liver cell suspensions were filtered through subsequent layers of nylon meshes [ELKO Filtering, Miami Gardens, FL] ranging from 500µm to 70µm. Suspensions were centrifuged for 5min at 300g and resuspended in cold William’s Medium E. Cell number and viability were determined by counting live and dead cells in a Neubauer chamber with TrypanBlue exclusion.
For injections with matrigel (in Baboon 3 only), the cell suspension was mixed with growth factor-reduced Corning Matrigel Basement Membrane Matrix [Discovery Labware, Bedford, MA] to a final concentration of matrigel of 4.2mg/ml. All final cell concentrations for injections were adjusted to approximately 100 million cells/mL.
In Baboon 1, 4.0 billion hepatocytes were isolated, and suspended in 33mL William’s Medium E (0.12 billion cells/mL), with a cell viability of 79%. In Baboon 2, 4.3 billion hepatocytes were obtained, and suspended in 45mL (0.096 billion cells/mL), with a viability of 95%. In Baboon 3, 3.8 billion hepatocytes were obtained, suspended in 40mL (20mL of William’s Medium E mixed with 20mL of matrigel; 0.095 billion cells/mL) with a viability of 94%. The hepatocytes were transplanted into the recipient within 60min after isolation.
Pig hepatocyte transplantation in baboons
Under full anesthesia, a midline abdominal incision was made. Pig hepatocytes (suspended in cold William’s Medium E), were injected directly into four sites:- (i) the parenchyma of the lower pole of the spleen, (ii) the subcapsular space of one or both kidneys, (iii) three mesenteric lymph nodes (and in Baboon 3 into a femoral lymph node through a small incision over the node), and (iv) the adipose tissue of the anterior abdominal wall (through the midline incision), all using an 18-gauge needle. The sites and numbers of injected hepatocytes in each baboon are shown in Table 1. Postoperative analgesia was with buprenorphine 0.01mg/kg i.m. x2 daily for 3 days.
Table 1.
Details of pig hepatocyte injections in baboons
| Baboon # | Injection sites | # of hepatocytes injected |
|---|---|---|
| Baboon 1 (tacrolimus+rapamycin-based immunosuppressive regimen) (b) | ||
| Total hepatocytes injected = 3.5 billion; viability 79% | ||
| Spleen | 1.7 billion | |
| Kidney capsule (left) | 0.4 billion | |
| Lymph node (mesenteric) | 0.4 billion | |
| Abdominal wall adipose tissue (right) | 0.6 billion | |
| Abdominal wall adipose tissue (left) | 0.4 billion | |
| Follow-up 35 days | ||
| Baboon 2 (anti-CD40mAb+rapamycin-based immunosuppressive regimen) (a) | ||
| Total hepatocytes injected = 4.3 billion; viability 95% | ||
| Spleen | 1.7 billion | |
| Kidney capsule (right) | 0.8 billion | |
| Lymph node (mesenteric) | 1.2 billion | |
| Abdominal wall adipose tissue (right) | 0.3 billion | |
| Abdominal wall adipose tissue (left) | 0.3 billion | |
| Follow-up 28 days | ||
| Baboon 3 (anti-CD40mAb+rapamycin-based immunosuppressive regimen) (a) | ||
| Total hepatocytes injected = 3.8 billion; viability 94% (+ matrigel) | ||
| Spleen | 1.9 billion | |
| Kidney capsule (right) | 0.3 billion | |
| Kidney capsule (left) | 0.3 billion | |
| Lymph node (mesenteric) | 0.4 billion | |
| Abdominal wall adipose tissue (right) | 0.3 billion | |
| Abdominal wall adipose tissue (left) | 0.4 billion | |
| Lymph node (femoral) | 0.2 billion | |
| Follow-up 28 days | ||
In Baboon 3, the femoral lymph node was excised on post-transplant day 7 (under full anesthesia) to determine the early survival of the injected pig hepatocytes.
Immunosuppressive and supportive therapy
All three baboons received anti-thymocyte globulin (ATG) induction and tapering methlprednisolone (Table 2). Baboon 1 received maintenance immunosuppressive therapy with tacrolimus and rapamycin. Whole blood levels of tacrolimus and rapamycin were measured at least x2 weekly and remained largely within the desired ranges (Table 2). Baboons 2 and 3 received an anti-CD40mAb/rapamycin-based regimen that prevents sensitization (i.e., an anti-pig IgM and IgG antibody response and a T cell proliferative response) to pig heart, kidney, and artery patch transplants in baboons (25–27). The anti-CD40mAb (2C10R4) was kindly provided by Dr. Keith Reimann at the NIH NHP Resource Center, Boston, MA. The levels of 2C10R4 were not measured, but previous studies by us and others have indicated that, at the dose used, the levels were higher than those considered in the therapeutic range (27–29).
Table 2.
Details of immunosuppressive and supportive therapy
| Dose | Duration | |
|---|---|---|
|
Induction therapy | ||
| Thymoglobulin (ATG) (Genzyme, Cambridge, MA) |
10mg/kg i.v. | day −2 |
| Methylprednisolone (Pfizer, New York, NY) |
5mg/kg i.v. | before ATG, and on day 0 |
|
Maintenance immunosuppressive therapy | ||
| Anti-CD40mAb (NIH NHP Resource Center, Boston, MA) |
50mg/kg i.v. | days −1, 0, 4, 7, 10, 14, then every 7 days |
| OR | ||
| Tacrolimus (LC Laboratories, Woburn, MA) |
0.03–0.05mg/kg i.m. x2/day |
to maintain a blood trough level of 8–12ng/mL |
|
Additional maintenance immunosuppressive therapy | ||
| Rapamycin (LC, Laboratories, Woburn, MA) |
0.01mg/kg i.m. x2/day | to maintain a blood trough level of 8–12ng/mL |
| Methylprednisolone | 5mg/kg i.v/day. | tapering to 0.25mg/kg/day i.m. by day 6 |
Immune monitoring of the recipient baboon
The baboon was sedated (ketamine 10–20mg i.m.) x2 weekly for blood draws and i.v. drug administration. Complete blood cell counts were monitored weekly.
Monitoring of baboon CD4+ and CD8+ T cells by flow cytometry
Blood samples were collected before any therapy, pre-transplantation and on days −1, 7, 28 (or 35). The numbers of CD3+CD4+, CD3+CD8+ T cells, and CD20+ B cells were determined by flowcytometry as previously described (26), and data were analyzed using Flowjo software [Tree Star, Ashland, OR].
Monitoring of anti-nonGal IgM and IgG antibodies by flow cytometry
Blood was drawn for measurement of anti-nonGal pig IgM and IgG antibodies by flow cytometry (using GTKO pAECs as target cells) - (i) before any immunosuppressive therapy was administered, (ii) immediately before hepatocyte transplantation (day −3), and (iii) on post-transplant days 7, 14, 21, 28 (or 35). The CD3+T and CD20+B cell counts were monitored at the same time-points. Negative controls were obtained by incubating the target cells with secondary anti-human antibodies only in the absence of serum. Sensitized baboon serum (from a baboon that had received a GTKO pig artery patch transplant without immunosuppressive therapy [30]) was used as a positive control.
CFSE-mixed lymphocyte reaction (MLR)
Blood was drawn for CFSE-mixed lymphocyte reaction (MLR) to determine the baboon’s cellular proliferative response, as previously described (31), (i) before any immunosuppressive therapy was administered, and (ii) between days 28 and 35 (before euthanasia).
Monitoring for hepatocyte survival and function
At the time of euthanasia, samples were taken from the sites of hepatocyte transplantation for (i) real-time PCR detection of porcine DNA, and (ii) real-time PCR for porcine gene expression. Pig albumin levels were measured in the baboon blood by pig albumin ELISA, using the method of Nagata et al (32).
DNA and RNA extraction
DNA and RNA were extracted using the AllPrep DNA/RNA mini kit [Qiagen, Hilden, Germany] from frozen tissue pieces that were snap-frozen in liquid nitrogen immediately after excision. Tissues were homogenized in RLTplus buffer using a TissueRuptor, and subsequently lysed using Qiashredder columns [all Qiagen]. DNA and RNA were simultaneously extracted from the same samples; RNA was treated with DNase [Qiagen] on extraction columns. Concentrations of eluted nucleic acids were determined with a Qubit fluorometer using Quant-iT dsDNA and RNA assay kits [Thermo Fisher Scientific].
Real-time PCR for detection of porcine DNA
Porcine DNA in baboon samples was quantified using Taqman probe-based real-time PCR for porcine specific cytochrome B DNA sequences, adapted and slightly modified from Tanabe et al. (33). PCR was performed with Applied Biosystems Master Mix and StepOnePlus 96-well Thermocycler real-time PCR and software (all Thermo Fisher Scientific). DNA (total 100ng) was implemented per reaction. Negative controls included no-template (water) PCR control and non-transplanted, commercial baboon tissue DNA control [Amsbio, Lake Forest, CA]. Standard curves were created from DNA extracted from pig liver tissue. All standards and samples from each specimen were run with three technical repeats. Standards were 0.1pg, 1pg, 10pg, 100pg, 1ng, and 10ng pig DNA. Within the StepOnePlus software, a linear standard curve was generated automatically by detection of the cycle threshold (Ct) value for each standard; sample concentrations were calculated and data were given as % pig DNA of total implemented DNA.
Real-time PCR for porcine gene expression
Reverse transcription of RNA to cDNA was performed with the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit [Thermo Fisher Scientific]. Porcine-specific gene expression was analyzed by real-time PCR using the StepOnePlus 96-well Thermocycler real-time PCR system and software, and pre-designed Taqman probe assay mix with Applied Biosystems gene expression master mix [Thermo Fisher Scientific]. Taqman assays included Sus scrofa beta-actin (ACTB, Ss03376081_u1), albumin (ALB, Ss03378640_u1), and alpha-fetoprotein (AFP, Ss03384005_u1). Per reaction, 25ng RNA was implemented. Linear standard curves were generated from pig liver tissue cDNA in tenfold serial dilutions, ranging from undiluted to 1:1,000. Other steps were as for porcine DNA (above). No-template (water) was used as PCR negative control.
Detection of pig albumin in baboon sera by enzyme linked immunosorbent assay (ELISA)
The pig albumin concentration in the baboon blood was measured using a pig albumin ELISA kit [Bethyl Laboratories, Montgomery, TX] following the manufacturer’s protocol. Briefly, 100µL of diluted standard or sera sample (neat, 1:50 and 1:200) were added to designated wells of a 96-well plate in duplicate, and incubated for 1h at room temperature. Biotinylated anti-albumin detection antibody was added and incubated for 1h, after which a streptavidin-conjugated horseradish peroxidase was added. After washing, 50µL of 3,3’,5,5’-tetramethylbenzidine (TMB) substrate was added for color development, which was terminated by the addition of diluted sulfuric acid. The absorbance was measured using a fluorescence plate reader at 450nm [1420 Multilabel Counter, PerkinElmer, Waltham, MA]. The albumin concentrations in the samples were quantified by interpolating their absorbance from the standard curve generated in each plate.
Histopathological examination
At the time of euthanasia (28–35 days), biopsies of the spleen, mesenteric lymph nodes, kidneys, and anterior abdominal wall fat were taken for histological examination for the presence of pig hepatocytes (and DNA). Biopsies were stored in formalin and OCT for immunostaining, and snap-frozen for mRNA. All major organs were inspected for signs of pathology and, if present on macroscopic examination, biopsies were taken for microscopic examination.
In addition, in Baboon 3 the single femoral lymph node injected with pig hepatocytes was excised on day 7 for histological examination.
RESULTS
Isolation of GTKO/CD46 pig hepatocytes
The isolation of pig hepatocytes from GTKO/hCD46 pig livers proved straightforward. The isolated hepatocytes were examined by immunofluorescence and flow cytometry for expression of Gal, SLA I, SLA II, and hCD46. They expressed no Gal but high levels of hCD46 (not shown). On cell isolates, expression of SLA I and SLA II was greater on CD31+ isolates (containing some vascular endothelial cells) than on CD31− isolates (not shown).
Both baboon and human IgM and IgG binding was reduced to hepatocytes from GTKO/hCD46 pigs (when compared with those from wild-type pigs [not shown]).
Pig hepatocyte transplantation in immunosuppressed baboons
Recipient baboon survival and clinical complications
All three baboons remained healthy and active throughout the period of follow-up which was electively for either 28 (n=2) or 35 (n=1) days (Table 1). There were no complications from the surgical procedure or immunosuppressive therapy.
Immunologic monitoring
T cell (CD4+, CD8+) and B cell (CD20+) numbers in baboon recipients
In all baboons, after the administration of ATG on day −2, a profound depletion of T cells (CD4+, CD8+) was obtained (not shown). In Baboon 1, there was slow recovery of CD8+ cells. In Baboons 2 and 3, T cell numbers were maintained low throughout the course of the experiment, with CD4+ and CD8+ cell numbers remaining <500 cells/µL. There was also a reduction of CD20+ B cells in Baboon 3.
Serum IgM and IgG responses to the transplantation of GTKO/hCD46 pig hepatocytes in baboons
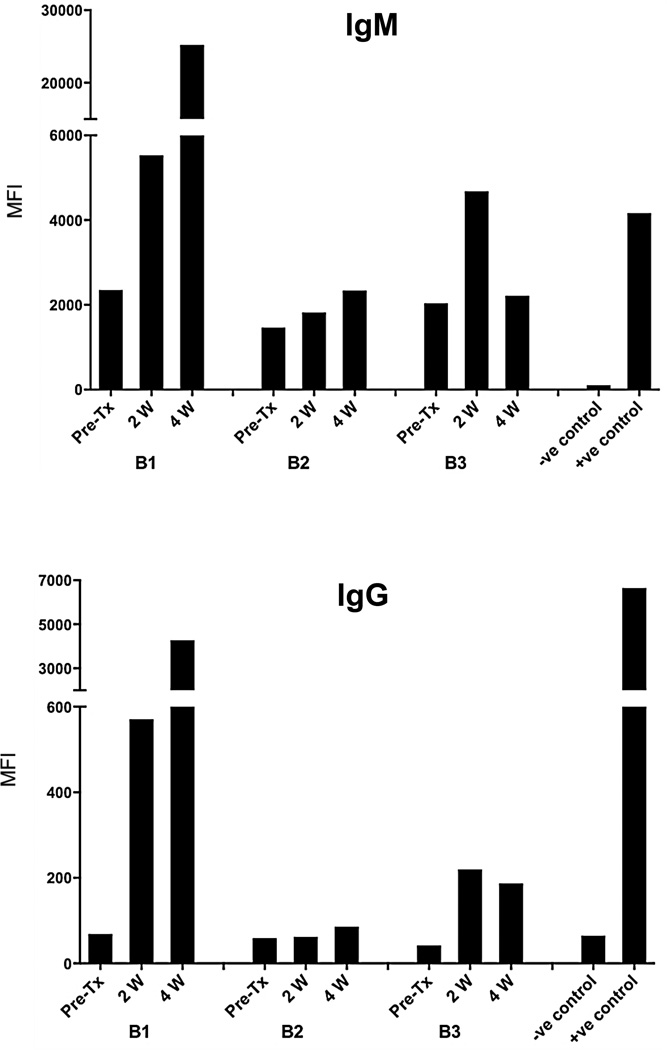
Baboons 1 and 3 demonstrated a significant increase in anti-pig IgM and an even greater increase in IgG after GTKO/hCD46 pig hepatocyte transplantation (Figure 1), indicating sensitization to pig antigens. Baboon 2 did not become sensitized.
Figure 1. Serum anti-pig IgM (above) and IgG (below) levels in baboon recipients of GTKO/hCD46 pig hepatocytes.
In Baboons 1 and 3 (B1 and B3), there were increases in IgM and IgG after pig hepatocyte transplantation, indicating sensitization to pig antigens. No significant sensitization occurred in Baboon 2 (B2).
Recipient baboon T cell proliferation in response to pig nonGal antigens
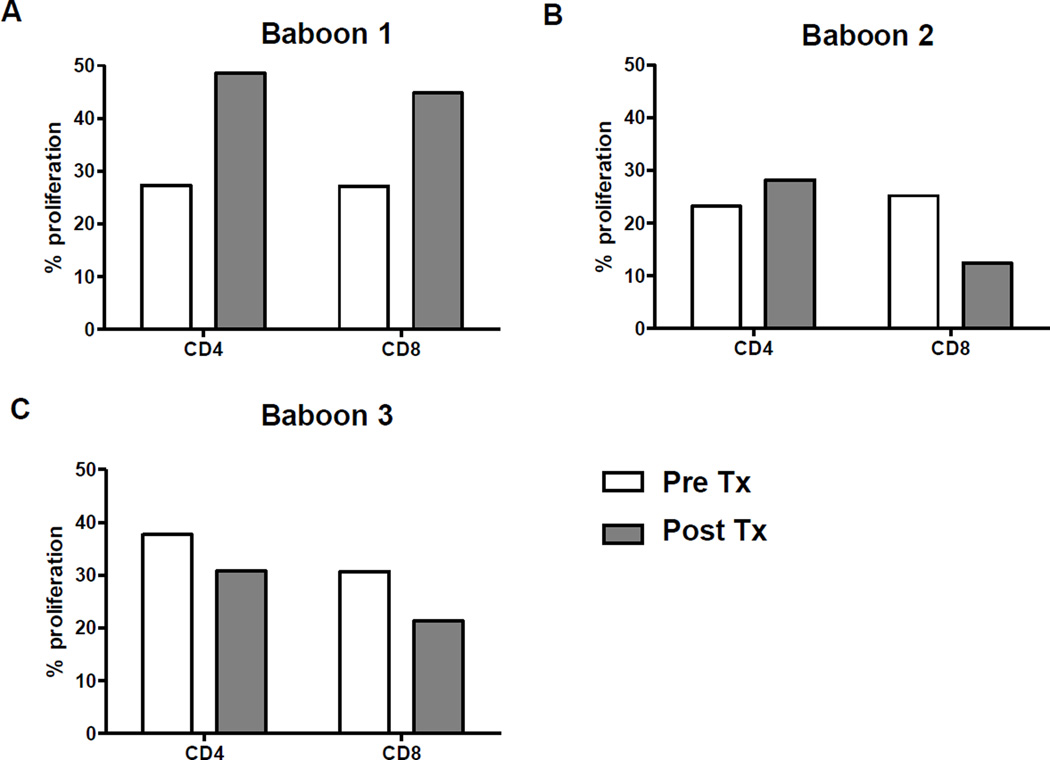
Baboon 1 demonstrated an approximate 70% increase in CD4+ and CD8+ proliferative responses (Figure 2). In contrast, Baboons 2 and 3 showed no or minimal increase in the T cell proliferative response.
Figure 2. Recipient baboon T cell proliferative responses to GTKO/hCD46 pig hepatocytes.
When a conventional immunosuppressive regimen (tacrolimus and rapamycin-based) was administered (Baboon 1), increases in CD4+ and CD8+ T cell proliferative responses were observed on MLR. When an anti-CD40mAb-based regimen was administered (Baboons 2 and 3), no increase in the T cell proliferative response was documented.
Monitoring for hepatocyte survival and function
Real time PCR for detection of porcine DNA
Very low levels or no pig DNA were detectable in any of the baboon tissues tested (Table 3). The femoral lymph node examined 7 days after the hepatocyte injection showed a relatively high percentage of pig DNA, but levels in all other tissues at 4 or 5 weeks was very low, except on one occasion. (The high expression in one mesenteric lymph node at 4 weeks in Baboon 1 cannot be explained.) In Baboon 2, that had no detectable immune response to the pig hepatocytes, the levels of pig DNA were particularly low.
Table 3.
Identification of porcine DNA in biopsies
| Samples | % Pig DNA of total DNA: Baboon1 |
Baboon2 | Baboon3 |
|---|---|---|---|
| Lymph node (mesenteric) | 4.928993 | 0.000000 | 0.030144 |
| Lymph node (mesenteric) | 0.020414 | 0.000000 | |
| Lymph node 2 (mesenteric) (non-injected control) |
0.000016 | ||
| Lymph node (femoral) | 0.000005 | ||
| Lymph node (femoral – one week after transplant) |
0.503526 | ||
| Fat (right) | 0.579307 | 0.000191 | 0.114621 |
| Fat (left) | 0.000871 | 0.120461 | 0.299991 |
| Spleen (upper) | 0.000000 | 0.000000 | 0.000000 |
| Spleen (middle) | 0.000047 | 0.000000 | 0.000010 |
| Spleen (lower) | 0.104509 | 0.000003 | 0.006618 |
| Kidney (right) | 0.109842 | 0.000034 | |
| Kidney (left) | 0.000060 | 0.000000 | |
| Kidney capsule (right) | 0.000000 | 0.118036 | |
| Kidney capsule (left) | 0.000014 | 0.000420 | |
| Liver (right lobe) | 0.000002 | 0.000015 | 0.000021 |
| Lung (right) | 0.000000 | 0.000018 | 0.000000 |
All biopsies were taken at 4 or 5 weeks after pig hepatocyte transplantation, except for one femoral lymph node biopsy in Baboon 3, which was examined after one week. Baboon tissues were analyzed for porcine DNA; data are given as means from 3 technical repeats as percentage of porcine DNA of the total DNA.
Real time PCR for detection of pig gene expression
Pig gene expression in the baboon tissues was equally low (Table 4), and correlated fairly well with the detection of pig DNA. In the majority of the tissues, no pig gene expression was detected.
Table 4.
Porcine gene expression in biopsies
| Baboon 1 | Baboon 2 | Baboon 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta- actin (% of adult pig liver cDNA) |
Albumi n (% of adult pig liver cDNA) |
AFP (% of adult pig liver cDNA) |
Beta- actin (% of adult pig liver cDNA) |
Albumi n (% of adult pig liver cDNA) |
AFP (% of adult pig liver cDNA) |
Beta- actin (% of adult pig liver cDNA) |
Albumin (% of adult pig liver cDNA) |
AFP (% of adult pig liver cDNA |
|
| Lymph node (mesenteric) |
0.001185 | 0.000196 | 0.000091 | 0.01660 | 0.00277 | ||||
| Lymph node (mesenteric) |
|||||||||
| Lymph node 2 (mesenteric) (non-injected control) |
|||||||||
| Lymph node (femoral - one week after transplant) |
117.92024 | 1.73812 | 11,838.36548 | ||||||
| Lymph node (femoral) |
|||||||||
| Fat (right) | 0.000022 | 0.30993 | 0.02061 | ||||||
| Fat (left) | 5.97875 4 | 0.00175 | 4.39360 | 0.61928 | 0.05174 | ||||
| Spleen (upper) | |||||||||
| Spleen (middle) | |||||||||
| Spleen (lower) | 0.000016 | 0.000002 | 0.00013 | ||||||
| Kidney (right) | |||||||||
| Kidney (left) | |||||||||
| Kidney capsule (right) |
5.662468 | 0.00005 | |||||||
| Kidney capsule (left) |
0.00992 | 0.00101 | |||||||
| Liver (right lobe) |
|||||||||
| Lung (right) | |||||||||
All biopsies were taken 4 or 5 weeks after pig hepatocyte transplantation, except for one femoral lymph node biopsy in Baboon 3, which was examined after one week. Baboon tissues were analyzed for porcine gene expression by real-time PCR; data are presented as means from three technical repeats in percentage of porcine liver cDNA.
AFP: alpha-fetoprotein
Detection of pig albumin in baboon sera by ELISA
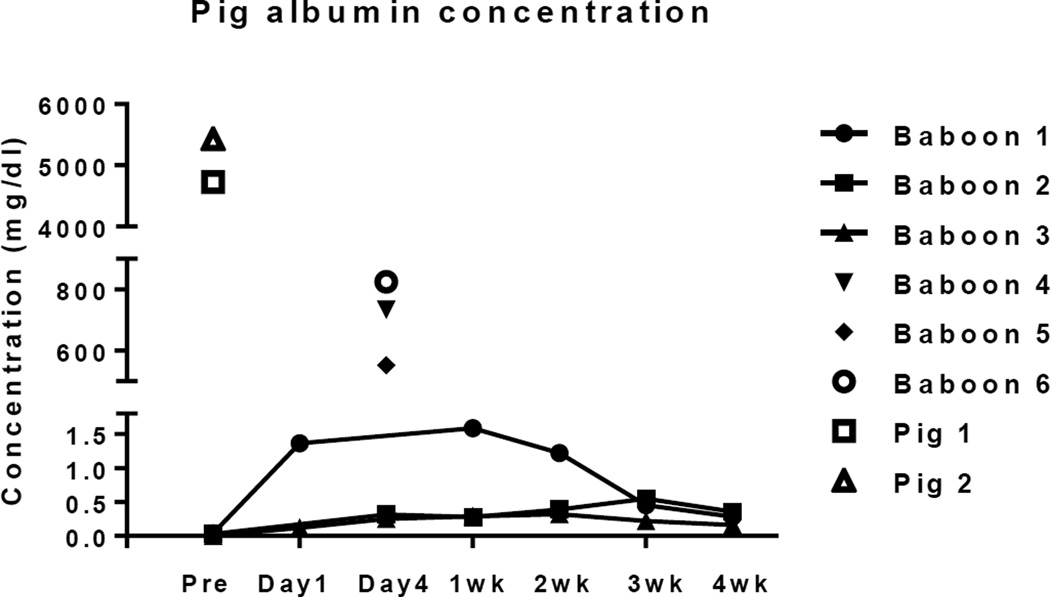
No pig albumin was detected in baboon sera before transplantation. After hepatocyte transplantation, pig albumin was detected at every time-point, but at very low levels (Figure 3). Baboon 1 demonstrated the highest pig albumin concentration at 1 week after transplantation, but the level then slowly decreased. Baboons 2 and 3 had even lower levels, with peak levels at 3 and 2 weeks, respectively. The results were compared with those from historic baboons with pig orthotopic liver grafts (n=3, Baboons 4–7) on day 3 post-transplant, which were several hundred-fold higher, and with levels from healthy pigs (n=2, Pigs 1 and 2), that were approximately 5–10-fold higher than in baboons with orthotopic pig liver grafts and >5,000-fold higher than in baboons with pig hepatocyte transplants.
Figure 3. Pig albumin levels in the sera of baboons with GTKO/hCD46 pig hepatocyte transplants.
Some pig albumin was detected in the serum of all 3 baboons, but the levels were very low. Also shown are levels in the sera of two healthy GTKO/hCD46 pigs (Pigs 1 and 2) and in the sera of 3 baboons on day 3 after pig orthotopic liver transplantation (Baboons 4, 5, and 6) (29). Following pig hepatocyte transplantation, the pig albumin levels were approximately 500–1,000-fold less than in baboons with pig liver grafts, and approximately 5,000–10,000-fold less than in healthy pigs.
Histopathology
Four or five weeks after hepatocyte transplantation, no cells with hepatocyte-like structure were identified in the spleen, mesenteric lymph nodes, kidney capsule, or subcutaneous fat in any of the 3 baboons (not shown). However, some hepatocyte-like cells were identified in the femoral lymph node 7 days after transplantation (not shown) No infiltrating mononuclear cells were identified in any biopsy (although identifying these would have been difficult in the spleen or lymph nodes).
DISCUSSION
Pig hepatocyte isolation proved straightforward from livers of GTKO/hCD46 pigs. However, even with the transplantation of hepatocytes from pigs genetically-engineered to provide some protection from the primate innate immune response (34), the results were extremely disappointing.
In all 3 baboons, there was minimal or no evidence of pig DNA or pig gene expression at any of the sites of hepatocyte injection, and minimal pig albumin was detected in the blood of the baboons. Since the half-life of endogenous albumin is approximately 3 weeks, while that of blood-derived albumin is only 12–16h (35), we anticipated that a significant level of pig albumin would be detected in the recipient at least for one day after transplantation. However, the levels of albumin measured were many 100-fold less than detected in baboons with orthotopic liver grafts (36) and many 1,000-fold less than in healthy pigs. Despite our opinion based on in vitro studies that the isolated hepatocytes were viable, our failure to carry out functional studies in rodents means that we cannot exclude the possibility of inadequate engraftment.
However, immune assays demonstrated that Baboons 1 and 3 became sensitized to pig antigens (increase in anti-pig IgG in both cases and T cell proliferative response in Baboon 1). In these two baboons, the grafts may well have been lost from an immune response (though no features were seen on histopathological examination at the sites of transplantation). In Baboon 1, the immunosuppressive regimen was clearly inadequate, but in Baboons 2 and 3 we had administered a costimulation blockade-based immunosuppressive regimen that has repeatedly been demonstrated to prevent a response to pig antigens after pig heart, kidney, and artery patch transplants in baboons that have been followed for up to 5 months (26–28). Furthermore, it prevented an anti-pig IgG response in Baboon 2.
The response documented in Baboon 3 may have been associated with the site of transplantation (i.e., spleen or lymph node versus direct blood vessel anastomosis in the abdomen) or to the immunogenicity of the transplanted tissue (i.e., hepatocytes versus organ). Transplantation of allogeneic hepatocytes into lymph nodes in immunosuppressed mice has been successful (12,13).
An alternative factor may have been that matrigel (a mouse tumor-derived extracellular protein extract) may not be as effective as the alginate (an algae-based polysaccharide) used by Nagata et al. (32). However, this specific alginate preparation does not appear to be available today, and the alternate alginate preparations we tested to create gels became solid once calcium was added (which is essential) and could not be used for injection. However, the matrigel we used has been shown to be effective in mouse hepatocyte and multiple other cell studies (12–14).
In Baboon 2, one reason for the disappointing result might have been because the hepatocytes had not been administered in a gel, as reported by Nagata et al (32). This may have resulted in rapid loss of hepatocytes from the sites of injection. In Baboon 3, therefore, we injected the hepatocytes in matrigel. Furthermore, we injected the pig hepatocytes into an additional site (a femoral lymph node) and examined this lymph node for the presence of pig hepatocytes after one week, in addition to the usual examination of all sites at 4 or 5 weeks.
In Baboon 3, at one week, the femoral lymph node demonstrated higher levels of pig DNA and gene expression than seen previously (at 4 weeks). Disappointingly, however, the results at 5 weeks were again almost identical to those in Baboons 1 and 2, with minimal evidence of pig hepatocyte survival, and minimal albumin levels in the blood. However, this prolonged exposure to the pig antigens may have increased the likelihood of sensitization.
As a result of this disappointing experience, the following points need to be considered.
Are pig hepatocytes more immunogenic than pig hearts, kidneys, artery patch grafts, or islets? To investigate this possibility, in future studies pig vascular endothelial cells could be transplanted into the spleen and/or lymph nodes, and the results compared with those obtained following hepatocyte transplants in these sites.
Does injection of pig cells (antigens) into the spleen and/or lymph nodes stimulate a greater immune response than when pig tissues are grafted at other sites? This would be in contrast to experience with hepatocyte transplantation into the lymph nodes in mice, which proved a good site under standard immunosuppression.
Did the presence of the recipient’s intact liver (with a full complement of hepatocytes) prevent survival and proliferation of pig hepatocytes (probably related to the absence or low levels of factors that stimulate hepatocyte growth). We suggest that this may be an important factor (15). Future studies should investigate pig hepatocyte transplantation in nonhuman primates that are in hepatic failure (e.g., after partial hepatectomy, portal vein embolization [37,38] and injury to the remaining hepatocytes). In Nagata’s study (18), no steps were taken to reduce the hepatocyte mass or function of the native liver, and so this was not a major difference from our own study. As the outcome of Nagata’s study was significantly better than ours, this suggests that rendering the recipient in hepatic failure may not be essential.
Is compatibility between pig CD47 and primate SIRP-α essential if phagocytosis of the hepatocytes is to be avoided? As porcine hepatocytes (20–30µm) (39) and primate macrophages (20–50µm) are of similar size, we suggest that the hepatocytes are too large to be phagocytosed by the macrophages. However, fragile hepatocytes could fragment, possibly from direct activation of recipient macrophages resulting in injury to the hepatocytes (40), and then be phagocytosed. In addition to the evidence of CD47/SIRP-α species incompatibility (40–42), natural killer (NK) cells can affect xenograft survival in mice through NK cell-mediated cytotoxicity (43).
Our results are in contrast to those of Nagata et al, who transplanted wild-type pig hepatocytes into the spleens of three immunosuppressed cynomolgus monkeys (32). Between 1–2 billion hepatocytes (in a 1% alginate matrix) were injected directly into the parenchyma of the spleens (comparable to the number transplanted into the spleen in the present study). The immunosuppressive regimen was intensive, but clinically-applicable, conventional therapy (including induction with thymoglobulin, an anti-CD25mAb, and methylprednisolone, with maintenance cyclosporine, FTY720, rapamycin, and methylprednisolone). Our own very limited data in the present study, and more extensive experience previously (44), suggest that conventional immunosuppressive therapy (based on calcineurin inhibition), unless very intensive, may be insufficient to prevent an adaptive immune response against even genetically-engineered pig hepatocytes. However, immunosuppressive regimens based on T cell costimulation blockade, although proven to be successful in pig vascularized solid organ Tx (25–28,45,46), did not completely prevent an adaptive immune response in the present study. Of course, if graft failure was associated with a lack of viability of the isolated hepatocytes, then the intensity of the immune response may have been irrelevant. Furthermore, in the Nagata study, the period of time from hepatocyte isolation to transplantation was approximately 20h, whereas in our study this was <60min.
In Nagata’s study (32), graft function was determined by the measurement of porcine albumin. A peak of porcine albumin was detected in the blood within the first month. Following a single injection, the pig hepatocytes functioned for between 25 days (limited by death of the monkey from a cytomegalovirus infection) and >80 days. Following retransplantation on two occasions in one of the monkeys, porcine albumin was detected for >253 days (the monkey dying from complications associated with replacement of a central venous catheter). Although pig hepatocyte transplantation was associated with a slight increase in anti-Gal IgG (considered to be within the normal range), there was no detectable increase in anti-nonGal antibody levels, suggesting that the transplantation of hepatocytes from GTKO pigs might induce a minimal humoral immune response. We could not confirm this hope in the present study.
It may be that transplantation into the spleen alone is not immunogenic, but that transplantation in other sites, e.g., lymph nodes, might be, although the similarities in structure and function between spleen and lymph nodes suggest that this is unlikely.
From experience gained from the present study, if initiating further studies, we would make the following changes to the experimental protocol. We would (i) carry out functional studies in rodents to confirm the functional viability of the hepatocytes, (ii) inject the hepatocytes into just one site, e.g., spleen, in case injection into other sites might be detrimental (as it is possible that hepatocytes injected into some sites are more immunogenic than others, and this might confuse the results), (iii) measure the cytokine and chemokine responses to the hepatocytes as an indicator of the innate immune response, and (iv) consider the transplantation of pig hepatocytes expressing human CD47 and/or HLA-E/G (47,48), as this might be beneficial in reducing the innate immune response. Based on our pig liver transplants into baboons (Figure 3) (36), we suggest that a minimal functional level of albumin might be at least 500mg/dL, but preferably >1g/dL.
In conclusion, the transplantation of genetically-engineered pig hepatocytes into multiple sites in immunosuppressed, but otherwise healthy, baboons was associated with very early graft failure even in the absence of an adaptive immune response. Considerable further study is required before clinical trials should be undertaken.
Acknowledgments
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and # PO1 HL107152 by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. We thank David Ayares and his colleagues at Revivicor, Inc., for providing the genetically-engineered pigs used as sources of hepatocytes in this study. The baboons used in the study were from the Oklahoma University Health Sciences Center Baboon Research Resources, which is supported by the Office of the Director, NIH, under Award Number P40OD010431 and P40OD010988. We would like to express our gratitude to Dr. Keith Reimann for providing us with anti-CD40mAb from the NHP Reagent Resource (contract HHSN272200900037C) and to Matthew Young for assistance during ex vivo liver perfusion.
ABBREVIATIONS
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- hCD46
human complement-regulatory protein CD46
- PBMC
peripheral blood mononuclear cells
Footnotes
Conflict of interest
No authors reports a conflict of interest.
References
- 1.Gridelli B, Vizzini G, Pietrosi G, et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl. 2012;18:226–237. doi: 10.1002/lt.22322. [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Liu H, Ezzelarab M, et al. Experimental hepatocyte xenotransplantation--a comprehensive review of the literature. Xenotransplantation. 2015;22:239–248. doi: 10.1111/xen.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier RP, Navarro-Alvarez N, Morel P, et al. Current status of hepatocyte xenotransplantation. Int J Surg. 2015;23:273–279. doi: 10.1016/j.ijsu.2015.08.077. [DOI] [PubMed] [Google Scholar]
- 4.Mazaris EM, Roussos CT, Papalois VE. Hepatocyte transplantation: a review of worldwide clinical developments and experiences. Exp Clin Transplant. 2005;3:306–315. [PubMed] [Google Scholar]
- 5.Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. J Hepatobiliary Pancreat Surg. 2009;16:97–100. doi: 10.1007/s00534-008-0023-0. [DOI] [PubMed] [Google Scholar]
- 6.Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–298. doi: 10.1038/nrgastro.2010.44. [DOI] [PubMed] [Google Scholar]
- 7.Hansel MC, Gramignoli R, SkvoraK KJ, et al. The history and use of human hepatocytes for the treatment of liver diseases: the first 100 patients. Curr Protoc Toxicol. 2014;62 doi: 10.1002/0471140856.tx1412s62. 14.12.1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, STORM SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54:162–177. doi: 10.1159/000369552. [DOI] [PubMed] [Google Scholar]
- 9.Koch CA, Kanazawa A, Nishitai R, et al. Intrinsic resistance of hepatocytes to complement-mediated injury. J Immunol. 2005;174:7302–7309. doi: 10.4049/jimmunol.174.11.7302. [DOI] [PubMed] [Google Scholar]
- 10.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 11.Hara H, Campanile N, Tai HC, et al. An in vitro model of pig liver xenotransplantation--pig complement is associated with reduced lysis of wild-type and genetically modified pig cells. Xenotransplantation. 2010;17:370–378. doi: 10.1111/j.1399-3089.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoppo T, Komori J, Manohar R, Stolz Db, Lagasse E. Rescue of lethal hepatic failure by hepatized lymph nodes in mice. Gastroenterology. 2011;140:656–666. doi: 10.1053/j.gastro.2010.11.006. e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komori J, Boone L, Deward A, Hoppo T, Lagasse E. The mouse lymph node as an ectopic transplantation site for multiple tissues. Nat Biotechnol. 2012;30:976–983. doi: 10.1038/nbt.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deward AD, Komori J, Lagasse E. Ectopic transplantation sites for cell-based therapy. Curr Opin Organ Transplant. 2014;19:169–174. doi: 10.1097/MOT.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi K, Kay MA, Yokoyama T, et al. Stability and repeat regeneration potential of the engineered liver tissues under the kidney capsule in mice. Cell Transplant. 2005;14:621–627. doi: 10.3727/000000005783982620. [DOI] [PubMed] [Google Scholar]
- 17.Le H, Cusick RA, Utsunomiya H, MA Px, Langer R, Vacanti JP. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer scaffolds. Tissue Eng. 2003;9:1227–1232. doi: 10.1089/10763270360728134. [DOI] [PubMed] [Google Scholar]
- 18.Phelps Cj, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai Y, Vaught TD, Boone J, et al. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat Biotechnol. 2002;20:251–255. doi: 10.1038/nbt0302-251. [DOI] [PubMed] [Google Scholar]
- 20.Azimzadeh A, Kelishadi S, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement-regulatory protein. Xenotransplantation. 2015;22:310–316. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach J, Grehn S, Neuhaus P. Endothelial cell kinetics after anoxia and hypothermia in preservation solutions as indicators of endothelial repair. Transplant Proc. 1993;25:1593–1594. [PubMed] [Google Scholar]
- 22.Gerlach JC, Brombacher J, Courtney JM, Neuhaus P. Nonenzymatic versus enzymatic hepatocyte isolation from pig livers for larger scale investigations of liver cell perfusion systems. Int J Artif Organs. 1993;16:677–681. [PubMed] [Google Scholar]
- 23.Gerlach J, Kloppel K, Schon MR, et al. Comparison of pig hepatocyte isolation using intraoperative perfusion without warm ischemia and isolation of cells from abattoir organs after warm ischemia. Artif Organs. 1993;17:950–953. doi: 10.1111/j.1525-1594.1993.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach Jc, Brombacher J, Kloppel K, Schnoy N, Neuhaus P. Comparison of four methods for mass hepatocyte isolation from pig and human livers. Transplantation. 1994;57:1318–1322. doi: 10.1097/00007890-199405150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2014;21:35–45. doi: 10.1111/xen.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwase H, Satyananda V, Zhou H, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32:99–108. doi: 10.1016/j.trim.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. doi: 10.1111/xen.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heart transplantation - first experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwase H, Hara H, Ezzelarab M, et al. Immunologic and physiologic observations in baboons with life-supporting genetically- engineered pig kidney grafts. Xenotransplantation. 2016 doi: 10.1111/xen.12293. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara H, Koike N, Long C, et al. Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs. Invest Ophthalmol Vis Sci. 2011;52:5278–5286. doi: 10.1167/iovs.10-6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagata H, Nishitai R, Shirota C, et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132:321–329. doi: 10.1053/j.gastro.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 33.TAnabe S, Hase M, Yano T, et al. A real-time quantitative PCR detection method for pork, chicken, beef, mutton, and horseflesh in foods. Biosci Biotechnol Biochem. 2007;71:3131–3135. doi: 10.1271/bbb.70683. [DOI] [PubMed] [Google Scholar]
- 34.Cooper DK, Satyananda V, Ekser B, et al. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liumbruno GM, Bennardello F, Latttanzio A, Piccoli P, Rossettias G. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7:216–234. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10:273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 37.Dagher I, Boudechiche L, Branger J, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82:1067–1073. doi: 10.1097/01.tp.0000236103.99456.8f. [DOI] [PubMed] [Google Scholar]
- 38.Lainas P, Boudechiche L, Osoroi A, et al. Liver regeneration and recanalization time course following reversible portal vein embolization. J Hepatol. 2008;49:354–362. doi: 10.1016/j.jhep.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 39.Stegemann JP, Raina S, Nicholson DT, et al. Comparison of analytical methods for quantitation of isolated porcine hepatocyte yields. Tissue Eng. 2000;6:253–264. doi: 10.1089/10763270050044434. [DOI] [PubMed] [Google Scholar]
- 40.Navarro-Alvarez N, Yang YG. Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft loss: a potential barrier to hepatocyte xenotransplantation. Cell Transplant. 2014;23:345–354. doi: 10.3727/096368913X663604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waern JM, Yuan Q, Rudrich U, et al. Ectopic expression of murine CD47 minimizes macrophage rejection of human hepatocyte xenografts in immunodeficient mice. Hepatology. 2012;56:1479–1488. doi: 10.1002/hep.25816. [DOI] [PubMed] [Google Scholar]
- 42.Zhang M, Wang H, Tan S, Navarro-Alvarez N, Zheng Y, Yang YG. Donor CD47 controls T cell alloresponses and is required for tolerance induction following hepatocyte allotransplantation. Sci Rep. 2016;6:26839. doi: 10.1038/srep26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawahara T, Douglas DN, Lewis J, et al. Critical role of natural killer cells in the rejection of human hepatocytes after xenotransplantation into immunodeficient mice. Transpl Int. 2010;10:934–943. doi: 10.1111/j.1432-2277.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 44.Buhler L, Awwad M, Basker M, et al. High-dose porcine hematopoietic cell transplantation combined with CD40 ligand blockade in baboons prevents an induced anti-pig humoral response. Transplantation. 2000;69:2296–2304. doi: 10.1097/00007890-200006150-00013. [DOI] [PubMed] [Google Scholar]
- 45.Mohiuddin MM, Singh AK, Corcoran PC, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148:1106–1113. doi: 10.1016/j.jtcvs.2014.06.002. discussion 1113–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohiuddin MM, Singh AK, Corcoran PC, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss EH, Lilienfeld BG, Muller S, et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation. 2009;87:35–43. doi: 10.1097/TP.0b013e318191c784. [DOI] [PubMed] [Google Scholar]
- 48.Esquivel EL, Maeda A, Eguchi H, et al. Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl immunol. 2015;32:109–115. doi: 10.1016/j.trim.2014.12.004. [DOI] [PubMed] [Google Scholar]