STRUCTURED ABSTRACT
BACKGROUND
Although beta-blockers are recommended for older adults after acute myocardial infarction (AMI), limited studies suggest that nursing home (NH) residents may often not receive beta-blockers in this setting.
OBJECTIVES
To evaluate how often beta-blockers were started after AMI in NH residents who previously did not use these drugs, and to evaluate which factors were associated with post-AMI use of beta-blockers.
DESIGN
Retrospective cohort using linked national Minimum Data Set assessments; Online Survey, Certification and Reporting (OSCAR) records; and Medicare claims.
SETTING
U.S. NHs.
PARTICIPANTS
National cohort of 15,720 residents aged ≥65 years who were hospitalized for an AMI May 2007–March 2010, had no beta-blocker usage for ≥4 months prior, and survived ≥14 days after NH readmission.
MEASUREMENTS
The outcome was beta-blocker initiation within 30 days of NH readmission.
RESULTS
Fifty-seven percent (n=8,953) of residents initiated a beta-blocker after AMI. After covariate adjustment, use of beta blockers declined with advancing age (down to odds ratio (OR) 0.65, 95% confidence interval (CI) 0.54–0.79 for ≥95 versus 65–74 years); and were used less often in adults with higher levels of functional impaired (dependent or totally dependent versus independent to limited assistance, OR=0.84, 95% CI=0.75–0.94) and medication use (≥15 versus ≤10 medications, OR=0.89, 95% CI=0.80–0.99). A wide variety of resident and NH characteristics were not associated with beta-blocker use, including sex, cognitive function, comorbidity burden, and NH ownership.
CONCLUSION
Almost one-half of older NH residents in the U.S. do not initiate a beta-blocker after AMI. The absence of observed factors that strongly predict beta-blocker use may indicate a lack of consensus on how to manage older NH residents suggesting the need to develop and disseminate thoughtful practice standards.
Keywords: Nursing homes, myocardial infarction, beta-blockers, elderly, drug utilization
INTRODUCTION
One and one-half million older Americans live in nursing homes (NHs), and over 50% of NH residents have cardiac disease.(1) NH residents are rarely included in randomized clinical trials (RCTs) used to inform practice guidelines for common conditions such as acute myocardial infarction (AMI).(2) The result is a profound lack of evidence to guide treatment decisions for older NH residents for whom the potential benefits of some interventions, like beta-blocker therapy, may be counterbalanced by adverse effects to which older adults are particularly susceptible.(2)
According to the 2013 American College of Cardiology Foundation/American Heart Association guidelines, oral beta-blocker therapy should be initiated for all patients within 24 hours after an AMI and continued for at least 3 years, in the absence of contraindications. (3–5) Post-hospitalization, beta-blockers are a mainstay of secondary prevention. The guideline recommendations are supported by RCTs that have demonstrated that the long-term use of beta-blockers following AMI substantially reduces mortality in individuals up to 75 years of age.(6–9) Observational studies have extended these findings to even older patients. Many studies report a mortality benefit in community-dwelling people up to and beyond age 85 years.(10–12)
Data from community-dwelling older adults have shown that use of beta-blockers after AMI decreases with increasing age. (13, 14) Studies of community-dwelling individuals have also presented conflicting data on whether functional limitations and geriatric syndromes are associated with even lower rates of beta-blocker use.(13, 14) However, much less is known about use of these agents in older NH residents, who often have different clinical characteristics and systems of care than their community-dwelling counterparts. A handful of studies on this topic have been conducted using data from the 1990s and in small, selected populations —these found low utilization of beta-blockers in the NH setting.(15–19) These studies have important limitations that hinder our understanding of current patterns of beta-blocker use. Because these studies were done using older data and selected samples, the generalizability to the current, national population of NH residents in the U.S. remains unclear. Further, little evidence is available to determine whether prescribing practices have changed in conjunction with general improvements in adherence to ischemic heart disease-related guideline recommendations in the U.S. Lastly, there is uncertainty about the extent to which previously observed rates of beta-blocker use are due to the continuation of beta-blocker therapy from before AMI (i.e., prevalent use), or represent new prescribing choices after AMI. Understanding recent prescribing practices in the NH setting is essential for identifying gaps in the quality of care and corresponding high leverage points to address gaps.
Therefore, the objective of this study was to describe the epidemiology of beta-blocker use after AMI within a national sample of U.S. nursing homes. We focused on individuals who were non-users of beta-blockers in order to understand how NH prescribers respond to widely accepted clinical practice guidelines that recommend initiating a beta-blocker after AMI.
METHODS
Data Sources
Using national Medicare data, we linked denominator (eligibility) information, Part A inpatient hospital claims, Part D prescription drug claims, and Minimum Data Set (MDS) 2.0 data for all fee-for-service beneficiaries in 2007–2010 who were eligible for inclusion. The MDS is a comprehensive, clinical assessment instrument used to document health status of nursing home residents, including demographic and medical information, and assessments of functional, psychological, and cognitive abilities.(20, 21) The Centers for Medicare and Medicaid Services (CMS) require that each certified U.S. nursing home conduct an MDS assessment of all residents on admission, quarterly thereafter, and with significant changes in clinical condition. (22) Online Survey Certification and Reporting (OSCAR) data was utilized for facility-level information, including nursing home characteristics and staffing levels.(23, 24) We employed a previously validated residential history file algorithm to track the timing and location of health service use.(25)
Study Population
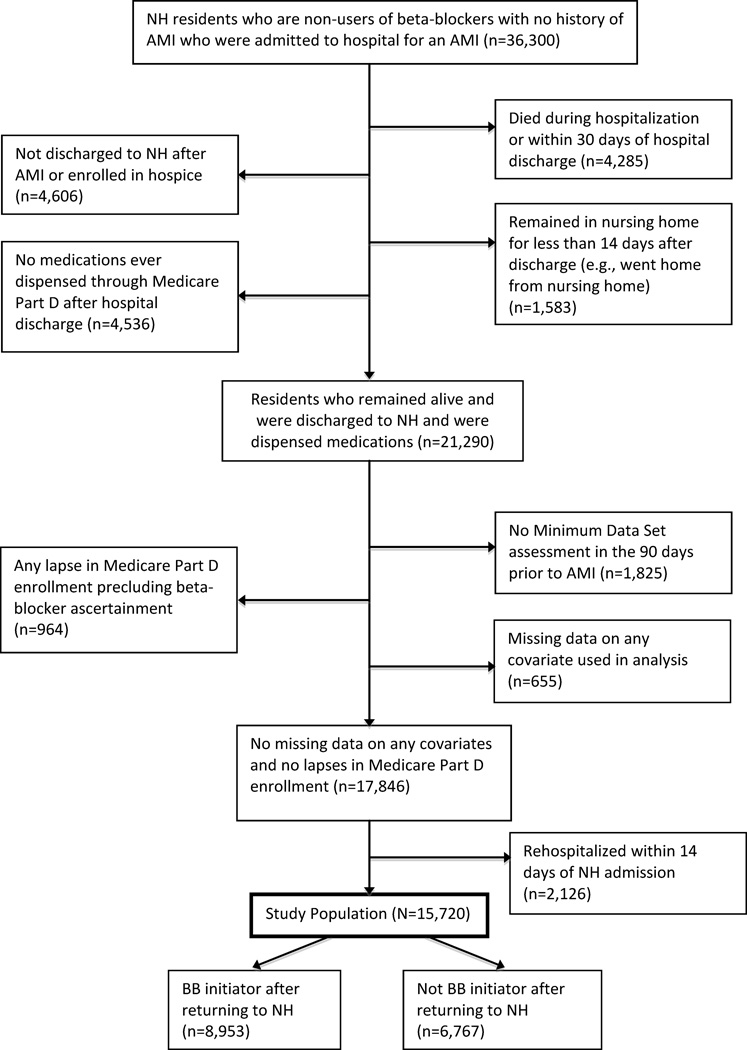
We conducted a retrospective inception cohort study of a national cohort of long-stay nursing home residents without a history of AMI who were hospitalized for AMI, had not previously taken beta-blockers for at least four months prior to AMI, and were re-admitted to U.S. nursing homes directly following hospital discharge between May 1, 2007 and December 31, 2010 (Figure 1). Our final sample consisted of 15,720 NH residents admitted to 8,349 NHs. Residents lived in all 50 U.S. states, the District of Columbia, Puerto Rico, and the Virgin Islands. We selected previous non-users to permit an evaluation of the decision to initiate beta-blockers after AMI, distinct from the decision to continue these agents in patients who had already been taking them before their AMI. We restricted our analysis to residents age ≥65 years who were in a nursing home ≥90 days and then hospitalized for an AMI, defined as a principal diagnosis on a Part A claim (International Classification of Diseases, Ninth Revision [ICD-9] codes 410.0–410.9), between May 1, 2007 and March 31, 2010. We required residents to have been continuously enrolled Medicare fee-for-service beneficiaries and have had no prescription for beta-blockers in the four months prior to the index AMI hospitalization. After the AMI, residents must have been discharged to a nursing home and not enrolled in hospice. Individuals with multiple AMIs entered the study on their first AMI during the study period. We excluded individuals for whom beta-blocker exposure could not be reliably ascertained after readmission to the NH, including individuals with lapses in use of Medicare Part D, individuals who died within 30 days of NH readmission, and individuals rehospitalized within 14 days of NH readmission. To maintain our study population of NH residents, we also excluded those who left the NH to receive care in other settings shortly after admission.
Figure 1.
Flow diagram of resident inclusion and exclusion in study cohort.
Measurement of Beta-blocker Use
We identified oral beta-blockers by generic name in Medicare Part D prescription drug claims, which contain a complete history of drug dispensings for this population, including date dispensed, dose, route, and days’ supply. Part D coverage is common in nursing homes with approximately 81% of residents covered.(26) Beta-blockers considered in this study included orally administered formulations of acebutolol, atenolol, betaxolol, bisoprolol, carvedilol, labetalol, metoprolol, nadolol, nebivolol, penbutolol, pindolol, propranolol, and timolol.
We used two complimentary approaches to ascertain beta-blocker exposure, based upon the initial NH care pathway (long-term care, or skilled nursing facility care) following the index AMI hospitalization. Individuals admitted directly from hospital to nursing home for long term care (LTC) were classified as beta-blocker users if there was at least one dispensing of a beta-blocker in Part D claims within the first 30 days after admission. In contrast, medication ascertainment required a different approach for people readmitted to the NH under the “skilled nursing facility” (SNF) care pathway. Many NH residents – even ones there for long-term residential care – return from the hospital to the NH on this time-limited SNF pathway. This pathway is paid for by Medicare Part A and covers additional services such as extra rehabilitative therapy and high-level nursing care.(27) While patients are receiving SNF care, medication purchases are covered under a single bundled payment.(27) Because of this, Part D claims are not generated while patients are on the SNF pathway, precluding direct observation of medication use. After completion of a defined period of SNF services (typically less than one month, with a maximum of 100 days), patients in the NH long-term revert back to the LTC pathway.(27, 28) At this point, those with Medicare Part D coverage again receive their drugs through the Part D program.
Therefore, to evaluate post-AMI beta-blocker utilization in patients who returned to the NH on SNF care, we evaluated whether or not these patients used beta-blockers once they had transitioned from SNF care back to LTC and Part D claims were once again available. To test whether this approach was valid, we conducted a separate validation study using prescription drug dispensing data from a large, national private nursing home chain (HCR ManorCare, LLC), in which complete drug dispensing data were available regardless of SNF or LTC status. We observed that nearly all residents (>94%) in this post-AMI cohort continued taking beta-blockers after transition from SNF to LTC. This suggested that, in our main cohort, it was reasonable to impute beta-blocker use in the early post-hospitalization SNF period based on beta-blocker usage following transition to LTC. Subsequently, this approach was employed for individuals admitted to the nursing home for SNF services following the index AMI hospitalization (N=11,283). Beta-blocker use was defined as any dispensing within 60 days of transition from SNF to LTC (the period during which medications are covered under Medicare Part D). Sixty days was selected as a sufficiently long period to allow for depletion of beta-blocker supplies obtained through Part A billing during SNF care, thus requiring a Part D dispensing. Of note, we were unable to determine if a beta blocker was started during the index hospitalization, and therefore if the nursing home physician was continuing a recommendation to prescribe or not prescribe the drug initiated by the inpatient physician. Regardless, at some level the nursing home physician was involved in the prescribing decision insofar as we only evaluated medications filled after return to the nursing home.
Measure of Resident and NH Characteristics
Variables that could potentially predict beta-blocker use included demographics (age, sex, and race) from Medicare enrollment files, concomitant medication use (e.g. calcium channel blockers, loop diuretics, opioid analgesics) from Part D claims, and comorbidities (e.g., asthma, chronic obstructive pulmonary disease, congestive heart failure) from Part A claims, all measured in the year prior to AMI. Part A claims were also used to document recent hospital course, severity of cardiovascular disease, and the Elixhauser Comorbidity Index score.(29)
A number of MDS items have been structured into reliable and valid measures of residents’ functional status.(30–32) The level of functional impairment for each resident was estimated with the MDS Activities of Daily Living (ADL) Scale score documented in the assessment closest to the AMI date in the 90 days prior to AMI. This summary measure indicates the degree of dependence on staff assistance in seven areas of ADL function (bed mobility, transfer, locomotion, dressing, eating, toilet use, personal hygiene), and ranges from 0 (no assistance required) to 28 (total dependence in ADL functioning). (33) Cognitive function was measured with the Cognitive Performance Scale; scores range from 0 (intact) to 6 (severe impairment).(31) Other geriatric syndromes and conditions (weight loss, falls, presence and frequency of pain, and Changes in Health, End-Stage Disease, Signs, and Symptoms Scale (CHESS) score) were also measured in the MDS. The CHESS score is a measure of poor prognosis and health instability and has been validated as a proxy for frailty.(34, 35) While an MDS assessment after the AMI hospitalization and before beta-blocker initiation might have been preferred to capture updated values of variables that could be altered by the AMI hospitalization, the first assessment occurred after beta blocker initiation in nearly all cases.
Facility characteristics and indicators of care quality were obtained from the most recent OSCAR survey before the acute AMI hospitalization, such as ownership (profit, non-profit, government), total number of beds, and total direct care hours/day/resident.
We hypothesized that poor functional status and cognition, frailty, and older age would be associated with lower rates of beta blocker use. We included pre-AMI medication use as markers of residents’ clinically active conditions and risk of future clinical events (e.g., residents prescribed statins may be at higher perceived risk of future cardiovascular events).
Analytic Approach
We first evaluated univariable associations between potential predictors and beta-blocker initiation using logistic regression to estimate odds ratios (OR). To test our hypothesis that certain individual and facility factors were independently associated with beta-blocker prescribing for residents after AMI, we used a multilevel multivariable logistic regression model.(36) Because residents are clustered within NHs facilities, we included random intercepts for facilities in the model to ensure more accurate standard errors.(37) Patient and facility characteristics were modeled as fixed effects. We divided the regression results into two tables for clarity, but multivariable analyses adjusted for the full set of variables shown in Tables 2 and 3, plus additional variables listed in Supplementary Table 2. Data were analyzed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and Stata, version 14.0 (Stata Corp., College Station, TX), software.
Table 2.
Univariable and Multivariable Analysis of Demographics and Geriatric Syndromes Associated with Beta-blocker Initiation after Resident Admission to Nursing Home after Acute Myocardial Infarction (N=15,720)
| Characteristic | Beta-blocker Initiation (n/n, %) |
Univariable Association (OR, 95% CI) |
Multivariable Association (OR, 95% CI)1 |
|---|---|---|---|
| All patients | 8,953 (57) | ||
| Age in years | |||
| 65 to <75 | 1,576/2,599 (61) | Reference | Reference |
| 75 to <85 | 3,291/5,720 (58) | 0.88 (0.80–0.97) | 0.89 (0.79–1.00) |
| 85 to <95 | 3,516/6,284 (56) | 0.83 (0.75–0.91) | 0.83 (0.73–0.94) |
| ≥95 | 570/1,117 (51) | 0.68 (0.59–0.78) | 0.65 (0.54–0.79) |
| Sex | |||
| Male | 2,649/4,580 (58) | Reference | Reference |
| Female | 6,304/11,140 (57) | 0.95 (0.89–1.02) | 0.97 (0.88–1.06) |
| Race | |||
| White, non-Hispanic | 7,232/12,829 (56) | Reference | Reference |
| Black, non-Hispanic | 1,158/1,914 (61) | 1.19 (1.08–1.31) | 1.13 (0.99–1.29) |
| Hispanic | 391/677 (58) | 1.06 (0.91–1.24) | 1.05 (0.86–1.28) |
| AI/AN/API | 172/300 (57) | 1.04 (0.83–1.31) | 0.98 (0.72–1.33) |
| Region | |||
| Northeast | 2,401/3,907 (62) | Reference | Reference |
| Midwest | 2,592/4,464 (58) | 0.87 (0.80–0.95) | 0.94 (0.83–1.07) |
| South | 2,902/5,451 (53) | 0.71 (0.66–0.78) | 0.81 (0.72–0.92) |
| West | 957/1,723 (56) | 0.78 (0.70–0.88) | 0.78 (0.67–0.92) |
| Caribbean | 101/175 (58) | 0.86 (0.63–1.16) | 0.98 (0.67–1.43) |
| CHESS score (health instability) | |||
| No instability | 5,093/8,836 (58) | Reference | Reference |
| Minimal | 2,562/4,524 (57) | 0.96 (0.89–1.03) | 0.94 (0.85–1.03) |
| Low | 1,066/1,941 (55) | 0.90 (0.81–0.99) | 0.85 (0.74–0.98) |
| Moderate to very high | 232/419 (55) | 0.91 (0.75–1.11) | 0.86 (0.67–1.12) |
| Cognition | |||
| Cognitively intact | 1,614/2,741 (59) | Reference | Reference |
| Mild dementia | 2,933/5,091 (58) | 0.95 (0.86–1.04) | 0.99 (0.88–1.12) |
| Moderate to severe dementia | 4,406/7,888 (56) | 0.88 (0.81–0.97) | 0.99 (0.87–1.13) |
| Activities of daily living status | |||
| Independent/limited assistance | 2,446/4,304 (57) | Reference | Reference |
| Extensive assistance | 4,145/7,196 (58) | 1.03 (0.96–1.11) | 0.95 (0.86–1.05) |
| Dependent/totally dependent | 2,362/4,220 (56) | 0.97 (0.89–1.05) | 0.84 (0.75–0.94) |
Abbreviations: OR, odds ratio; AI/AN/API, American Indian/Alaskan Native/Asian Pacific Islander; CHESS, Changes in Health, End-Stage Disease, Signs, and Symptoms Scale; ADLs, Activities of Daily Living.
Multivariable analyses also adjusted for a wide range of variables not shown here including demographics, clinical conditions, baseline medications, geriatric syndromes, AMI characteristics, and nursing home characteristics; see Supplementary Table 2 for complete list.
Table 3.
Univariable and Multivariable Analysis of Clinical Conditions and Myocardial Infarction Characteristics Associated with Beta-blocker Initiation after Resident Admission to Nursing Home after Acute Myocardial Infarction (N=15,720)
| Characteristic | Beta-blocker Initiation (n/n, %) |
Univariable Association (OR, 95% CI) |
Multivariable Association (OR, 95% CI)1 |
|---|---|---|---|
| All patients | 8,953 (57) | ||
| Clinical Conditions at Baseline | |||
| Atrial fibrillation | |||
| No | 6,817/11,913 (57) | Reference | Reference |
| Yes | 2,136/3,807 (56) | 0.96 (0.89–1.03) | 1.01 (0.91–1.12) |
| Angina pectoris | |||
| No | 8,279/13,692 (61) | Reference | Reference |
| Yes | 674/2,028 (33) | 0.33 (0.30–0.36) | 0.29 (0.26–0.34) |
| Unstable angina | |||
| No | 8,145/14,086 (58) | Reference | Reference |
| Yes | 808/1,634 (50) | 0.71 (0.64–0.79) | 0.57 (0.50–0.66) |
| Asthma | |||
| No | 8,838/15,482 (57) | Reference | Reference |
| Yes | 115/238 (48) | 0.70 (0.54–0.91) | 0.78 (0.56–1.08) |
| COPD | |||
| No | 6,735/11,560 (58) | Reference | Reference |
| Yes | 2,218/4,160 (53) | 0.82 (0.76–0.88) | 0.90 (0.81–0.99) |
| CHF | |||
| No | 4,419/8,135 (54) | Reference | Reference |
| Yes | 4,534/7,585 (60) | 1.25 (1.17–1.33) | 1.30 (1.20–1.42) |
| Depression | |||
| No | 7,852/13,781 (57) | Reference | Reference |
| Yes | 1,101/1,939 (57) | 0.99 (0.90–1.09) | 1.02 (0.90–1.16) |
| Elixhauser score | |||
| 0–2 | 2,561/4,268 (55) | Reference | Reference |
| 3–4 | 4,979/8,604 (58) | 1.11 (1.03–1.19) | 1.02 (0.93–1.13) |
| ≥5 | 1,413/2,488 (57) | 1.06 (0.96–1.17) | 0.98 (0.84–1.14) |
| Characteristics of Acute Myocardial Infarction Hospitalization | |||
| Length of stay, days | |||
| 0–4 | 2,383/4,534 (53) | Reference | Reference |
| 5–9 | 4,438/7,597 (58) | 1.27 (1.18–1.37) | 1.10 (0.99–1.22) |
| ≥10 | 2,132/3,589 (59) | 1.32 (1.21–1.44) | 1.02 (0.89–1.16) |
| CCU or ICU use, days | |||
| 0 | 3,385/6,662 (51) | Reference | Reference |
| 1–3 | 2,425/4,014 (60) | 1.48 (1.37–1.60) | 1.35 (1.22–1.49) |
| ≥4 | 3,143/5,044 (62) | 1.60 (1.49–1.72) | 1.23 (1.11–1.36) |
Abbreviations: OR, odds ratio; COPD, Chronic Obstructive Pulmonary Disease; CHF, Congestive Heart Failure; CCU/ICU, Coronary Care Unit/Intensive Care Unit.
Multivariable analyses also adjusted for a wide range of variables not shown here including demographics, clinical conditions, baseline medications, geriatric syndromes, AMI characteristics, and nursing home characteristics; see Supplementary Table 2 for complete list.
The study protocol was approved by the Institutional Review Board of Brown University, the University of California San Francisco, and the San Francisco VA Health Care System.
RESULTS
Residents readmitted to the NH after AMI had a mean age of 83 years; 29% were male, 82% were non-Hispanic white race, and 72% returned to the NH on the Medicare SNF benefit (Table 1 and Supplementary Table S1). Extensive or greater assistance with ADLs was required by 73% of the cohort and some degree of cognitive impairment was present in 83%. Most residents (71%) had at least three comorbidities. Of the 8,349 unique NHs that residents returned to after AMI, 73% were operated for-profit and 70% had 100 beds or more. The number of residents returning to each NH post-AMI ranged from 1 to 22, with an average of two residents per NH.
Table 1.
Selected Characteristics1 of Study Nursing Home Residents (N=15,720)
| Characteristic | n (%) |
|---|---|
| Age in years, mean (SD) | 83 (8) |
| Male | 4,580 (29) |
| Race/ethnicity | |
| White, non-Hispanic | 12,829 (82) |
| Black, non-Hispanic | 1,914 (12) |
| Hispanic | 677 (4) |
| Other | 300 (2) |
| Nursing home length of stay in days, median (IQR) | 570 (160–1277) |
| Primary or secondary diagnoses (prior year) | |
| Congestive heart failure | 7,585 (48) |
| Angina pectoris | 2,028 (13) |
| Unstable angina | 1,634 (10) |
| Asthma | 238 (2) |
| Chronic obstructive pulmonary disease | 4,160 (27) |
| CHESS score (overall health stability) | |
| No instability | 8,836 (56) |
| Minimal instability | 4,524 (29) |
| Low instability | 1,941 (12) |
| Moderate to very high instability | 419 (3) |
| Cognitive performance | |
| Cognitively intact | 2,741 (17) |
| Mild dementia | 5,091 (32) |
| Moderate to severe dementia | 7,888 (50) |
| Activities of daily living status | |
| Independent to limited supervision | 4,304 (27) |
| Extensive assistance required | 7,196 (46) |
| Dependent or totally dependent | 4,220 (27) |
| Statin medications | 4,528 (29) |
| Antiplatelet medications | 2,618 (17) |
| Warfarin | 1,930 (12) |
| Number of Medications (last MDS assessment), mean (SD) | 12 (5) |
| MI index hospitalization characteristics | |
| Length of stay in days, median (IQR) | 6 (4–9) |
| One or more days in CCU or ICU | 9,058 (58) |
| Initial Post-MI Type of Care | |
| Skilled Nursing Facility | 11,283 (72) |
| Long-Term Care | 4,437 (28) |
SD, standard deviation; IQR, interquartile range; MI, myocardial infarction; CCU, coronary care unit; ICU, intensive care unit.
All characteristics measured before the acute myocardial infarction unless otherwise noted.
Of the 15,720 residents in the study population, 8,953 (57%) initiated a beta-blocker after returning to the NH after AMI. The proportion of residents newly prescribed a beta-blocker remained stable in each year of the study period: 56.8% in 2007, 57.3% in 2008, 56.6% in 2009, and 57.2% in 2010 (chi squared p value=0.90). A beta-blocker was dispensed to 60% of those who returned to the NH on the Medicare SNF benefit and 51% of those discharged directly to LTC. There was variation in beta-blocker use by geographic region, ranging from 53.2% of residents in the South to 61.5% of residents in the Northeast receiving beta-blocker post-AMI. The most frequently prescribed beta-blockers were metoprolol (69%), carvedilol (25%), and atenolol (4%).
In univariable analyses, few factors were meaningfully associated with beta-blocker use, with most ORs between 0.9 and 1.1 (Table 2, Table 3, and Supplementary Table S2). Residents aged ≥95 years were less likely to receive beta-blockers (OR 0.68, 95%CI 0.59–0.78) compared to age 65 to 74. A diagnosis of angina pectoris or unstable angina in the year before AMI were predictive of not receiving beta-blockers (OR 0.33, 95%CI 0.30–0.36 for angina pectoris versus no; OR 0.71, 95%CI 0.64–0.79 for unstable angina versus no). Residents who spent any time in a coronary care or intensive care unit (CCU or ICU) during the AMI hospitalization were more likely to receive beta-blockers (OR 1.48, 95%CI 1.37–1.60, for 1–3 days, and OR 1.60, 95%CI 1.49–1.72, for ≥4 days compared to 0 days).
Functional impairment before the AMI was not predictive of beta-blocker use in univariable analyses. However, in multivariable analyses, residents with severe functional impairment were less likely to receive beta-blockers after returning to the NH post-AMI (OR 0.84, 95%CI 0.75–0.94). Although older age was already a significant predictor of beta-blocker non-use in univariable analyses, the strength of the association increased in multivariable analyses. Sex, cognitive functioning, CHESS score, and Elixhauser Comorbidity Index were not independently associated with beta-blocker use (Tables 2 and 3). The broad set of NH characteristics examined was also not independently associated with beta-blocker use (Supplementary Table 2). When the analyses were stratified by initial post-AMI type of NH care, the independent associations between predictors and beta-blocker initiation were similar for residents who returned to the NH through the SNF and LTC pathways of care (data not shown).
DISCUSSION
In this national sample of older nursing home residents, 43% of older nursing home (NH) residents did not receive beta-blockers within 30 days of returning to the NH after acute myocardial infarction (AMI). Some of the variation in beta-blocker use was explained by advanced age and measures of frailty and functional dependence in the NH population. However, few factors were strongly predictive of beta-blocker use.
The absence of characteristics that were strongly predictive of beta-blocker use in our study may suggest a lack of clarity and consensus among providers about how to manage treatment for NH residents after AMI. While beta-blockers are well-tolerated in younger patients and those who are typically included in clinical trials (38–40), little data are available about the tolerability of beta-blockers in those who are frail, physically impaired, multimorbid, or very advanced in age. Without such data, clinicians may have concerns about the safety of these drugs in vulnerable older adults.(38, 41–44) However, it is interesting to note that markers of frailty and vulnerability, like the CHESS score and ability to carry out ADLs, were only weakly predictive in our models while age was more strongly predictive. This suggests that providers are not heavily basing their treatment decisions on functional status or other markers of frailty. Instead, providers might have embraced a patient-centered approach that respects patients’ and their families’ preferences to determine who would receive beta blockers. Our findings reveal an opportunity for future research to improve the evidence base for using beta-blockers post-AMI in the NH setting.
Unstable angina and angina pectoris at baseline were notable exceptions to the absence of strong associations with beta-blocker use. The multivariable-adjusted OR was 0.57 (95%CI 0.50–0.66) for unstable angina and 0.29 (95%CI 0.26–0.34) for angina pectoris. Since both conditions are symptomatic expressions of ischemic heart disease, it could be that many patients with these preexisting diagnoses were previously considered for beta-blocker therapy and rejected (e.g. due to contraindications or intolerance). In this setting, the decision to not prescribe BBs had already been made prior to the AMI, and so was not affected by that event.
Prior studies done in ambulatory populations have reported underutilization of beta-blockers for secondary prevention post-AMI.(3, 10, 12, 14, 45–48) Likewise, studies done in the NH populations have reported underuse.(15–19) Many of the estimates are similar to those of our study even though our data was more recent. For example, Vitagliano and colleagues used Cooperative Cardiovascular Project data from February 1994 through November 1995 to examine beta-blocker use among elderly Medicare beneficiaries discharged from acute care hospitals in the United States after AMI.(14) They found that 50% of patients were prescribed a beta-blocker at the time of discharge, an estimate similar to what we report. The similarity of their estimate to ours, despite their younger and less multimorbid study population, may be due to the use of earlier data reflecting the in-process dissemination of evidence about beta-blocker use in older adults. It may also be due in part to the use of prescribing data, which may have overestimated beta-blocker receipt among those who were not actually dispensed a beta-blocker. The study by Vitagliano and colleagues also reported qualitatively similar relationships between functional status and beta-blocker use, whereby older adults with worse functional status were less likely to receive beta-blockers after AMI. Using the same data as Vitagliano et al., Levy and colleagues isolated a subset of individuals admitted to the hospital from the NH who were “ideally eligible” to receive beta-blockers.(16) They found that 43.8% of NH residents who were ideally eligible were prescribed beta-blockers. In contrast, beta-blockers were prescribed to 61.5% of the ideally eligible community-dwelling cohort upon discharge. Our study confirms that more recent, nationwide prescribing practices are similar to those described in more selected, earlier cohort studies.
This study has some limitations. First, aside from the validation cohort from HCR ManorCare, LLC, beta-blocker use was unobservable during the SNF stay in the NH. As a consequence, we may have misclassified the use of beta-blockers for some NH residents receiving SNF care. However, our validation cohort from HCR ManorCare, LLC, for whom beta-blocker use during SNF stay was observable, suggests that our approach to classifying beta-blocker use will be accurate for all but a small minority of patients. Second, our data was from 2007–2010, but given the lack of substantial changes in guidelines, guideline dissemination, or nursing home standards of practice, it is unlikely that prescribing practices have changed markedly in the intervening years. Third, it is important to note that we focused our study on people who were not using beta-blockers before AMI, so as to evaluate new prescribing decisions about these drugs. Since individuals who use beta-blockers prior to AMI are likely to continue these drugs after AMI, overall use of beta-blockers after AMI is likely to be higher, and we are unable to directly compare our observed rates with other studies that combine incident and prevalent beta-blocker use. Also, our inclusion criteria that patients live for at least 30 days after hospital discharge has potential to create a selection bias, likely enriching our population with more beta-blocker users. However, such bias is likely to be small. Fourth, we excluded people without Medicare fee-for-service coverage, people without Medicare Part D prescription drug insurance, and several others factors, although it is unlikely that insurance coverage has a major impact on use of these inexpensive and ubiquitous medications. Finally, although our variables included several potential contraindications to beta blocker use, including obstructive lung disease and concurrent use of calcium channel blockers with atrioventricular node-blocking activity, our data sources are unable to robustly capture other contraindications such as symptomatic bradycardia or hypotension.
In summary, we report that many elderly NH residents do not receive beta-blockers after AMI. The absence of observed factors that strongly predict beta-blocker use may indicate a lack of consensus on how to manage NH residents with AMI. This is not surprising given the absence of evidence documenting the benefits and harms of beta-blockers in older NH residents, and the relatively low use may suggest ongoing concern about the balance of these benefits and harms. Given practical and ethical considerations, it is unlikely that any randomized controlled trials to study this question will be forthcoming. Rather, rigorous observational studies will be critical for developing an evidence base to evaluate the effectiveness, including mortality and rehospitalization, and potential adverse events, including functional outcomes, of beta-blockers in older NH residents.
Supplementary Material
Selected Characteristics of Study Nursing Home Residents.
Univariable and Multivariable Analysis of Characteristics Associated with Beta-blocker Initiation after Resident Admission to Nursing Home after Acute Myocardial Infarction.
Acknowledgments
Funding: Financial support for this study was provided by the National Heart, Lung, and Blood Institute (5R01HL111032) and National Institute on Aging (K24AG049057). Dr. Zullo is supported by an Agency for Healthcare Research and Quality award (5K12HS022998).
The authors would like to thank HCR ManorCare, LLC, for generously providing data that was used in the study.
Sponsor’s Role: The funding organization had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Appendix
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts |
A.R.Z. | Y.L. | L.A.D. | V.M. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
|
Employment or Affiliation |
X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
|
Personal Relationship |
X | X | X | X | ||||
| Elements of Financial/Personal Conflicts |
W.J.B. | D.D.D. | Y.M. | K.F. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
|
Employment or Affiliation |
X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
|
Personal Relationship |
X | X | X | X | ||||
| Elements of Financial/Personal Conflicts |
K.K. | M.A.S. | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
|
Employment or Affiliation |
X | X | ||
| Grants/Funds | X | X | ||
| Honoraria | X | X | ||
| Speaker Forum | X | X | ||
| Consultant | X | X | ||
| Stocks | X | X | ||
| Royalties | X | X | ||
| Expert Testimony | X | X | ||
| Board Member | X | X | ||
| Patents | X | X | ||
|
Personal Relationship |
X | X | ||
Conflict of Interest Explanations:
V.M.
V.M.’s research is in a related area to that of several different paid activities. V.M. also periodically serves as a paid speaker at national conferences where he discusses trends and research findings in long term and post-acute care. V.M. holds stock of unknown value in PointRight, Inc. an information services company providing advice and consultation to various components of the long term care and post-acute care industry, including suppliers and insurers. PointRight sells information on the measurement of nursing home quality to nursing homes and liability insurers. V.M. was a founder of the company but has subsequently divested much of his equity in the company and relinquished his seat on board. In addition, V.M. Chairs the Independent Quality Committee for HRC Manor Care, Inc., a nursing home chain, for which he receives compensation in the $20,000–$40,000 range. V.M. also serves as chair of a Scientific Advisory Committee for NaviHealth, a post-acute care service organization, for which he also receives compensation in the $20,000–40,000 per year range. V.M. serves as a Technical Expert Panel member on several Centers for Medicare/Medicaid quality measurement panels. V.M. is a member of the board of directors of Tufts Health Plan Foundation; Hospice Care of Rhode Island; and The Jewish Alliance of Rhode Island.
D.D.D.
D.D.D. is an employee of Optum and stockholder in UnitedHealth Group, Optum’s parent company.
M.A.S.
M.A.S. is a paid consultant for iodine.com.
Footnotes
Prior Presentation: This research was presented in part at the 2015 American Geriatrics Society Annual Meeting Epidemiology Paper Session.
Author Contributions: Study concept and design: Steinman, Mor, Boscardin. Acquisition of data: Mor, Steinman, Dore, Zullo, Daiello, Miao, Fung, Komaiko. Analysis of data: Zullo, Lee. Interpretation of results: Zullo, Lee, Daiello, Mor, Boscardin, Steinman, Miao, Fung, Komaiko. Preparation of initial draft of manuscript: Zullo, Steinman. Critical review of manuscript: All authors.
REFERENCES
- 1.Khera S, Kolte D, Gupta T, Mujib M, Aronow WS, Agarwal P, et al. Management and outcomes of ST-elevation myocardial infarction in nursing home versus community-dwelling older patients: a propensity matched study. Journal of the American Medical Directors Association. 2014;15(8):593–599. doi: 10.1016/j.jamda.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. Journal of the American College of Cardiology. 2016;67(20):2419–2440. doi: 10.1016/j.jacc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freemantle N, Cleland J, Young P, Mason J, Harrison J. Beta Blockade after myocardial infarction: systematic review and meta regression analysis. Bmj. 1999;318(7200):1730–1737. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):529–555. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 5.Smith SC, Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 6.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA : the journal of the American Medical Association. 1982;247(12):1707–1714. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 7.The Beta-Blocker Pooling Project (BBPP): subgroup findings from randomized trials in post infarction patients. The Beta-Blocker Pooling Project Research Group. European heart journal. 1988;9(1):8–16. [PubMed] [Google Scholar]
- 8.Gundersen T, Abrahamsen AM, Kjekshus J, Ronnevik PK. Timolol-related reduction in mortality and reinfarction in patients ages 65–75 years surviving acute myocardial infarction. Prepared for the Norwegian Multicentre Study Group. Circulation. 1982;66(6):1179–1184. doi: 10.1161/01.cir.66.6.1179. [DOI] [PubMed] [Google Scholar]
- 9.Improvement in prognosis of myocardial infarction by long-term beta-adrenoreceptor blockade using practolol. A multicentre international study. British medical journal. 1975;3(5986):735–740. doi: 10.1136/bmj.3.5986.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A, Marciniak TA. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA : the journal of the American Medical Association. 1998;280(7):623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. The New England journal of medicine. 1998;339(8):489–497. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- 12.Soumerai SB, McLaughlin TJ, Spiegelman D, Hertzmark E, Thibault G, Goldman L. Adverse outcomes of underuse of beta-blockers in elderly survivors of acute myocardial infarction. JAMA : the journal of the American Medical Association. 1997;277(2):115–121. [PubMed] [Google Scholar]
- 13.Gnjidic D, Bennett A, Le Couteur DG, Blyth FM, Cumming RG, Waite L, et al. Ischemic heart disease, prescription of optimal medical therapy and geriatric syndromes in community-dwelling older men: A population-based study. International journal of cardiology. 2015;192:49–55. doi: 10.1016/j.ijcard.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 14.Vitagliano G, Curtis JP, Concato J, Feinstein AR, Radford MJ, Krumholz HM. Association between functional status and use and effectiveness of beta-blocker prophylaxis in elderly survivors of acute myocardial infarction. Journal of the American Geriatrics Society. 2004;52(4):495–501. doi: 10.1111/j.1532-5415.2004.52153.x. [DOI] [PubMed] [Google Scholar]
- 15.Fahey T, Montgomery AA, Barnes J, Protheroe J. Quality of care for elderly residents in nursing homes and elderly people living at home: controlled observational study. Bmj. 2003;326(7389):580. doi: 10.1136/bmj.326.7389.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy CR, Radcliff TA, Williams ET, Hutt E. Acute myocardial infarction in nursing home residents: adherence to treatment guidelines reduces mortality, but why is adherence so low? Journal of the American Medical Directors Association. 2009;10(1):56–61. doi: 10.1016/j.jamda.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Joseph J, Koka M, Aronow WS. Prevalence of use of antiplatelet drugs, beta blockers, statins, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in older patients with coronary artery disease in an academic nursing home. Journal of the American Medical Directors Association. 2008;9(2):124–127. doi: 10.1016/j.jamda.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Ziesmer V, Aronow WS. Underutilization of aspirin, beta blockers, angiotensin-converting enzyme inhibitors, and lipid-lowering drugs and overutilization of calcium channel blockers in older persons with coronary artery disease in an academic nursing home. The journals of gerontology Series A, Biological sciences and medical sciences. 2002;57(6):M398–M400. doi: 10.1093/gerona/57.6.m398. [DOI] [PubMed] [Google Scholar]
- 19.Aronow WS. Prevalence of use of beta blockers and of calcium channel blockers in older patients with prior myocardial infarction at the time of admission to a nursing home. Journal of the American Geriatrics Society. 1996;44(9):1075–1077. doi: 10.1111/j.1532-5415.1996.tb02941.x. [DOI] [PubMed] [Google Scholar]
- 20.Straker JK, Bailer AJ. A review and characterization of the MDS process in nursing homes. Journal of gerontological nursing. 2008;34(10):36–44. doi: 10.3928/00989134-20081001-01. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Medicare and Medicaid Services. Long-Term Care Facility Resident Assessment Instrument 2.0 User's Manual. 2009 Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/NHQIMDS20.html.
- 22.Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle & nerve. 2001;24(3):403–409. doi: 10.1002/1097-4598(200103)24:3<403::aid-mus1013>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Feng Z, Katz PR, Intrator O, Karuza J, Mor V. Physician and nurse staffing in nursing homes: the role and limitations of the Online Survey Certification and Reporting (OSCAR) system. Journal of the American Medical Directors Association. 2005;6(1):27–33. doi: 10.1016/j.jamda.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Kash BA, Hawes C, Phillips CD. Comparing staffing levels in the Online Survey Certification and Reporting (OSCAR) system with the Medicaid Cost Report data: are differences systematic? The Gerontologist. 2007;47(4):480–489. doi: 10.1093/geront/47.4.480. [DOI] [PubMed] [Google Scholar]
- 25.Intrator O, Hiris J, Berg K, Miller SC, Mor V. The residential history file: studying nursing home residents' long-term care histories(*) Health services research. 2011;46(1 Pt 1):120–137. doi: 10.1111/j.1475-6773.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briesacher BA, Soumerai SB, Field TS, Fouayzi H, Gurwitz JH. Nursing home residents and enrollment in Medicare Part D. Journal of the American Geriatrics Society. 2009;57(10):1902–1907. doi: 10.1111/j.1532-5415.2009.02454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare and Medicaid Services. Medicare Claims Processing Manual, Chapter 6—SNF Inpatient Part A Billing and SNF Consolidated Billing. [cited 2016 April 27]; Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c06.pdf.
- 28.Grebla RC, Keohane L, Lee Y, Lipsitz LA, Rahman M, Trivedi AN. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health affairs. 2015;34(8):1324–1330. doi: 10.1377/hlthaff.2015.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Medical care. 2004;42(4):355–360. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter GI, Hastie CL, Morris JN, Fries BE, Ankri J. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC geriatrics. 2006;6:7. doi: 10.1186/1471-2318-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JN, Fries BE, Mehr DR, Hawes C, Phillips C, Mor V, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 32.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Medical care. 2004;42(4 Suppl):III50–III59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 33.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(11):M546–M553. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 34.Hirdes JP, Frijters DH, Teare GF. The MDS-CHESS scale: a new measure to predict mortality in institutionalized older people. Journal of the American Geriatrics Society. 2003;51(1):96–100. doi: 10.1034/j.1601-5215.2002.51017.x. [DOI] [PubMed] [Google Scholar]
- 35.Mor V, Intrator O, Unruh MA, Cai S. Temporal and Geographic variation in the validity and internal consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0. BMC health services research. 2011;11:78. doi: 10.1186/1472-6963-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vittinghoff E. Regression methods in biostatistics : linear, logistic, survival, and repeated measures models. 2nd. xx. New York: Springer; 2012. p. 509. [Google Scholar]
- 37.Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata. 3rd. College Station, Tex: Stata Press Publication; 2012. [Google Scholar]
- 38.Andrus MR, Loyed JV. Use of beta-adrenoceptor antagonists in older patients with chronic obstructive pulmonary disease and cardiovascular co-morbidity: safety issues. Drugs & aging. 2008;25(2):131–144. doi: 10.2165/00002512-200825020-00005. [DOI] [PubMed] [Google Scholar]
- 39.Mangoni AA. Cardiovascular drug therapy in elderly patients: specific age-related pharmacokinetic, pharmacodynamic and therapeutic considerations. Drugs & aging. 2005;22(11):913–941. doi: 10.2165/00002512-200522110-00003. [DOI] [PubMed] [Google Scholar]
- 40.Ko DT, Hebert PR, Coffey CS, Curtis JP, Foody JM, Sedrakyan A, et al. Adverse effects of beta-blocker therapy for patients with heart failure: a quantitative overview of randomized trials. Archives of internal medicine. 2004;164(13):1389–1394. doi: 10.1001/archinte.164.13.1389. [DOI] [PubMed] [Google Scholar]
- 41.Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. Bmj. 2003;326(7382):196. doi: 10.1136/bmj.326.7382.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips SM, Marton RL, Tofler GH. Barriers to diagnosing and managing heart failure in primary care. Med J Aust. 2004;181(2):78–81. doi: 10.5694/j.1326-5377.2004.tb06178.x. [DOI] [PubMed] [Google Scholar]
- 44.Steinman MA, Patil S, Kamat P, Peterson C, Knight SJ. A taxonomy of reasons for not prescribing guideline-recommended medications for patients with heart failure. The American journal of geriatric pharmacotherapy. 2010;8(6):583–594. doi: 10.1016/S1543-5946(10)80007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rochon PA, Anderson GM, Tu JV, Clark JP, Gurwitz JH, Szalai JP, et al. Use of beta-blocker therapy in older patients after acute myocardial infarction in Ontario. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 1999;161(11):1403–1408. [PMC free article] [PubMed] [Google Scholar]
- 46.Brand DA, Newcomer LN, Freiburger A, Tian H. Cardiologists' practices compared with practice guidelines: use of beta-blockade after acute myocardial infarction. Journal of the American College of Cardiology. 1995;26(6):1432–1436. doi: 10.1016/0735-1097(95)00362-2. [DOI] [PubMed] [Google Scholar]
- 47.Gurwitz JH, Goldberg RJ, Chen Z, Gore JM, Alpert JS. Beta-blocker therapy in acute myocardial infarction: evidence for underutilization in the elderly. The American journal of medicine. 1992;93(6):605–610. doi: 10.1016/0002-9343(92)90192-e. [DOI] [PubMed] [Google Scholar]
- 48.Sial SH, Malone M, Freeman JL, Battiola R, Nachodsky J, Goodwin JS. Beta blocker use in the treatment of community hospital patients discharged after myocardial infarction. Journal of general internal medicine. 1994;9(11):599–605. doi: 10.1007/BF02600301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selected Characteristics of Study Nursing Home Residents.
Univariable and Multivariable Analysis of Characteristics Associated with Beta-blocker Initiation after Resident Admission to Nursing Home after Acute Myocardial Infarction.