Abstract
Background
Nogo-B (Reticulon 4B) is an endoplasmic reticulum (ER) resident protein that regulates ER structure and function. Since ER stress is known to induce M2 macrophage polarization, we examined whether Nogo-B regulates M1/M2 polarization of Kupffer cells and alters the pathogenesis of alcoholic liver disease (ALD).
Methods
M1 and M2 phenotypes were assessed in relation to Nogo-B expression and disease severity in liver specimens from ALD patients (NCT01875211). Liver specimens from wild-type (WT) and Nogo-B knockout (KO) mice fed control or Lieber-DeCarli ethanol liquid diet (5% ethanol) for 6 weeks were analyzed for liver injury and steatosis. Kupffer cells isolated from WT and Nogo-B KO mice were assessed for M1 and M2 activation.
Results
A significant positive correlation was observed between Nogo-B positive Kupffer cells and disease severity in ALD patients (n=30, r=0.66, p=0.048). Further, Nogo-B positive Kupffer cells correlated with M1 activation (iNOS) (r=0.50, p=0.05) and negatively with markers of M2 status (CD163) (r=−0.48, p=0.07) in these patients. WT mice exhibited significantly increased liver injury (p<0.05) and higher hepatic triglyceride levels (p<0.01), compared to Nogo-B KO mice in response to chronic ethanol feeding. Nogo-B in Kupffer cells promoted M1 polarization, whereas absence of Nogo-B increased ER stress and M2 polarization in Kupffer cells.
Conclusion
Nogo-B is permissive for M1 polarization of Kupffer cells, thereby accentuating liver injury in ALD in humans and mice. Nogo-B in Kupffer cells may represent a new therapeutic target for ALD.
Keywords: steatosis, inflammation, ER stress, Reticulon 4B, alcoholic liver disease patients
Chronic alcohol consumption leads to hepatic steatosis, a marker of liver injury. Hepatic steatosis is the abnormal accumulation of lipids in hepatocytes, and may stem from a host of insults with metabolic and viral etiologies being among the most common.(1, 2) Kupffer cells (liver resident macrophages) mediate inflammatory responses in alcoholic liver disease, but also contribute to the development of steatosis in a paracrine manner.(3–7) Macrophages have different functional states with pro-inflammatory M1 type and anti-inflammatory M2 type.(8–10) The mechanisms that govern this M1/M2 polarization remain to be elucidated.
Nogo-B, also known as Reticulon 4B, is an endoplasmic reticulum (ER) resident protein that maintains ER structure.(11–13) Nogo-B belongs to the Reticulon 4 family composed of three isoforms, Nogo-A (200kDa), Nogo-B (55kDa in humans, 45kDa in rats/mice) and Nogo-C (25kDa).(14) Nogo-A and Nogo-C are expressed in the central nervous system(15) with Nogo-C also expressed in skeletal muscles.(15, 16) Nogo-B is expressed in most tissues. In the liver, Nogo-B is the only isoform that has been found and is expressed in non-parenchymal cells and cholangiocytes, but not in hepatocytes.(17) We showed that Nogo-B increases liver fibrosis through facilitating TGFβ signaling (17) and inhibiting apoptosis of myofibroblasts (activated hepatic stellate cells).(18) The role of Nogo-B in Kupffer cells is unknown, although Nogo-B-deficient monocytes/macrophages have defects in cell migration and induction of inflammatory cytokines.(19)
M1 macrophages release pro-inflammatory enzymes and cytokines such as inducible nitric oxide synthase (iNOS), interleukin(IL)-1beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), and can be induced by interferon-gamma (IFNγ) and lipopolysaccharide (LPS).(20–22) Importantly, iNOS, IL1β and TNFα have been reported to enhance hepatic steatosis in alcoholic or non-alcoholic settings.(6, 7, 23) In contrast, M2 macrophages express anti-inflammatory enzymes and cytokines such as CD163, IL-10, arginase-1, scavenging receptor, and mannose receptors, and are implicated in tissue repair, remodeling, and immune regulation. M2 macrophage polarization can be induced by IL-4 and IL-13 stimulation.(20–22) Studies also implicate ER stress as a key factor that causes M2 polarization.(24, 25) Given that Nogo-B is highly expressed in Kupffer cells and that Nogo-B regulates ER structure and function(13), we investigated the role of Nogo-B in M1/M2 Kupffer cell polarization and the development of ethanol-induced steatosis in human liver specimens and mice.
Materials and Methods
Human alcoholic liver specimens
Liver specimens from a clinical trial investigating alcoholic hepatitis were provided by Moon Young Kim (Yonsei University Wonju College of Medicine, South Korea).(26, 27) This patient study excluded patients who showed more than F3 fibrosis grade according to the METAVIR fibrosis scoring system because it focused on hepatic inflammation and early fibrosis than advanced hepatic fibrosis. Patients’ clinical profiles are summarized in Supplementary Table 1. For our study, 30 liver samples were used. Each liver sample was scored in terms of steatosis (0: <5%, 1: 5–33%, 2: 33–66%, 3: >66%), inflammation (0: 0 foci, 1: <2 foci, 2: 2–4 foci, 3: >4 foci per 200x field), ballooning (0: none, 1: few balloon cells, 2: prominent ballooning), necrosis (0: none, 1: mild, 2: moderate, 3: severe) and fibrosis (0: none, 1: perisinusoidal or periportal only, 2: perisinusoidal and periportal, 3: bridging fibrosis, 4: cirrhosis) based on the histological scoring system by Kleiner et al.(28) Then, all values were combined as final liver histological scores, which categorized these samples into three groups.
Mild group (n=10): combine liver histology score from 0 to 4.
Moderate group (n=10): combine liver histology score from 4 to 11.
Severe group (n=10): combine liver histology score from 11 to 14.
Animals
Two-month old, male, Nogo-B knockout (Nogo-A/B −/−, C57BL/6 background) and their age-matched littermate wild-type (WT) mice were used. All animal experiments were approved by the Institutional Animal Care and Use Committees of Yale University and the Veterans Affairs Connecticut Healthcare System, and performed in accordance with the National institutes of Health Guide for the Care and Use of Laboratory Animals. Nogo-A/B KO mice were a gift from Stephen Strittmatter (Yale University, New Haven, CT) and Mark Tessier-Lavigne (Rockefeller University, New York, NY).
Single LPS injection
To induce acute liver inflammation, WT and Nogo-B KO mice were administered a single LPS injection (500ng/g body weight, IP). Nine hours after LPS injection, mice were sacrificed and samples (plasma and liver tissues) were harvested for protein and RNA extraction as well as histological analysis.
Ethanol diet feeding
Wild-type and Nogo-B KO mice were fed with a Lieber-DeCarli liquid diet (F1258SP, Bio-Serv, Flemington, NJ) containing 5% ethanol for 6 weeks. For controls, WT and Nogo-B KO mice were given a control diet (F1259SP, Bio-Serv), which matched ethanol-induced calorie with maltose dextrin. These diets were prepared daily and provided in the late afternoon before a 12-hour dark cycle. Mice were pair-fed with all diets. Nine hours before sacrifice, mice received a single intraperitoneal LPS injection (500ng/g body weight) to promote hepatic inflammation. Liver tissues and plasma were collected.
Histological and immunohistochemical analyses
Formalin-fixed and paraffin-embedded liver tissue blocks were cut into 5µm thick sections and stained with hematoxylin and eosin (H&E) solution for histopathological analysis. All liver tissue slides were scored for hepatic steatosis and lobular inflammation based on a scoring system described by Kleiner DE et al.(28) For immunohistochemistry, paraffin sections were microwaved for 3 minutes and heated in citric acid buffer (pH 6.0) for 30 minutes for antigen retrieval, and blocked with goat serum for 1 hour at room temperature. To investigate the infiltration of neutrophils into liver tissues, immunohistochemical analysis was performed using anti-myeloperoxidase (MPO) antibody (Biocare Medical, Concord, CA) and visualized with avidin-biotin-peroxidase complex method using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and 3,3-diaminobenzidine (Vector Laboratories).
For immunofluorescence of human liver samples, we used anti-Nogo-B antibody conjugated with 555 fluorochrome (red color) and anti-CD68 antibody conjugated with 488 fluorochrome (green color). As the threshold for the differentiation of Nogo-B “high” and “low”, we only counted “yellow color” (red + green = yellow color) as a high signal of Nogo-B and “green color” as a low signal of Nogo-B (Fig. 1B, right panel). Kupffer cells with a high level of Nogo-B are shown in yellow due to the overlap of Nogo-B (red) and CD68+ Kupffer cells (green), while a low level of Nogo-B appears predominantly in green (indicating only CD68 expression). We used 5 fields at 200X magnification per one human liver sample for analysis. Five fields were sufficient to cover almost the entire sample area, since liver biopsies were conducted with a very tiny needle.
Figure 1. Nogo-B levels in Kupffer cells correlate with severity of alcoholic liver disease (ALD) in humans.
(A) Representative images of H&E staining and Nogo-B immunohistochemistry of liver specimens from patients with mild, moderate and severe ALD. Scale bar = 50µm. (B) Representative images of immunohistochemistry and immunofluorescence of Nogo-B (red) and CD68-positive (green) Kupffer cells. Co-localization (yellow) of Nogo-B with Kupffer cells was observed, but Nogo-B expression differed among Kupffer cells. Kupffer cells with low levels of Nogo-B appear predominantly in green (right upper panel), while Kupffer cells with high levels of Nogo-B are shown in yellow (right lower panel). Scale bar = 50µm. (C) Immunofluorescence of Nogo-B and CD68-positive Kupffer cells in mild, moderate and severe ALD. Scale bar = 50µm. (D) A positive correlation was observed between the ratio of Nogo-Bhigh Kupffer cells to total Kupffer cells and ALD severity (n=30, r=0.66, p=0.048).
Measurement of hepatic and plasma triglycerides as well as plasma alanine aminotransferase (ALT
Please refer to Supplementary materials
Macrophage adoptive transfer
Please refer to Supplementary materials
Kupffer cell isolation
Kupffer cells were isolated from WT and Nogo-B KO mice using the collagenase liver perfusion system as previously described.(29) Briefly, the liver was perfused with a digestion buffer containing collagenase for 5 minutes, chopped finely and filtered through a 70µm mesh. The hepatocyte fraction was discarded after centrifugation at 50g for 5 minutes. Non-parenchymal cell fractions were carefully layered on the top of Percoll gradients composed of 25% and 50% Percoll layers. After centrifugation at 2300rpm for 30 minutes, the Kupffer cell fraction was isolated from between the two different Percoll gradient layers. Isolated Kupffer cells were cultured with RPMI media containing 10% FBS for 1–2 days.
Nogo-B silencing in Raw 264.7 cells and induction of ER stress
Raw 264.7 cells (murine macrophage cell lines) were plated on 6-well dish plates at 2×105 cells/well and cultured in a humidified 5% CO2 incubator at 37°C for 24 hours. For transfection of Nogo-B siRNA (Santa Cruz Biotechnology, Santa Cruz, CA), cells were incubated in 750µl Opti-MEM (Life Technologies, Grand Island, NY) containing 50nM Nogo-B siRNA or control scrambled siRNA with 3.75µl of transfection agent (ScreenFect A, Wako Chemicals USA, Richmond, VA) for 6 hours. Then, 750µl of DMEM was added to each well. After 48 hours, the cells were treated with tunicamycin at the final concentration of 2µg/ml for 24 hours to induce ER stress.
LPS or IL-4 stimulation of isolated Kupffer cells and Raw 264.7 cells for M1 and M2 polarization
Isolated Kupffer cells and Raw 264.7 cells were plated on 6-well dish plates at 2×105 cells/well and cultured in a humidified 5% CO2 incubator at 37°C for 24 hours. To determine the effect of Nogo-B deletion on M1 and M2 polarization in Kupffer cells and macrophages, WT and Nogo-B KO Kupffer cells and Raw 264.7 cells with scrambled siRNA or Nogo-B siRNA were incubated with 100ng/ml of LPS, an M1 inducer, or 20ng/ml of IL-4, an M2 inducer, for 24 hours.
Immunofluorescence
Liver tissues fixed in 10% neutral buffered formalin were processed routinely and embedded in paraffin. Paraffin-embedded tissues were sectioned at thickness of 5µm. For immunofluorescence of isolated Kupffer cells, the cells were plated on coverslips and fixed in 4% paraformaldehyde (PFA) for 15 minutes. For immunofluorescence of CD68 (1:100; MCA1957; AD Serotec; Raleigh, NC) and CHOP (1:200; 2895; Cell sign; Beverly, MA.), liver tissues were fixed in 4%-PFA, dehydrated in 30% sucrose solution and sectioned at thickness of 6µm. After washing with phosphate-buffered saline (PBS), liver sections and cells were incubated with 0.1% Triton X-100 in PBS for 20 minutes for permeabilization. Then, sections or cells were blocked with 5% donkey serum for 1 hour, followed by incubation with primary antibodies including goat anti-Nogo-B (1:200, Santa Cruz Biotechnology, Dallas, TX), rabbit anti-CD68 IgG (1:200, Santa Cruz Biotechnology), mouse anti-iNOS (1:200, Santa Cruz Biotechnology), mouse anti-CD163 (Santa Cruz Biotechnology) and mouse anti-BiP/GRP78 (1:100, BD biosciences, San Jose, CA) overnight at 4°C. After washing with PBS three times each for 5 minutes, samples were treated with Alexa Fluor 488 donkey anti-goat IgG (1:500, Life Technologies), Alexa Fluor 488 donkey anti-rabbit IgG (1:500, Life Technologies), Alexa Fluor 555 donkey anti-mouse IgG (1:500, Life Technologies), Alexa Fluor 647 donkey anti-rabbit IgG (1:500, Life Technologies), Alexa Fluor 647 donkey anti-mouse IgG (1:500, Life Technologies), or biotin anti-rat with Alexa Fluor 568 streptavidin (1:1000, Life Technologies) for 1 hour at room temperature. Some Kupffer cells were incubated with Alexa rhodamine phalloidin (1:500, Life Technologies) for 30 minutes at room temperature. Sections and cells on coverslips were mounted with Antifade Reagent with DAPI (Cell Signaling Technology, Danvers, MA).
Quantitative real-time polymerase chain reaction
Please refer to Supplementary materials and Supplementary Table 2
Western blot analysis
Please refer to Supplementary materials
Statistical analysis
Data obtained from all experiments were expressed as mean ± standard error of mean (SEM). Statistical significance among multiple groups was determined by performing one-way analysis of variance (ANOVA) or Student’s t test. P values < 0.05 were considered statistically significant.
Results
Elevated levels of Nogo-B are found in Kupffer cells in patients with alcoholic liver disease and correspond to disease severity
Immunohistochemistry of liver specimens from patients with alcoholic liver disease (ALD) demonstrated that Nogo-B levels dramatically increased with severity of ALD (Fig. 1A). Nogo-B was mainly found in sinusoidal areas but not in hepatocytes (Fig. 1A). Immunofluorescent staining showed co-localization of Nogo-B with CD68, a Kupffer cell marker in the liver. However, the levels of Nogo-B expression varied among Kupffer cells (Fig. 1B). The ratio of Kupffer cells exhibiting high levels of Nogo-B to the total Kupffer cells correlated (r=0.66, p=0.048) with severity of ALD evaluated by histopathological scores (Figs. 1C & D). The total number of Kupffer cells did not change with disease severity (Supplementary Fig. 1). Further, those Kupffer cells tended to show crown-like structure (Fig. 1C, arrows and insets), which is typically observed in advanced steatohepatitis.(30) These results indicate that Nogo-B labeling in Kupffer cells increases with the severity of ALD.
Kupffer cells with high Nogo-B levels correlate positively with M1 type Kupffer cells and negatively with M2 type Kupffer cells in patients with ALD
To determine whether a correlation between Nogo-B expression and Kupffer cell polarization was present, we examined Nogo-B, iNOS (M1 marker) and CD163 (M2 marker) labeling of liver specimens from ALD patients with varying degrees of inflammation. Both Nogo-B and iNOS levels increased with severity of ALD (Fig. 2A). iNOS-positive Kupffer cells correlated positively with Nogo-BHigh Kupffer cells (r=0.50, p=0.05) (Fig. 2B) and negatively with Nogo-BLow Kupffer cells (r=−0.56, p=0.03) (Fig. 2C). In contrast, CD163 levels decreased with severity of ALD (Fig. 2D). CD163-positive Kupffer cells correlated negatively with Nogo-BHigh Kupffer cells (r=−0.48, p=0.07) (Fig. 2E) and positively with Nogo-BLow Kupffer cells (r=0.51, p=0.05) (Fig. 2F). These data suggest that Nogo-B levels in Kupffer cells have a role in M1/M2 polarization of Kupffer cells in ALD.
Figure 2. Kupffer cells with high levels of Nogo-B correlate positively with M1 type Kupffer cells and negatively with M2 type Kupffer cells in patients with ALD.
(A) Representative immunofluorescence images of Nogo-B (green), iNOS (red) and CD68 (purple, a macrophage marker). Scale bar = 50µm. (B) A positive correlation between the number of Nogo-Bhigh Kupffer cells and iNOS-positive (M1 type) Kupffer cells (n=15, p=0.05). (C) A negative correlation between the number of Nogo-Blow Kupffer cells and iNOS-positive Kupffer cells (n=15, p=0.03). (D) Representative immunofluorescence images of Nogo-B (green), CD163 (red) and CD68 (purple). Scale bar = 50µm. (E) A negative correlation between the number of Nogo-Bhigh Kupffer cells and CD163-positive (M2 type) Kupffer cells (n=15, p=0.07). (F) A positive correlation between the number of Nogo-Blow Kupffer cells and CD163-positive Kupffer cells (n=15, p=0.05).
Nogo-B increases ethanol-induced hepatic steatosis and injury in mice
We next examined whether genetic deletion of Nogo-B in mice altered ethanol-induced hepatic steatosis and injury. WT and Nogo-B KO mice were fed the Lieber-DeCarli (LD) ethanol or control diet for 6 weeks. Histological analysis demonstrated that ethanol feeding significantly increased hepatic steatosis in both WT (steatosis score = 2.0) and Nogo-B KO mice (steatosis score = 0.83), compared to their respective control pair-fed mice (WT: steatosis score = 0.16; Nogo-B KO: steatosis score = 0) (Figs. 3A & B). However, WT mice showed significantly higher steatosis (2.4-fold, p<0.05) than Nogo-B KO mice (Fig. 3B). Livers from WT mice exhibited macrovesicular steatosis that was predominantly peri-central (Fig. 3A, inset, black arrows). In contrast, mild fatty change was observed in the livers of Nogo-B KO mice (Fig. 3A, inset, yellow arrows). Consistent with the histological findings, hepatic and plasma triglyceride levels were significantly higher in WT mice (2.5-fold, p<0.01 and 1.57-fold, p<0.01, respectively) than Nogo-B KO mice in response to chronic ethanol diet feeding (Figs. 3C & D).
Figure 3. Nogo-B increases ethanol-induced hepatic steatosis in mice.
(A) Representative images of H&E staining of liver specimens from WT and Nogo-B KO mice fed control or Lieber-DeCarli ethanol (5% ethanol) diet for 6 weeks. Significantly higher fat accumulation was observed around the central vein area of WT livers (macrovesicular fatty changes, black arrows) compared to Nogo-B KO livers (mild fatty changes, yellow arrows). Scale bar = 100µm. (B) Histological scores of steatosis. (C) Hepatic triglyceride (TG) levels (mg/g of liver). (D) Plasma TG levels (mg/dl). All data are shown as mean ± SEM (n=3–6 per group, *p<0.05, **p<0.01).
Further, hepatic inflammation scores (Fig. 4A, p=0.05), plasma ALT levels (Fig. 4B, p=0.05), and neutrophil infiltration assessed by immunohistochemistry of myeloperoxidase (MPO) (Fig. 4C, p<0.01) were all significantly higher in WT mice than Nogo-B KO mice.
Figure 4. Nogo-B increases ethanol-induced liver inflammation in mice.
Samples were collected from WT and Nogo-B KO mice fed control or Lieber-DeCarli ethanol (LD) diet for 6 weeks. (A) Histological scores of hepatic lobular inflammation. (B) Plasma ALT levels (IU/L). (C) Representative photomicrographs of myeloperoxidase (MPO) immunohistochemistry (IHC) as a marker of neutrophils. Scale bar = 100µm. Quantification of MPO-positive cells (right panel). MPO-positive cells were counted in >10 fields of 400× magnification for each sample and the mean number of MPO-positive cells was compared. All data are shown as mean ± SEM (n=3–6 per group, **p<0.01). (D) Experimental scheme. NGB: Nogo-B, BMMϕ: bone marrow macrophages. (E) FACS plots of Kupffer cells/macrophages (CD45.2) isolated from CD45.1 recipient mouse. (F) H&E staining of livers and plasma ALT values in recipient mice fed LD diet for 10 days followed by gavage administration of ethanol (31.5%, body weight × 0.02ml). Scale bar; 50µm. (G) MPO levels as an indicator of neutrophil infiltration and inflammation. Frozen liver sections were stained for myeloperoxidase (pink) and nucleus (blue). n=4. Scale bar; 30µm.
Supplementary Fig. 2 shows the expression of Nogo-B in the liver of mouse fed control or LD diet. Nogo-B was highly positive in non-parenchymal cells and cholangiocytes, but not in hepatocytes, except for some hepatocytes around the portal vein in the LD diet group with very faint staining. These observations are consistent with our previous studies.(17, 31)
Nogo-B in Kupffer cells facilitates ethanol-induced liver injury in mice
We performed adoptive transfer of macrophages, in which bone marrow-derived macrophages from WT and Nogo-B KO mice were injected to B6 CD45.1 mice (The Jackson Laboratory, Bar Harbor, ME)(Fig. 4D). These mice were fed with LD diet for 10 days, followed by gavage administration of ethanol in accordance with the NIAAA model.(32) Fig. 4E shows that approximately 30% of F4/80-positive Kupffer cells/macrophages in the recipient mouse originated from the donor mouse. Nogo-B KO macrophage adoptive transfer demonstrated reduced liver injury, indicated by H&E staining and lower ALT values (p=0.01) (Fig. 4F), and MPO levels (p=0.002) as an indicator of neutrophil infiltration and inflammation (Fig. 4G), compared to WT macrophages.
Further, a co-culture experiment demonstrated significantly higher levels of triglycerides (by 30%, p<0.05) in hepatocytes incubated with WT Kupffer cells than those with Nogo-B KO Kupffer cells (Supplementary Fig. 3). This also suggests that Nogo-B in Kupffer cells promotes hepatic steatosis. Furthermore, despite similar levels of Nogo-B expression between LSECs and Kupffer cells in response to LPS, co-culture of hepatocytes with conditioned medium collected from LSECs of WT or Nogo-B KO mice resulted in no differences in fat accumulation in hepatocytes (Supplementary Fig. 4). These results indicate that the presence of Nogo-B in LSECs is not likely to be a factor in the lipid accumulation in hepatocytes.
Nogo-B promotes M1 and inhibits M2 polarization of Kupffer cells in mice fed ethanol
We determined expression of a pro-inflammatory M1 macrophage marker (iNOS) and anti-inflammatory M2 macrophage markers, including IL10, CD163, MRC1 and MRC2, in livers from WT and Nogo-B KO mice fed LD or control diet. As shown in Fig. 5A, ethanol-fed WT mice demonstrated significantly increased numbers of iNOS-positive Kupffer cells compared to ethanol-fed Nogo-B KO mice. In contrast, WT livers exhibited decreased expression of IL-10 (2.2-fold, p<0.05), CD163 (2.9-fold, p<0.05), MRC1 (2.2-fold, p=0.09) and MRC2 (2–fold, p=0.09), compared to KO livers in response to ethanol (Fig. 5B).
Figure 5. Nogo-B increases M1 and decreases M2 polarization of Kupffer cells in mice fed ethanol.
(A) Representative immunofluorescence images of iNOS (red) and F4/80 (green, a macrophage marker) in liver specimens from WT and Nogo-B KO mice fed Lieber-DeCarli ethanol diet for 6 weeks. WT livers showed significantly increased iNOS (an M1 marker) induction in Kupffer cells with ethanol feeding. Scale bar = 50µm. The bar chart represents the number of iNOS and F4/80 positive Kupffer cells per field at 200× magnification. (B) Expression of IL-10 (an inducer of M2 polarization) and M2 markers (CD163, MRC1 and MRC2) in liver tissues of WT and Nogo-B KO mice fed control or Lieber-DeCarli ethanol diet for 6 weeks. WT livers exhibited significantly decreased levels of IL-10 and CD163 genes in response to ethanol. (C) Expression of pro-inflammatory genes and anti-inflammatory genes in liver tissues isolated from WT and Nogo-B KO mice 9 hours after single LPS (500ng/g) ip injection. Nogo-B increased pro-inflammatory gene levels and decreased anti-inflammatory gene levels. All data are shown as mean ± SEM (n=3–6 per group, *p<0.05, **p<0.01).
The same pattern was observed in Kupffer cells isolated from WT and Nogo-B KO mice fed LD diet for 6 weeks. FACS analysis demonstrated higher iNOS (M1, p=0.002) and lower arginase-1 (M2, p=0.0007) levels in WT Kupffer cells and lower iNOS (M1) and higher arginase-1 (M2) levels in Nogo-B KO Kupffer cells (Supplementary Fig. 5).
Given that elevated blood levels of LPS are a hallmark of alcoholic liver disease(33–35) and that LPS is a major inducer of M1 polarization(25), we also determined M1/M2 polarization in WT and Nogo-B KO mice in response to LPS (500ng/g body weight). Nine hours after LPS injection, WT livers demonstrated increased expression of pro-inflammatory genes, including iNOS (0.4-fold, p=0.06), IL-6 (0.69-fold, p=0.06), MIP-2a (0.58-fold, p<0.05), MIP-1a (0.6-fold, p<0.05), MIP-1b (0.65-fold, p<0.05), E-selectin (CD62E) (0.57-fold, p<0.05), and CD11b (0.69-fold, p<0.05), compared to Nogo-B KO livers (Fig. 5C). In contrast, anti-inflammatory genes such as arginase-1 (ARG-1) and IL-10 were significantly higher in Nogo-B KO livers than WT livers (Fig. 5C). FACS analysis confirmed this trend with Kupffer cells isolated from WT and Nogo-B KO mice treated with LPS, showing higher levels of IL1β (p<0.0001) and TNFα (p<0.0001) in WT Kupffer cells than in Nogo-B KO Kupffer cells (Supplementary Fig. 6). These results support the notion that Nogo-B regulates pro-inflammatory M1 Kupffer cell polarization in ALD. Absence of Nogo-B therefore promotes Kupffer cell polarization toward M2 and away from M1 polarization.
Nogo-B promotes M1 polarization of Kupffer cells in vitro
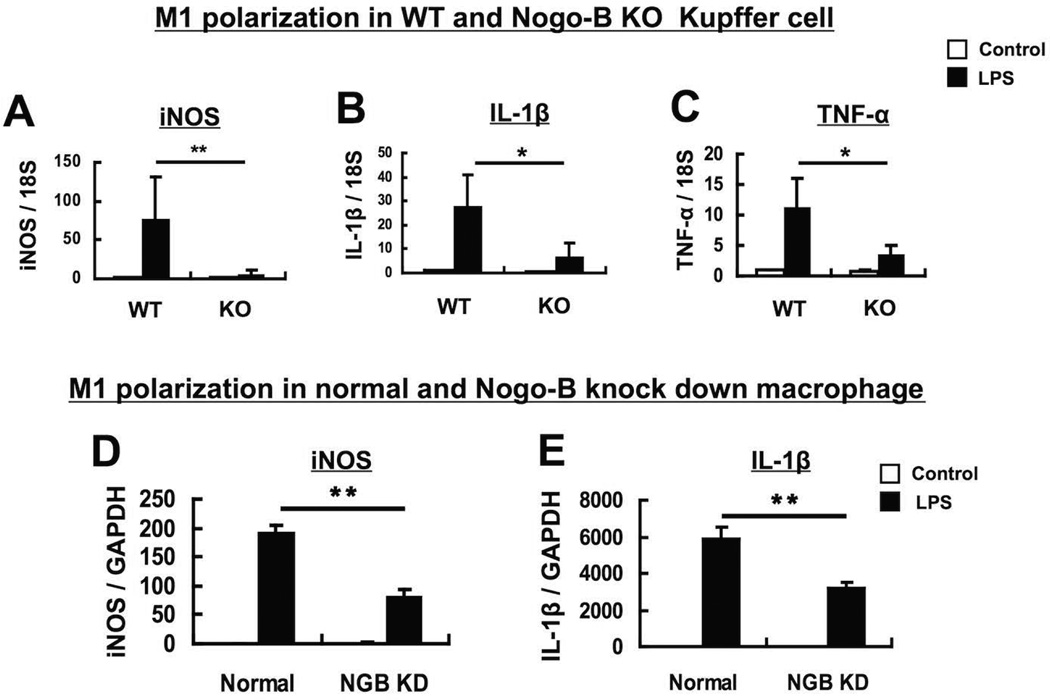
The role of Nogo-B in Kupffer cell activation was next confirmed in vitro using primary Kupffer cells and RNA interference of endogenous Nogo-B. Kupffer cells were isolated from WT and Nogo-B KO mice and treated with LPS (100ng/ml), a strong M1 inducer, for 24 hours. Then, expression of M1 type macrophage markers, iNOS, IL-1β and TNF-α, were examined. Expression of all these M1 markers was significantly increased in WT Kupffer cells [12.8-fold for iNOS (p<0.01), 3.9-fold for IL-1β (p<0.05), and 3.1-fold for TNF-α (p<0.05)], compared to Nogo-B KO Kupffer cells (Figs. 6A, B & C). Further, silencing Nogo-B by Nogo-B siRNA significantly decreased expression of iNOS (1.7-fold, p<0.01) and IL-1β (1.8-fold, p<0.01) in a macrophage cell line (Raw 264.7 cells) in response to LPS treatment at 100ng/ml for 24 hours (Figs. 6D & E). These results indicate that Nogo-B regulates markers of M1 activation.
Figure 6. Nogo-B increases expression of M1-type (pro-inflammatory) genes in Kupffer cells and macrophages in vitro.
Kupffer cells were isolated from WT and Nogo-B KO mice (A to C). WT Kupffer cells showed significantly increased expression of iNOS (A), IL-1β (B) and TNF-α (C), compared to Nogo-B KO Kupffer cells in response to LPS (100ng/ml), a strong M1 inducer, for 24 hours. Nogo-B expression was suppressed by Nogo-B siRNA in Raw 264.7 cells (a macrophage cell line) (D & E). Nogo-B silencing resulted in significantly decreased expression of iNOS (D) and IL-1β (E) in response to LPS (100ng/ml) for 24 hours. Data were normalized against 18S ribosomal RNA expression (A to C) or GAPDH mRNA expression (D & E), and are shown as mean ± SEM from 3–5 independent experiments (*p<0.05, **p<0.01).
Lack of Nogo-B facilitates M2 polarization of Kupffer cells through increased ER stress
Immunofluorescence microscopy of livers from ethanol-fed WT and Nogo-B KO mice showed a prominent increase in the levels of BiP/GRP78, an ER stress marker, in WT hepatocytes (Fig. 7A). Since Nogo-B is not expressed in hepatocytes (17), the increase in BiP labeling in WT hepatocytes may reflect the 2.5-fold increase in lipid accumulation found in WT hepatocytes compared to those from Nogo-B KOmice. In sharp contrast, levels of BiP/GRP78 in Kupffer cells were much higher in Nogo-B KO livers than WT livers (Fig. 7A, arrowheads) (5–fold, p<0.01). Immuno-labeling of CHOP, another ER stress marker, and CD68 demonstrated significantly increased levels of CHOP-positive Kupffer cells in Nogo-B KO mice fed LD diet, compared to their WT counterparts (Fig. 7B, arrowheads, 2-fold, p=0.005), indicating increased ER stress in Kupffer cells in the absence of Nogo-B.
Figure 7. Nogo-B decreases ethanol-induced ER stress in Kupffer cells, which determines M1 versus M2 polarization.
(A) Representative immunofluorescence images of BiP/GRP78 (red, an ER stress marker) in liver tissues of WT and Nogo-B KO mice fed control or Lieber-DeCarli ethanol diet for 6 weeks. Scale bar = 20µm. The bar chart represents the number of BiP-positive Kupffer-like cells per field at 200× magnification. (B) Representative immunofluorescence images of CHOP (green, an ER stress marker) and CD68 (red, a macrophage marker) in liver tissues of WT and Nogo-B KO mice fed control or Lieber-DeCarli ethanol diet for 6 weeks. Scale bar = 20µm. The bar chart represents the percentage of CHOP-positive Kupffer cells to the total Kupffer cells per field at 200× magnification. n=5. (C) A murine macrophage cell line (Raw 264.7 cell) was treated with tunicamycin (2µg/ml), an ER stress inducer, for 24 hours after scrambled control siRNA or Nogo-B siRNA treatment for 48 hours. Western blot analysis for control and Nogo-B silenced macrophages after tunicamycin treatment. Silencing Nogo-B in macrophages significantly increased BiP levels in response to tunicamycin. The bar chart represents quantification of BiP levels. (n=3 per group, **p<0.01) (D) Nogo-B decreased BiP levels in response to IL4, an M2 inducer, in vitro. Twenty four hours after isolation, WT and Nogo-B KO Kupffer cells were treated with IL-4 (20ng/ml) for 24 hours. Protein levels of BiP, Nogo-B and β-actin were assessed by western blot analysis. The bar chart represents quantification of BiP levels. (n=3 per group, **p<0.01). (E) Kupffer cells were isolated from WT and Nogo-B KO mice. WT Kupffer cells showed significantly decreased expression of M2 markers, IL-10, CD163 and MRC1, compared to Nogo-B KO Kupffer cells in response to IL-4 (20ng/ml) for 24 hours. All data are shown as mean ± SEM (n=3 per group, *p<0.05, **p<0.01). (F) Raw 264.7 cells were treated with tunicamycin (2ug/ml) for 2 hours before LPS treatment (100ng/ml) for 24 hours. Tunicamycin treatment significantly decreased mRNA levels of M1 markers, iNOS, IL-1β and TNF-α, but increased CD163, an M2 marker. All data are shown as mean ± SEM (n=3 per group, *p<0.05, **p<0.01).
Consistent with these findings from liver sections, Nogo-B knockdown using Nogo-B siRNA increased BiP/GRP78 (by 25%, p<0.01, Fig. 7C) and CHOP (6–fold, p<0.01, Supplementary Fig. 7) in Raw 264.7 macrophages in response to ER stress inducers, tunicamycin (2ug/ml for 24 hours) and thapsigargin (1 µM, 2 hours), respectively. Tunicamycin had no toxicity toward Raw 264.7 cells at 2µg/ml over the time frame of the experiment (24 hours). This concentration was chosen as an optimal concentration after multiple concentrations (0.5, 1, 2, 5, 10 and 20µg/ml) were tested. These findings indicate that Nogo-B mediates ER stress in Kupffer cells in the setting of chronic ethanol feeding.
To further examine the link among Nogo-B, ER stress and M2 polarization, Kupffer cells from WT and Nogo-B KO mice were treated with IL-4 (20ng/ml for 24 hours) to induce M2 polarization, and ER stress was determined. BiP/GRP78 levels were significantly higher in Nogo-B KO Kupffer cells than in WT Kupffer cells (Fig. 7D). Further, expression of an anti-inflammatory cytokine, IL-10 (2.9-fold, p<0.05), and M2 markers, CD163 (22.1-fold, p<0.01) and mannose receptor C type 1 (MRC1) (2.1-fold, p<0.01), was significantly increased in Nogo-B KO Kupffer cells in response to IL-4, compared to WT Kupffer cells (Fig. 7E).
To determine the effect of ER stress on M1 polarization, Raw 264.7 cells were treated with tunicamycin for 2 hours before treatment with LPS, an M1 inducer, for 24 hours. Tunicamycin-pretreatment decreased expression of iNOS (3.5-fold, p<0.01), IL-1β (16–fold, p<0.01) and TNF-α (16.6-fold, p<0.01), but increased CD163 (4–fold, p=0.06) even in the presence of LPS (Fig. 7F).
Along with a study that showed CHOP to induce M2 polarization(25), our results suggest that lack of Nogo-B facilitates cytokine-induced M2 polarization of Kupffer cells by accentuating ER stress.
Discussion
This study demonstrated that Nogo-B accentuates injury in alcoholic liver disease (ALD) by promoting M1 polarization and inhibiting M2 polarization in Kupffer cells. Liver specimens from patients with ALD showed that Nogo-B levels increased with severity of hepatic injury and steatosis. Further, the human liver specimens showed that Nogo-B levels in Kupffer cells are positively correlated to M1 polarization and negatively to M2 polarization. Consistent with the human data, presence of Nogo-B in mice resulted in: (1) increased hepatic steatosis and inflammation; (2) increased M1 polarization; (3) decreased M2 polarization; and (4) reduced ER stress levels in Kupffer cells in response to ethanol. Mechanistically, our study suggests that increased ER stress in Kupffer cells due to absence of Nogo-B promotes M2 polarization of Kupffer cells and decreases liver steatosis, inflammation and injury in response to ethanol feeding.
Macrophage polarization plays an important role in disease progression and regression.(8, 9, 36) M1 macrophages express high levels of pro-inflammatory enzymes and cytokines, such as iNOS, IL1β, TNFα and IL-12, while M2 macrophages induce anti-inflammatory enzymes and cytokines, including arginase, CD163 and IL-10. Multiple factors are involved in M1/M2 polarization. Among such factors, ER stress is known as a critical inducer of M2 polarization.(24, 25) A prior study showed that ER stress was necessary to generate M2 phenotype through JNK activation and increased peroxisome proliferator-activated receptor gamma (PPARγ) expression.(25) M2 Kupffer cell polarization by C/EBP homologous protein (CHOP)-induced ER stress was reported to ameliorate steatohepatitis in mice.(37, 38) These studies support our findings. Our result demonstrating inhibition of LPS-induced M1 polarization by tunicamycin pretreatment (Fig. 7F) may be important. This suggests that ER stress not only facilitates M2 polarization, but also suppresses M1 polarization, implicating ER stress as a critical determinant of M1/M2 polarization of Kupffer cells.
Nogo-B, as an ER resident protein, plays an essential role in maintaining ER structure. Lack of Nogo-B leads to a reduction in the number of ER tubules and an expansion of peripheral sheets(12, 13) One function of ER sheets is to aid in the unfolded protein response (UPR)(11), which may predispose to developing ER stress in response to stimuli.(13) This may explain higher ER stress seen in Nogo-B KO cells. Further investigation of ER structure in the presence and absence of Nogo-B may identify specific structural differences that promote ER stress responses.
A previous study demonstrated that BiP suppresses ER stress in hepatocytes and reduces hepatic steatosis in obese rodents.(39) In our study, despite increased levels of BiP in Nogo-B KO Kupffer cells, BiP did not appear to protect Nogo-B KO Kupffer cells from ER stress. This was indicated by increased levels of CHOP, another ER stress marker, in Kupffer cells in livers from Nogo-B KO mice with LD diet (Fig. 7B) and in macrophages with Nogo-B suppression as presented in Supplementary Fig. 8. The reduced hepatic steatosis and injury associated with lack of Nogo-B may be related at least in part to increased M2 polarization through ER stress/CHOP mediated signaling. Further studies evaluating the relationship between Nogo-B and ER stress may suggest a broader role for nogo-B in a broad array of disease processes where ER stress is a prominent feature.
This study has identified Nogo-B as a novel regulator of Kupffer cell polarization in response to ethanol and a promoter of ethanol-induced hepatic steatosis. We previously demonstrated that Nogo-B regulates liver fibrosis/cirrhosis.(17, 18) Global deletion of Nogo-B gene in mice resulted in decreased liver fibrosis/cirrhosis and prevented the development of portal hypertension.(17, 18) Plasma Nogo-B levels were also found increased in cirrhotic patients, suggesting a role of Nogo-B in the progression of liver cirrhosis.(40) The current study, along with these other observations, suggest that targeted deletion of Nogo-B in Kupffer cells could be a novel therapeutic strategy for ethanol-induced hepatic injury.
This study identifies a paracrine-mediated effect of Kupffer cells on hepatic lipid accumulation. Chronic alcohol consumption leads to lipid accumulation in hepatocytes by increasing fatty acid synthesis, decreasing fatty acid oxidation, and reducing triglyceride export from the liver.(41, 42) The role of non-parenchymal cells in mediating lipid accumulation is less understood. Kupffer cells have crucial roles in the inflammatory responses of alcoholic liver disease, but also appear to contribute to the development of steatosis in a paracrine manner.(3–7) Depletion of Kupffer cells in mice by clodronate liposomes was reported to decrease steatosis in a non-alcoholic setting.(6) Pro-inflammatory M1 markers such as iNOS, IL1β and TNFα have also been implicated in the development of alcoholic and non-alcoholic hepatic steatosis.(6, 7, 23, 43) Cannabinoid CB2 receptor in Kupffer cells reduced ethanol-induced hepatic steatosis by inhibiting M1 polarization and favoring M2 polarization.(38) Further, M2 Kupffer cells were shown to facilitate apoptosis of M1 Kupffer cells, thereby mitigating hepatic steatosis.(22) Given that Nogo-B is not expressed in hepatocytes, the current study demonstrates that Kupffer cells can contribute to hepatic steatosis in a paracrine manner.
We have suggested that Nogo-B regulates Kupffer cell polarization through ER stress. However, there may be other mechanisms by which Nogo-B exerts its effect on Kupffer cell polarization. For example, IL4 is known to induce M2 polarization not only through ER stress but also through other pathways including the induction of oxidative stress, autophagy and PPARγ.(24, 25) Nogo-B may also be involved in these processes, thereby contributing to Kupffer cell polarization. Similarly, presence of Nogo-B resulted in increased levels of M1 markers such as iNOS, IL1β and TNFα in response to ethanol. These pro-inflammatory enzyme and cytokines are induced by NFkB. Therefore, Nogo-B may also facilitate M1 polarization through NFkB activation. These possibilities should be examined to further clarify the role of Nogo-B in Kupffer cell polarization.
In conclusion, we have demonstrated that Nogo-B worsens the severity of alcoholic liver disease by promoting M1 polarization and inhibiting M2 polarization in Kupffer cells. Histological assessment of Nogo-B levels and its association with severity in patients with ALD confirms this association. Therefore, targeted deletion of Nogo-B in Kupffer cells may represent a potential therapeutic strategy for alcoholic liver disease.
Supplementary Material
Acknowledgments
The authors thank Kathy Harry and the Yale Liver Center for assistance of Kupffer cell isolation and Jinah Han for technical assistance.
Financial support: This work was supported by NIH grants R01 DK082600, P30 DK045735, R21AA023599 and Connecticut DPH grant #2015-0901 (YI), VA Merit Grant (CC), and JSPS KAKENHI 26440055 of Ministry of Education, Sport, Culture, Science and Technology in Japan (AS).
Footnotes
Competing interests: None
Contributors: JP & MS performed experiments, analyzed data and wrote the manuscript; MK collected patients’ samples, analyzed patients’ data and wrote the manuscript; SB collected patients’ samples and analyzed data; MC prepared patients’ samples and performed pathological analysis; TU planned and performed experiments and critically revised the manuscript; AS planned experiments and revised the manuscript; XO supervised experiments; CC critically revised the manuscript; YI planned and supervised the study, revised and approved the manuscript.
References
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen JI, Nagy LE. Pathogenesis of alcoholic liver disease: interactions between parenchymal and non-parenchymal cells. J Dig Dis. 2011;12:3–9. doi: 10.1111/j.1751-2980.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797. doi: 10.1002/cphy.c120026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju C, Mandrekar P. Macrophages and Alcohol-Related Liver Inflammation. Alcohol Res. 2015;37:151–162. [PMC free article] [PubMed] [Google Scholar]
- 6.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, et al. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 7.Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-alpha production. J Biol Chem. 2012;287:40161–40172. doi: 10.1074/jbc.M112.417014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman JR, Voeltz GK. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 2011;21:709–717. doi: 10.1016/j.tcb.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell. 2006;124:573–586. doi: 10.1016/j.cell.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 13.Jozsef L, Tashiro K, Kuo A, Park EJ, Skoura A, Albinsson S, Rivera-Molina F, et al. Reticulon 4 is necessary for endoplasmic reticulum tubulation, STIM1-Orai1 coupling, and store-operated calcium entry. J Biol Chem. 2014;289:9380–9395. doi: 10.1074/jbc.M114.548602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oertle T, Schwab ME. Nogo and its paRTNers. Trends Cell Biol. 2003;13:187–194. doi: 10.1016/s0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 15.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 2002;22:3553–3567. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 17.Zhang D, Utsumi T, Huang HC, Gao L, Sangwung P, Chung C, Shibao K, et al. Reticulon 4B (Nogo-B) is a novel regulator of hepatic fibrosis. Hepatology. 2011;53:1306–1315. doi: 10.1002/hep.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tashiro K, Satoh A, Utsumi T, Chung C, Iwakiri Y. Absence of Nogo-B (reticulon 4B) facilitates hepatic stellate cell apoptosis and diminishes hepatic fibrosis in mice. Am J Pathol. 2013;182:786–795. doi: 10.1016/j.ajpath.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Fernandez-Hernando C, Suarez Y, Schleicher M, Hao Z, Wright PL, DiLorenzo A, et al. Reticulon 4B (Nogo-B) is necessary for macrophage infiltration and tissue repair. Proc Natl Acad Sci U S A. 2009;106:17511–17516. doi: 10.1073/pnas.0907359106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 21.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–142. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 23.Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid Redox Signal. 2011;14:2121–2135. doi: 10.1089/ars.2010.3263. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor N, Niu J, Saad Y, Kumar S, Sirakova T, Becerra E, Li X, et al. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, et al. Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation. J Biol Chem. 2012;287:11629–11641. doi: 10.1074/jbc.M111.338673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang YO, Kim YJ, Baik SK, Kim MY, Eom YW, Cho MY, Park HJ, et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver Int. 2014;34:33–41. doi: 10.1111/liv.12218. [DOI] [PubMed] [Google Scholar]
- 27.Moon KM, Kim G, Baik SK, Choi E, Kim MY, Kim HA, Cho MY, et al. Ultrasonographic scoring system score versus liver stiffness measurement in prediction of cirrhosis. Clin Mol Hepatol. 2013;19:389–398. doi: 10.3350/cmh.2013.19.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Soroka CJ, Mennone A, Rahner C, Harry K, Pypaert M, Boyer JL. Radixin is required to maintain apical canalicular membrane structure and function in rat hepatocytes. Gastroenterology. 2006;131:878–884. doi: 10.1053/j.gastro.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh M, Kato H, Suganami T, Konuma K, Marumoto Y, Terai S, Sakugawa H, et al. Hepatic crown-like structure: a unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLoS One. 2013;8:e82163. doi: 10.1371/journal.pone.0082163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao L, Utsumi T, Tashiro K, Liu B, Zhang D, Swenson ES, Iwakiri Y. Reticulon 4B (Nogo-B) facilitates hepatocyte proliferation and liver regeneration in mice. Hepatology. 2013;57:1992–2003. doi: 10.1002/hep.26235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 34.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 35.Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 36.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H. Macrophages in inflammation and its resolution. Front Immunol. 2012;3:324. doi: 10.3389/fimmu.2012.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, Pecker F, et al. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217–1226. doi: 10.1002/hep.24524. [DOI] [PubMed] [Google Scholar]
- 39.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Men R, Wen M, Dan X, Zhu Y, Wang W, Li J, Wu W, et al. Nogo-B: A potential indicator for hepatic cirrhosis and regulator in hepatic stellate cell activation. Hepatol Res. 2015;45:113–122. doi: 10.1111/hepr.12324. [DOI] [PubMed] [Google Scholar]
- 41.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 42.Crabb DW, Galli A, Fischer M, You M. Molecular mechanisms of alcoholic fatty liver: role of peroxisome proliferator-activated receptor alpha. Alcohol. 2004;34:35–38. doi: 10.1016/j.alcohol.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.