Abstract
Antimicrobial peptides such as cathelicidins are an important component of innate immune defence against inhaled microorganisms and have demonstrated antimicrobial activity against Mycobacterium tuberculosis with in vitro models. Despite this, little is known about the regulation and expression of cathelicidin during tuberculosis in vivo. We sought to determine whether the cathelicidin-related antimicrobial peptide (Cramp) gene, the murine functional homologue of the human cathelicidin gene (CAMP or LL-37), is required for regulating protective immunity during M. tuberculosis infection in vivo. We used Cramp−/− mice in a validated model of pulmonary tuberculosis and conducted cell-based assays with macrophages from these mice. We evaluated the in vivo susceptibility of Cramp−/− mice to infection and further dissected various pro-inflammatory immune responses against M. tuberculosis. We observed increased susceptibility of Cramp−/− mice to M. tuberculosis compared to wild type mice. Macrophages from Cramp−/− mice were unable to control M. tuberculosis growth in an in vitro infection model, were deficient in intracellular calcium influx and were defective in stimulating T-cells. Additionally, CD4 and CD8 T-cells from Cramp−/− mice produced less IFNβ upon stimulation. Furthermore, bacterial-derived cyclic-AMP modulated cathelicidin expression in macrophages. Our results demonstrate that cathelicidin is required for innate resistance to M. tuberculosis in a relevant animal model and is a key mediator in regulating the levels of pro-inflammatory cytokines by calcium and cyclic nucleotides.
Keywords: Cathelicidin Anti-microbial Peptide, Tuberculosis, Cyclic AMP
Introduction
The lack of a successful vaccine coupled with the emergence of multi- and extensively drug-resistant (MDR and XDR) strains of Mycobacterium tuberculosis (M. tuberculosis) underscores the need for new and potent strategies to counter the loss of millions of human lives. Cathelicidins are evolutionarily conserved antimicrobial peptides present in all mammals, and the human cationic antimicrobial protein 18 (LL-37, hCAP18) is the only known member of the human cathelicidin family of antimicrobial peptides. Murine cathelicidin-related antimicrobial peptide (mCRAMP) is the murine orthologue of the only structurally related human cathelicidin, LL-37/hCAP18 [1]. Originally recognized as a neutrophil specific peptide, it is now known that cathelicidin is expressed by many cell types like macrophages, circulating monocytes, NK, T and B cells and by squamous epithelium [2].
Antimicrobial peptides (AMPs), such as cathelicidin (LL-37), are important innate immune effectors for protection against M. tuberculosis infection. It has been shown that Vitamin D-mediated antimicrobial activity against M. tuberculosis in humans is dependent on the induction of antimicrobial peptides including cathelicidin [3–5]. Cathelicidin has also been demonstrated to possess dose-dependent antimicrobial activity against M. Tuberculosis [6, 7]. As cathelicidin-deficient mice are more susceptible to a range of infections, we hypothesized that Cramp is required for regulating protective immunity to M. tuberculosis in the mouse. Earlier observations provide insights that cyclic AMP (cAMP) plays a major role in butyrate-mediated cathelicidin LL-37 induction in human epithelial cells [8]. However, transcriptional repression of cathelicidin has been found through the activation of cellular signal transduction pathways including cAMP accumulation by cholera toxin [9]. In this study, we found that cathelicidin limits pro-inflammatory responses to M. tuberculosis by decreasing the levels of key cytokines and important second messengers in infected macrophages. Comparing survival in infected wild type and Cramp−/− mice, we found that wild type mice survive longer than Cramp−/− mice. Finally, we determined that transcriptional regulation of cathelicidin in macrophages may be due to differential cAMP accumulation in the host cell.
Methods
Animals
Approximately 4–6 week-old female Balb/c mice were purchased from The Charles River Laboratory (Wilmington, MA). Cramp−/− (Cnlp−/−) mice were bred from Cramp−/− knockout Balb/c breeding pairs at the Johns Hopkins animal facility [31, 32]. Mice were backcrossed at least 70 times. Infected mice were maintained in an animal biosafety level 3 laboratory at all times. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University, and covers all animal procedures.
Bacterial and Cell Culture Stocks
M. tuberculosis CDC1551 passaged in mice was obtained from the Johns Hopkins Center for Tuberculosis Research stocks (Baltimore, MD). Mutant lacking the adenylate cyclase gene Rv0386 (M. tuberculosis strain JHU-0386) and the complemented strain (JHU-0386-Comp) were also obtained from laboratory stocks [11]. For preparation for infections, mycobacteria were grown to an O.D. 600 nm of approximately 1.0 in 7H9 Middlebrook liquid medium supplemented with oleic acid-albumin-dextrose-catalase (Becton Dickinson, Sparks, MD), 0.5% glycerol and 0.05% Tween-80 (Sigma, St. Louis, MO).
Aerosol infection procedure
Aerosol infections were performed with M. tuberculosis CDC1551 diluted appropriately to achieve the desired inoculum using the Glas-col Inhalation Exposure System in 3 consecutive runs as previously described [33]. All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee. At least three animals from each infection cycle were sacrificed the following day to determine the day 1 lung implantation. For bacterial burden, lung and spleens were dissected and homogenized at specific time points and serially diluted in PBS and plated on selective 7H11 agar plates (Becton-Dickinson). CFUs were counted after 3 wk of incubation at 37 °C.
Generation of macrophages and DCs
Macrophages and DCs were differentiated with either M-CSF or GM-CSF respectively, as described before [34]. Briefly, bone marrow from the tibias and femurs of BALB/c mice were flushed out and cells were cultured in RPMI 1640 medium containing 10% FCS, 0.05 M 2-mercaptoethanol, 1 mM sodium pyruvate plus either 15 ng/ml M-CSF or GM-CSF for 3 days. This method gives a homogenous population that is 99% macrophages or DCs with negligible contaminating monocytes.
Intracellular macrophage growth assay
Bone marrow derived macrophages were plated at a density of 5×105 cells per well. Bacterial cultures were sedimented, washed with PBS and resuspended in RPMI media, and were used to infect at MOI = 2. At 4 h post-infection, monolayers were washed with PBS and replaced in fresh RPMI media. At the indicated times, monolayers were lysed in 1 ml of PBS with 0.5% triton X-100 and bacterial counts were enumerated.
Estimation of intracellular calcium levels
Intracellular calcium levels were monitored essentially as previously described [34]. Briefly, either 2×107 DCs were loaded with 1 uM fluo-3-AM for 45 min at 37°C in culture medium. The cells were thoroughly washed with RPMI and suspended in fresh culture medium. An aliquot of cells was diluted in culture medium, and when required, stimulated with M. tuberculosis CDC1551 cell extract (obtained from BEI resources, NIAID) at 100 seconds, and real-time increase in intracellular calcium concentration ([Ca2+]i) was monitored immediately over a total period of 600 seconds by FACS using FACSCalibur (BD Biosciences) and the data were analyzed using CellQuest software.
T cell enrichment for T cell responses
Mice were immunized with M. tuberculosis CDC1551 cell extract (obtained from BEI resources) subcutaneously at the base of the tail. Seven days later, inguinal lymph nodes were extracted and B cells, macrophages and DCs were removed following MACS using anti-B220+, anti-CD11c+ and anti-CD11b+ microbeads. The negatively selected T cells were 98% pure as determined by CD90-PE staining. Previously infected BMDCs from wild type and Cramp−/− mice were then co-cultured with the enriched T cells in 2:1 ratio (T cells:BMDCs) from different groups at 37°C in a CO2 incubator for 48 h. The levels of the proinflammatory cytokines IFNγ, IL12p40 and TNFα were measured using ELISA (R&D Systems).
Apoptosis Assay
CD4+ and CD4+ T cells were purified using the CD4+ and CD4+ T cell isolation kit (Miltenyi Biotec) as described by the manufacturer. The extent of apoptosis was assessed by measuring Caspase-3 activity by using the Caspase-3 Colorimetric Assay kit (R&D Systems BF3100), according to the manufacturer’s instructions. Briefly, cells were lysed and supernatants incubated with reaction buffer containing dithiothreitol and substrates at 37°C. The reaction was measured by determining the change in absorbance at 405 nm using a microplate reader.
Quantitative PCR
Total RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. RNA was employed in real time quantitative RT-PCR using SYBRgreen on a Bio-Rad iCycler. The mRNA level was calculated using 2−ΔCt (ΔΔCt) method, using GAPDH as housekeeping gene. Primer sets used for quantifying relative cathelicidin expression are 5′-ACGAGGATCCAGATACTCCCAAGT-3′ (forward) and 5′-TTCCTTGAAGGCACATTGCTCAGG-3′ (reverse).
Statistical analysis
Lung CFU counts were log10 transformed before analysis. Mean CFU counts were compared using Student t-test. The proportions of mice relapsing were compared using Fisher’s exact test (STATA 8.2, College Station, TX). All measures of statistical variation are expressed as standard deviations (±SD).
Results
Higher bacterial burden in Cramp−/− mice during pulmonary tuberculosis
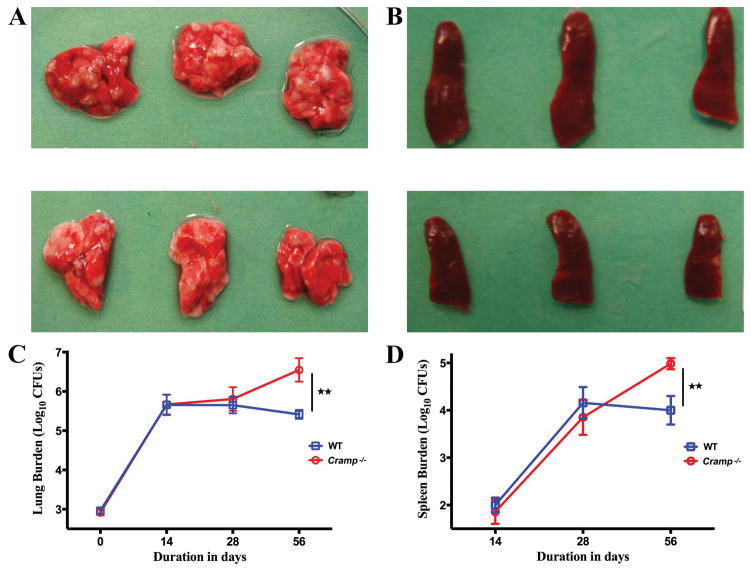
We hypothesized that cathelicidin expression regulates bacterial burden during M. tuberculosis infection. To test this, we infected Cramp−/− mice on a Balb/c background and wild type Balb/c control mice with M. tuberculosis by the aerosol route and monitored organ burdens of bacteria over time. Gross pathology revealed that wild type mouse lungs had fewer visible lesions than Cramp−/− 8 weeks after infection (Figure 1A). Similarly, Cramp−/− mice had visibly enlarged spleens compared to wild type mice (Figure 1B). Cramp−/− mice were not capable of controlling bacterial growth as efficiently as wild type mice. Indeed, the bacterial CFU counts in lungs of M. tuberculosis infected Cramp−/− mice is 10-fold higher than wild-type mice 56 days after infection (Figure 1C). Both genotypes demonstrated bacterial dissemination to, and growth within, the spleen. The pattern of bacterial growth in spleen was similar to lungs; with control mice exhibiting approximately 10-fold lower bacteria counts compared to Cramp−/− spleens 56 days after infection (Figure 1D). The mice formed well-developed characteristic granulomatous lesions in lungs with more acid-fast bacilli in the peripheral rim of giant cells from Cramp−/− mice compared to wild type (Figure S1).
Figure 1. Cramp−/− mice are unable to contain M. tuberculosis proliferation after aerosol infection.
Gross pathology of lungs (A) and spleens (B) at 8 weeks infection in Cramp−/− (upper panels) and wild type mice (lower panels). Lung (C) and spleen (D) CFU counts in wild type and Cramp−/− mice infected with 3.0 log10 units of M. tuberculosis CDC1551 by the aerosol route. The lungs were homogenised, diluted and plated for CFU counts expressed as Log10 (±S.D.). ** p <0.01.
Impaired mycobacterial growth inhibition in Cramp−/− bone marrow-derived macrophages
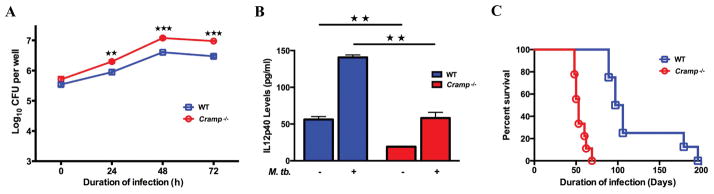
Murine bone marrow-derived macrophages (BMDM) are able to contain the growth of M. tuberculosis after infection. We evaluated the ability of BMDMs from wild type and Cramp−/− mice to restrict the growth of M. tuberculosis. BMDMs were exposed to M. tuberculosis at an MOI of 2 for 4 h. After extensive washing, the cultures were monitored daily for CFU, revealing higher M. tuberculosis intracellular growth in the infected BMDMs of Cramp−/− mice than wild type at 24, 48 and 72 h after bacterial infection (Figure 2A).
Figure 2. Cramp−/− and wild type macrophages differ in intracellular growth of bacteria and IL12p40 production upon M. tuberculosis infection leading to accelerated progression of TB disease in Cramp−/− mice.
BMDMs from wild type and Cramp−/− mice were infected in triplicate with M. tuberculosis. (A) Macrophages were lysed and bacteria plated for intracellular growth at different time points. (B) 1 million BMDM were infected with CDC1551 (MOI=2) and levels of IL12p40 was measured at 24 hrs by ELISA. (C) Time to death of wild type and Cramp −/− mice after aerosol infection. For A and B, ★★ and ★★★ denotes p < 0.01 and <0.001 respectively. For C, p <0.0001 from Fisher’s exact test.
Cramp−/− infected macrophages secrete lower levels of the proinflammatory cytokine IL12p40
IL-12p40 is known to play a dominant role in regulating pro-inflammatory responses from antigen presenting cells like macrophages and dendritic cells that are crucial for mediating protection against M. tuberculosis infection. To evaluate whether the reduced ability of Cramp−/− mice to control tuberculosis infection is due to an attenuated Th1 cell-mediated immune response, we measured levels of IL-12p40 in M. tuberculosis infected BMDMs. IL-12p40 levels increased significantly in wild type BMDMs upon infection with M. tuberculosis. In contrast, Cramp−/− macrophages had decreased IL12p40 levels 24 h after infection (140.72 ± 3.33 pg/ml versus 58.19 ± 7.74 pg/ml; p = 0.005; Figure 2B).
Cramp−/− mice are more susceptible to an aerosol M. tuberculosis challenge
Both wild type and Cramp−/− mice were challenged with M. tuberculosis through an aerosol route and analysed for time-to-death. The median time-to-death of infected Cramp−/− mice was 53 days while that of the infected wild type mice was 101 days. All infected Cramp−/− mice had died at 69 days post-infection, while the first wild type infected mouse died at 89 days post-infection (Figure 2C) (p < 0.0001 Log-rank (Mantel-Cox) test).
Cramp−/− mice generate less IFNγ, IL12p40 and TNFα during T-cell recall responses to M. tuberculosis
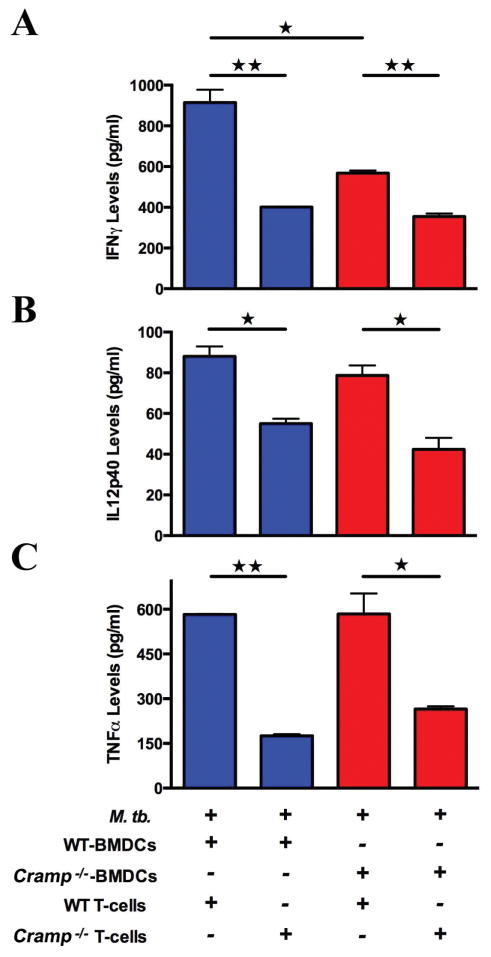
We next sought to investigate the effect of cathelicidin on the immune response induced by dendritic cells upon infection with M. tuberculosis. We isolated BMDCs from Cramp−/− and wild type mice and infected them with M. tuberculosis at MOI of 2. The M. tuberculosis infected dendritic cells were extensively washed and co-cultured for 48 h with M. tuberculosis primed T cells derived from mice injected with M. tuberculosis cell extract. As seen in Figure 3, when T-cells from the wild type mice were co-cultured with infected BMDCs either from wild type or knockout mice, the levels of IFNγ levels decreased by approximately 50% in the groups having Cramp−/− BMDCs as compared to WT BMDCs. BMDCs from Cramp−/− and wild type controls did not secrete IFNγ, while Cramp −/− BMDCs had decreased IL12p40 and TNFα levels compared to wild type (49.7 ± 0.55 pg/ml versus 26.9 ± 2.0 pg/ml IL12p40 levels). Wild type murine BMDCs co-cultured with T-cells from wild type mice produced higher levels of IFNγ, IL12p40 and TNFα compared to co-culturing with T-cells from Cramp−/− mice (Figure 3). Similarly, Cramp−/− BMDCs with wild type T-cells generated higher levels of IFNγ, IL12p40 and TNFα compared to co-culture with T-cells from Cramp −/− mice. Together, these observations demonstrate reduced secretion of IFNγ, IL12p40 and TNFα from Cramp−/− T-cells.
Figure 3. Cramp−/− mice have restricted T-cell responses to M. tuberculosis infection.
T-cells were isolated from immunized mice and co-cultured with M. tuberculosis CDC1551 infected dendritic cells (T-cell:DCs 2:1). IFNγ (A), IL12p40 (B) and TNFα (C) were measured using ELISA of the co-culture supernatant. Data shown are representative of two independent experiments. ★ and ★★ denotes p < 0.05 and <0.01 respectively.
Stimulated Cramp −/− splenocytes are defective in Type I interferon secretion
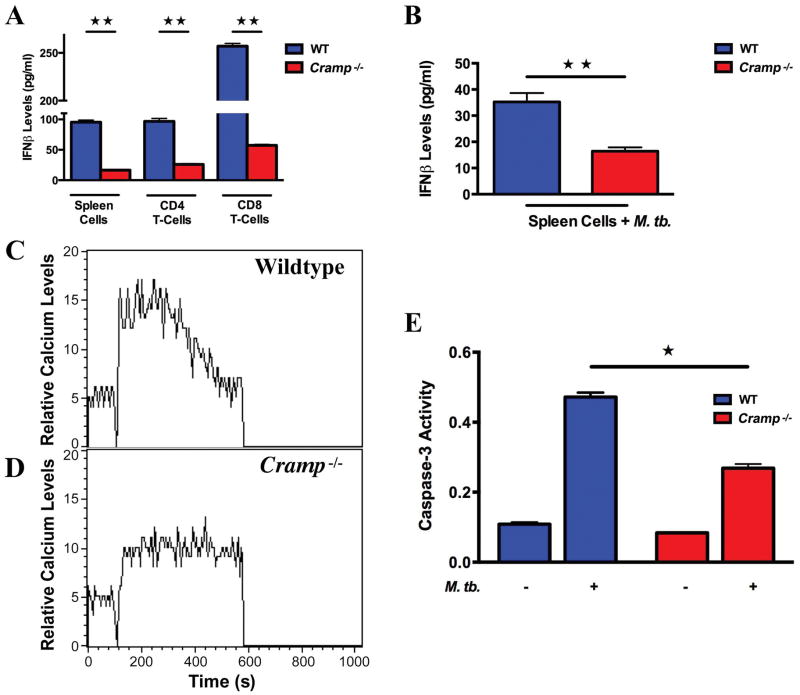
Type I interferons are important for protection against many viral infections and defence against bacterial pathogens and are believed to play a critical role in disease pathology and progression during tuberculosis. To evaluate whether Cramp −/− mice are able to mount an effective Type 1 interferon response upon exposure to M. tuberculosis, we harvested spleens on day14 post-infection and used them to derive splenocytes as well as CD4+ and CD8+ splenic T-cells. The levels of IFNβ from the splenocytes and from the CD4 and CD8 T-cells that were co-stimulated with bound CD3 and CD28 antibodies were then determined. Stimulated splenocytes from infected Cramp−/− mice generated negligible amounts of IFNβ (16 ± 1.05 pg/ml) compared to splenocytes from wild type mice (95.35 ± 3.55 pg/ml; p=0.002). Similarly, the levels of IFNβ from both the CD4 and CD8 T-cell compartments were higher in the wild type mice compared to Cramp−/− mice. Splenocytes from Cramp−/− mice secreted lower amounts of IFNβ (16.4 ± 1.5 pg/ml) than wild type mice (35.2 ± 3.4 pg/ml; p= 0.006) upon M. tuberculosis stimulation (Figure 4A,B). These studies revealed that CRAMP−/− T cells have a diminished capacity to mount a Type 1 interferon response following M. tuberculosis infection.
Figure 4. Cramp−/− cells mount an altered Type 1 interferon response, calcium influx and apoptosis signalling following M. tuberculosis infection.
(A) Splenocytes and splenic T-cells from the indicated group were stimulated with CD3 and CD28 antibodies for 48 h and supernatant collected for determination of IFNβ levels by ELISA. (B) Splenocytes from wild type and Cramp−/− mice were stimulated with M. tuberculosis for 48 h and supernatant collected for IFNβ ELISA. (C,D) Real time intracellular calcium influx using Fluo-3 AM dye in wild type (C) and Cramp −/− (D) BMDMs stimulated with 20 μg of M. tuberculosis cell extract. (E) Apoptosis (caspase-3 activity) in CD8+ T-cells. ★ and ★★ denote p < 0.05 and <0.01 respectively.
Decreased calcium levels in Cramp−/− bone marrow derived-dendritic cells upon Mycobacterium tuberculosis infection
Calcium levels modulate the generation of proinflammatory responses and consequently regulate the survival of M. tuberculosis in macrophages. We sought to investigate the effect of cathelicidin on calcium influx and protective immunity to M. tuberculosis infection by comparing the M. tuberculosis-induced calcium influx in infected BMDCs between wild type and Cramp−/− mice. We isolated BMDCs from Cramp−/− and wild type mice and loaded them with fluo-3-AM indicator dye for 45 min. When stimulated with M. tuberculosis cell extract at 100 seconds, wildtype BMDCs exhibited robust increase (208 nM) in intracellular calcium levels while Cramp−/− BMDCs had a poor response (83 nM) (Figure 4C,D). These results demonstrated an important role of cathelicidin in the calcium influx in BMDCs.
Decreased apoptosis in the CD4 and CD8 T-cell compartments of CRAMP−/− mice
It is known that M. tuberculosis infection inhibits apoptosis of infected phagocytic cells thereby facilitating intracellular survival of the bacillus and blocking antigen presentation [10]. To determine the effect of cathelicidin expression on apoptosis of T cells, CD4+ and CD8+ T cells were isolated from uninfected and M. tuberculosis infected animals and the activity of the caspase-3 class of proteases was monitored by colorimetric detection. Caspase-3 activity was similar in CD4+ cells (Figure S2), while we observed 2-fold higher activity in the cytotoxic CD8+ T-cell compartments from infected wild type mice compared to Cramp−/− mice (Figure 4E).
Cathelicidin levels are regulated by cAMP accumulation
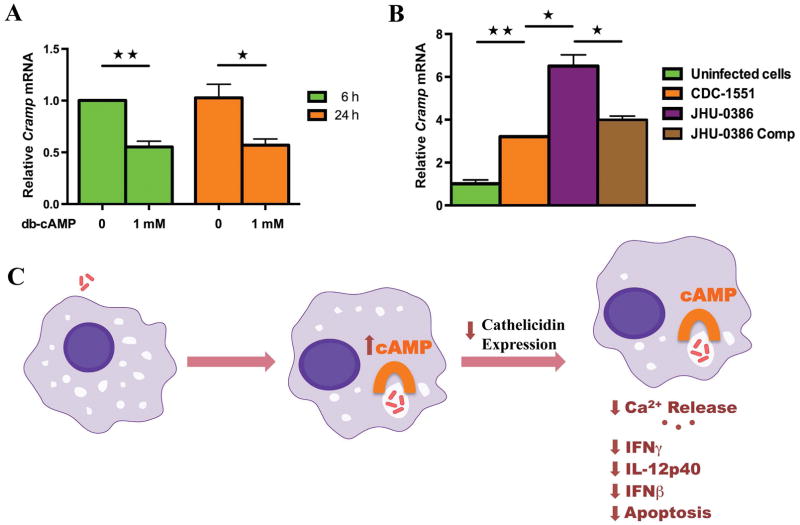
It is known that the cAMP accumulation can lead to transcriptional repression of cathelicidin in epithelial cells [9]. To analyse whether cAMP can decrease the expression of cathelicidin, we used a cell-permeable analogue of cAMP that activates cAMP-dependent protein kinases. Treatment of macrophage cells with dibutyryl-cAMP reduced the expression of cathelicidin mRNA to 50% as early as 6 h post exposure and levels remained unchanged at 24 h (Figure 5A). We further employed different recombinant strains of M. tuberculosis with altered ability to deliver cAMP to infected macrophage cytoplasm. Macrophages infected with M. tuberculosis strain JHU-0386 (mutant lacking the adenylate cyclase gene Rv0386 [11]), which produces a reduced intramacrophage cAMP burst and is attenuated for virulence, induced higher cathelicidin mRNA expression. The complemented strain JHU-0386-Comp induced similar levels of cathelicidin mRNA as wild type M. tuberculosis (Figure 5B). We further determined the relative CRAMP protein levels in cells infected by the different bacterial strains by western blot (Figure S4). Similar to the mRNA data, macrophages infected with of M. tuberculosis strain JHU-0386 induced higher cathelicidin protein levels. Collectively, our data indicate that cAMP signalling regulates the levels of cathelicidin in response to mycobacterial infection in macrophages and that M. tuberculosis-derived cAMP acts to inhibit LL-37 transcription. These results indicate that M. tuberculosis mediated cAMP secretion is a microbial subversion mechanism that serves to inhibit the antibacterial host defence system mediated by cathelicidin (Figure 5C).
Figure 5. Cathelicidin expression is regulated by cAMP accumulation in macrophages.
(A) RT-PCR of macrophages treated with dibutyryl-cAMP. (B) RT-PCR of macrophages 24 h after infection with different recombinant strains of bacteria.JHU-0386 is CDC1551 containing a transposon insertion in the adenylate cyclase gene Rv0386 while JHU-0386-Comp is JHU-0386 complemented with a wild type copy of Rv0386. (C) Scheme of cAMP dependent cathelicidin expression inhibition and consequent reductions in immune control pathways. Data shown are representative of three independent experiments. ★ and ★★ denotes p < 0.05 and <0.01 respectively.
Discussion
This study has two major findings. First, we demonstrate that the mouse Cramp gene is required for control of tuberculosis and that this control is likely mediated by CRAMP-dependent apoptosis and CRAMP-dependent cytokine responses known to be essential for containment of TB (IL-12, IFN-γ, and TNF-α). Second, we observed that the cAMP burst mediated by M. tuberculosis has an inhibitory effect on Cramp transcription. Thus, in addition to alterations in TNF-α secretion by bacterial-derived cAMP as previously described (11), M. tuberculosis subversion of host cAMP signalling also reduces Cramp transcription and may hence lead to reduced Ca+2 responsiveness, decreased apoptosis, and lower expression of IL-12, IFN-γ and other cytokines.
The cathelicidin family is one of the major classes of mammalian antimicrobial peptides that are known to be an important component of the innate immune system providing a non-specific, rapid response to foreign pathogens. Although it has been demonstrated that cathelicidins have direct antimicrobial effects against mycobacteria in vitro [12], little is known about cathelicidin-dependent immunoregulation of other immune responses within the innate and adaptive arms beyond the demonstration that they serve as chemotactic factors for CD4 T cells, neutrophils and monocytes [13, 14]. In this study, we evaluated the ability of cathelicidin-deficient macrophages and T cells to express known immune mechanisms - apart from direct killing of bacilli. Using cell-based assays, we observed defects in Ca2+ responsiveness, apoptosis and cytokine expression. There was a significant increase in nitric oxide production after infection in macrophages but this increase was similar in both Cramp−/− and wild type macrophages (Figure S3). Since these reduced responses in Cramp−/− immune cells were observed ex vivo, our results suggest that the phenotypes are not due to reduced direct killing of M. tuberculosis by cathelicidin, but rather to defective immune signalling in the absence of Cramp expression. Thus, the Cramp gene appears to play an immunoregulatory role in the triggering both innate and adaptive immune responses, independent of the anti-bacterial effects of the cathelicidin peptide itself.
Cyclic AMP is an important second messenger in mammalian cells that initiates intracellular cascade of cAMP-dependent cell signalling which involves activation of protein kinase A and the cAMP receptor-binding protein, CREB, which transduces elevated cAMP levels to the nucleus leading to elevated transcription of key genes in a cell-type dependent manner. We demonstrate that a cAMP-independent calcium signalling pathway as well as a cAMP-dependent pathway are responsible for CRAMP-dependent immunomodulation. Cathelicidin down-regulation in our studies was likely due to accumulation of cAMP in the host cell due to the cAMP burst associated with M. tuberculosis entry into macrophages. We demonstrated alterations in Cramp transcription in a cAMP-dependent manner both by exogenous addition of the cell-permeable cAMP-mimetic, dibutyryl-cAMP, and also by using recombinant M. tuberculosis that express a reduced cAMP burst due to loss of the Rv0386 adenylate cyclase. Higher cathelicidin expression in macrophages infected with recombinant strains of M. tuberculosis that produce a reduced intramacrophage burst of cAMP indicates the critical role played by cAMP in antimicrobial peptide regulation. Loss of Rv0386 has been linked to reduced intramacrophage cAMP levels resulting in the reduction of TNF-α production via the protein kinase A and CREB pathway, decreased immunopathology in animal tissues, and diminished bacterial survival [11].
Calcium plays a determinative role in the generation of proinflammatory responses [15] and is also required for host containment of mycobacteria in macrophages [16]. Calcium-dependent phagosome maturation is inhibited during M. tuberculosis infection by a mechanism that involves mycobacterial inhibition of sphingosine kinase [16]. Reduced phagosome maturation and the concomitant failure to mount lysosomal killing directly contributes to survival of M. tuberculosis within human macrophages. We found that there is decreased Ca2+ mobilisation in Cramp−/− bone marrow derived dendritic cells upon M. tuberculosis infection compared to that in wild type cells. It has been reported that human cathelicidin, LL-37, induces Ca2+ mobilization in human monocytes [14] and neutrophils [17]. In addition to its microbicidal activity, LL-37 may contribute to innate and adaptive immunity by recruiting neutrophils, monocytes and T cells to sites of microbial invasion by interacting with FPRL1 [18]. Another antimicrobial peptide, gomensin, also increased intracellular calcium mobilization and this increase was inhibited by calcium channel blockers [19]. Our observation that M. tuberculosis infection reduces Cramp transcription and that Cramp is required for normal Ca2+ mobilization in BMDCs is consistent with a mechanism of pathogen-mediated subversion of the cellular calcium signal transduction system to influence immune cell function.
Production of proinflammatory cytokines like IL-12 and TNF-α are critical for defence against mycobacterial infection. IL-12 plays a crucial role in activating the protective Th1 immune response against mycobacteria [20]. We found that IL12p40 levels were significantly reduced in Cramp−/− macrophages. It has been previously reported that exposure of macrophages to LL-37 resulted in increased IL-12p40 levels and reduced IL-10 expression on LPS stimulation [21]. These LL-37 effects on macrophage function required internalization of the peptide. In mice, control of M. tuberculosis infection is dependent on a Th1 response and the production of INF-γ, a type II interferon. Owing to their unique ability to prime naïve and memory T-cells, dendritic cells play an important role in the initiation and maintenance of protective immune responses following infection [22]. In our BMDC and T-cell co-culture experiments, Cramp−/− mouse are defective in producing IFN-γ, IL12p40 and other cytokines compared to wild type BMDC co-cultured with T cells. These observations support a role for CRAMP in immunoregulation of Th1 cytokine production.
Type I interferons are critical for resistance to viruses, while there are conflicting reports regarding their role during tuberculosis [35, 36]. Type I interferon responses have been shown to enhance activation of cytolytic CD8 T cells that are crucial during M. tuberculosis infection [37]. A type I interferon response has also been shown to limit lung infectivity of target cells by M. tuberculosis in animals devoid of type II interferon [38], while loss of the IFNAR1 receptor confers resistance to tuberculosis suggesting that type I interferon responses are detrimental in tuberculosis [39]. Human tuberculosis transcriptome analysis of peripheral blood revealed that genes associated with both the type I and type II interferon responses are upregulated during infection, suggesting common and dynamic roles of both types of interferon in tuberculosis pathogenesis [40]. Dendritic cells sense viral and microbial DNA through endosomal Toll-like receptors to produce Type I interferons. In a recent study, DNA-LL37 complexes stimulate Type I interferon production and are delivered to and retained within early endocytic compartments in dendritic cells to trigger Toll-like receptor 9 expression [23]. Furthermore, keratinocytes exposed to LL-37 and treated with TLR9 ligands increase production of Type I IFNs [24]. Our observation of reduced Type I interferon secretion in Cramp−/− cells infected with M. tuberculosis is compatible with these reports and further implicate cathelicidin as a mediator for Type I interferon responses. It was also found that cathelicidin expression was induced in Mycobacterium avium infected BMDMs in a TLR2- and TNF-dependent manner [25]. However, that study using intravenous M. avium infection in mice reported that cathelicidin was not required for the bacteriostasis of bacteria in vivo.
Apoptosis of CD4+ and CD8+ T cells has been suggested to lead to decline of effective immune response during tuberculosis [26] and other bacterial infections [27]. Apoptosis of both CD4+ and CD8+ T cells, but not other splenic cells, increased proportionally with the progression of mycobacteria infection [28]. Moreover, M. tuberculosis infected PBMCs from TB patients had significantly higher levels of apoptosis of CD4 T-cells [29]. It has been suggested that LL-37–induced apoptosis of CD8+ T cells is mediated via multiple different mechanisms in a caspase and granzyme-dependent manner [30]. Our findings of decreased apoptosis in CD8 T-cells from M. tuberculosis infected Cramp−/− mice further these observations, indicating that cathelicidin is required for normal apoptotic signalling in cytotoxic T lymphocytes. Thus, in addition to the direct antimicrobial activity by cathelicidins as previously described [12], altered apoptotic signalling may lead to immune modulation in Cramp−/− mice.
Our work strongly suggests that cathelicidins are an important multifunctional host immune regulator with not only direct anti-mycobacterial activity, as previously documented, but also direct immunoregulatory influences on adaptive immune responses against M. tuberculosis via modulation of pro-inflammatory cytokine responses, apoptosis and calcium influx. This pivotal role for cathelicidin in governing protective responses during TB infection, which depends on the M. tuberculosis-mediated cAMP burst in macrophages, offers insights into TB pathogenesis and suggests that further dissection of cathelicidin-mediated immune regulation are warranted.
Supplementary Material
More acid-fast bacilli in Cramp−/− mice
Cramp−/− cells mount an altered apoptosis signalling following M. tuberculosis infection
Similar NO production from Cramp−/− and wild type BMDM upon M. tuberculosis challenge
Cathelicidin expression is regulated by cAMP accumulation in macrophages
Acknowledgments
Supported by grants AI 079590, 037856 and 036973 from NIAID and the Howard Hughes Medical Institute.
Footnotes
Conflict of interest: None of the authors declare conflicting commercial interests.
Author contribution statement
SG conceived and designed the experiments. SG and KW carried out experiments and analysed data. SG, KW and WRB interpreted the data. RG generated and provided tools for experiments. SG and WRB drafted the manuscript. All authors have given final approval of the submitted and published versions.
References
- 1.Pestonjamasp VK, Huttner KH, Gallo RL. Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides. 2001;22:1643–1650. doi: 10.1016/s0196-9781(01)00499-5. [DOI] [PubMed] [Google Scholar]
- 2.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 3.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 4.Liu PT, Stenger S, Tang DH, et al. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Sonawane A, Santos JC, Mishra BB, et al. Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol. 2011;13:1601–1617. doi: 10.1111/j.1462-5822.2011.01644.x. [DOI] [PubMed] [Google Scholar]
- 7.Martineau AR, Wilkinson KA, Newton SM, et al. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 8.Chakraborty K, Maity PC, Sil AK, et al. cAMP stringently regulates human cathelicidin antimicrobial peptide expression in the mucosal epithelial cells by activating cAMP-response element-binding protein, AP-1, and inducible cAMP early repressor. J Biol Chem. 2009;284:21810–21827. doi: 10.1074/jbc.M109.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakraborty K, Ghosh S, Koley H, et al. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008;10:2520–2537. doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 10.Velmurugan K, Chen B, Miller JL, et al. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal N, Lamichhane G, Gupta R, et al. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460:98–102. doi: 10.1038/nature08123. [DOI] [PubMed] [Google Scholar]
- 12.Martineau AR, Newton SM, Wilkinson KA, et al. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roby KD, Nardo AD. Innate immunity and the role of the antimicrobial peptide cathelicidin in inflammatory skin disease. Drug Discov Today Dis Mech. 2013;10:e79–e82. doi: 10.1016/j.ddmec.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noble A, Truman JP, Vyas B, et al. The balance of protein kinase C and calcium signaling directs T cell subset development. J Immunol. 2000;164:1807–1813. doi: 10.4049/jimmunol.164.4.1807. [DOI] [PubMed] [Google Scholar]
- 16.Malik ZA, Thompson CR, Hashimi S, et al. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J Immunol. 2003;170:2811–2815. doi: 10.4049/jimmunol.170.6.2811. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Cherryholmes G, Chang F, et al. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol. 2009;39:3181–3194. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurosaka K, Chen Q, Yarovinsky F, et al. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 19.Soletti RC, del Barrio L, Daffre S, et al. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem Biol Interact. 2010;186:135–143. doi: 10.1016/j.cbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Khader SA, Partida-Sanchez S, Bell G, et al. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Does AM, Beekhuizen H, Ravensbergen B, et al. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J Immunol. 2010;185:1442–1449. doi: 10.4049/jimmunol.1000376. [DOI] [PubMed] [Google Scholar]
- 22.Sinha A, Salam N, Gupta S, et al. Mycobacterium tuberculosis and dendritic cells: recognition, activation and functional implications. Indian J Biochem Biophys. 2007;44:279–288. [PubMed] [Google Scholar]
- 23.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 24.Morizane S, Yamasaki K, Muhleisen B, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Invest Dermatol. 2012;132:135–143. doi: 10.1038/jid.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos JC, Silva-Gomes S, Silva JP, et al. Endogenous cathelicidin production limits inflammation and protective immunity to Mycobacterium avium in mice. Immun Inflamm Dis. 2014;2:1–12. doi: 10.1002/iid3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–953. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 27.Fuse Y, Nishimura H, Maeda K, et al. CD95 (Fas) may control the expansion of activated T cells after elimination of bacteria in murine listeriosis. Infect Immun. 1997;65:1883–1891. doi: 10.1128/iai.65.5.1883-1891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbertson B, Zhong J, Cheers C. Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with Mycobacterium avium. J Immunol. 1999;163:2073–2080. [PubMed] [Google Scholar]
- 29.Hirsch CS, Johnson JL, Okwera A, et al. Mechanisms of apoptosis of T-cells in human tuberculosis. J Clin Immunol. 2005;25:353–364. doi: 10.1007/s10875-005-4841-4. [DOI] [PubMed] [Google Scholar]
- 30.Mader JS, Marcet-Palacios M, Hancock RE, et al. The human cathelicidin, LL-37, induces granzyme-mediated apoptosis in cytotoxic T lymphocytes. Exp Cell Res. 2011;317:531–538. doi: 10.1016/j.yexcr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni MM, Barbi J, McMaster WR, et al. Mammalian antimicrobial peptide influences control of cutaneous Leishmania infection. Cell Microbiol. 2011;13:913–923. doi: 10.1111/j.1462-5822.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta S, Tyagi S, Almeida DV, et al. Acceleration of tuberculosis treatment by adjunctive therapy with verapamil as an efflux inhibitor. Am J Respir Crit Care Med. 2013;188:600–607. doi: 10.1164/rccm.201304-0650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Salam N, Srivastava V, et al. Voltage gated calcium channels negatively regulate protective immunity to Mycobacterium tuberculosis. PloS One. 2009;4:e5305. doi: 10.1371/journal.pone.0005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manca C, Tsenova L, Bergtold A, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc Natl Acad Sci U S A. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dey B, Dey RJ, Cheung LS, et al. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat Med. 2015;21:401–406. doi: 10.1038/nm.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179:6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188:6205–6215. doi: 10.4049/jimmunol.1200255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorhoi A, Yeremeev V, Nouailles G, et al. Type I IFN signaling triggers immunopathology in tuberculosis-susceptible mice by modulating lung phagocyte dynamics. Eur J Immunol. 2014;44:2380–2393. doi: 10.1002/eji.201344219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
More acid-fast bacilli in Cramp−/− mice
Cramp−/− cells mount an altered apoptosis signalling following M. tuberculosis infection
Similar NO production from Cramp−/− and wild type BMDM upon M. tuberculosis challenge
Cathelicidin expression is regulated by cAMP accumulation in macrophages