Abstract
Background
Inflammation is known to preclude tolerance after transplantation. We have previously shown that systemic inflammation in xenograft recipients (SIXR) precedes activation of coagulation in the absence of T cell responses. Accordingly, SIXR may amplify innate and adaptive immune responses against xenografts after pig to primate xenotransplantation, even with efficient immunosuppressive therapy. We evaluated the impact of anti-inflammatory agents on pro-inflammatory cytokines and chemokines in pig artery patch and heart xenograft recipients.
Methods
Baboons received an artery patch (Group 1, n=8) or heart (Group 2, n=4) from genetically engineered pigs. All baboons received lymphodepletion with thymoglobulin (ATG) and costimulation blockade-based immunosuppression (anti-CD40 and/or CTLA4Ig). In Group 1, baboons received either (i) no anti-inflammatory agents (n=2), (ii) cobra venom factor (CVF, n=2), (iii) α1-antitrypsin (AAT, n=2), or (iv) interleukin (IL)-6 receptor antagonist (IL-6RA, n=2). In Group 2, all baboon received corticosteroids, either without (n=2) or with (n=2) IL-6RA. Serum IFNγ, TNFα, IL-1β, IL-17, IL-6, IL-8, MCP-1, sCD40L levels were measured by Luminex. Fibrinogen, D-Dimers, and C-reactive protein (C-RP) were also measured. Recipient baboon T cell proliferation was evaluated by mixed lymphocyte reaction (MLR) before and after transplantation. Pig and baboon tissue factor (TF) mRNA levels in heart xenografts were measured by RT-PCR.
Results
In no recipient was a marked increase in T cell response to pig cells observed after transplantation. In Groups 1 and 2, post-transplantation levels of IFNγ, TNFα, IL-1β, and IL-17 remained comparable to or lower than pre-transplant levels, except in one heart recipient that succumbed to CMV infection. In Group 1, when no anti-inflammatory agent was administered, post-transplant levels of IL-6, IL-8, and MCP-1 were elevated. After CVF, IL-6, IL-8, and MCP-1 remained low. After IL-6RA, IL-6 and MCP-1 were elevated. After AAT, IL-8 was elevated. sCD40L became elevated intermittently in most recipients irrespective of the administered anti-inflammatory agent. In Group 2, IL-6 was transiently elevated, particularly after IL-6RA administration. MCP-1 gradually increased by 2 months in Group 2 recipients. sCD40L generally remained low except in one recipient. In Group 1 and Group 2 recipients, C-RP levels were elevated except after IL-6RA administration, while D-Dimers were elevated regardless of administration of anti-inflammatory agent. In Group 2, pig TF mRNA levels were increased in heart xenografts compared to naive pig hearts, irrespective of IL-6 receptor antagonist administration. Additionally, baboon TF mRNA levels were detectable in heart xenografts, but not in naive pig hearts.
Conclusions
Some pro-inflammatory cytokines and chemokines are elevated in xenograft recipients, even with efficient T cell-directed immunosuppressive therapy. Persistent elevation of D-Dimers, and individual cytokines and chemokines suggest a continuous inflammatory response, despite administration of anti-inflammatory agents. Systemic administration of combined anti-inflammatory agents as well as complement regulation may be essential to prevent SIXR after transplantation.
Keywords: Chemokines, Cytokines, Heart, Inflammation, Pig, Xenotransplantation
INTRODUCTION
Pro-inflammatory cytokines and chemokines are essential for protective immunity. However, excessive or prolonged production of these factors can be associated with various pathological conditions. Inflammation is known to prevent regulation of T cells and tolerance to allografts after transplantation, which in turn precludes long-term allograft survival.1 For example, inflammation early post transplant is associated with the development of allograft vasculopathy.2
Additionally, there is considerable interaction between innate inflammatory responses and activation of the coagulation system,3 where the role of pro-inflammatory cytokines in the activation of coagulation is well studied.4 Cytokines are known to induce and/or upregulate tissue factor expression on various cell types. For example, interleukin (IL)-1, IL-6, and tumor necrosis factor alpha (TNFα) induce tissue factor expression by endothelial cells and circulating monocytes.5,6 We have shown that systemic inflammation in xenograft recipients (SIXR) precedes dysregulation of coagulation,7 and is sustained in xenograft recipients.8 In organ xenograft recipients, innate immune cells infiltrating organ xenografts express tissue factor, a key factor in the activation of coagulation.9 Furthermore, recipient immune cells and platelets in peripheral blood upregulate tissue factor expression early after xenotransplantation.10
Complement activation is detrimental to xenograft survival, and regulation of the complement system is associated with prevention of early xenograft failure 11 and of delayed xenograft rejection.12 We have previously demonstrated that upregulation of IL-6 levels in xenograft recipients is associated with elevated levels of the inflammatory marker, C-reactive protein (C-RP),7 while the administration of IL-6 receptor blockade prevents the upregulation of C-RP.8 Additionally, administration of α1-antitrypsin (AAT) has been shown to prevent inflammatory responses in monkey recipients of allografts.13
Prevention of increased pro-inflammatory cytokine production after xenotransplantation is likely to protect against dysregulation of the coagulation system, and may allow reduction of immunosuppressive therapy after xenotransplantation. Accordingly, we evaluated the therapeutic effect of various anti-inflammatory agents on cytokine and chemokine levels, the inflammatory marker C-reactive protein (C-RP), and coagulation factors in pig artery patch and heart xenograft recipient baboons receiving T cell-directed immunosuppressive therapy.14,15
METHODS
Animals
Baboons (n=12) (Oklahoma University Health Sciences Center, Oklahoma City, OK) received either an artery patch graft (Group 1, n=8), 8,14 or a heart graft (Group 2, n=4) 16 from genetically-engineered pigs (Revivicor, Blacksburg, VA).17,18
Immunosuppression and anti-inflammatory agents
All baboons received a T cell-directed immunosuppressive regimen based on costimulation blockade (anti-CD40 and/or CTLA4Ig). Group 1 baboons received either (i) no anti-inflammatory agents (n=2), (ii) cobra venom factor (CVF, n=2; Complement Technology, Tyler, TX), (iii) α1-antitrypsin (AAT, n=2; Aralast NP, Baxter, Westlake Village, CA), or (iv) an IL-6 receptor antagonist (IL-6RA, n=2; tocilizumab, Genentech, South San Francisco, CA) (Table 1). In Group 2, two baboons received IL-6RA, while two recipients did not. Group 1 recipients received no corticosteroids, while Group 2 recipients were given maintenance corticosteroids.
TABLE 1.
Experimental groups, immunosuppressive therapy, donor pig genetic modifications (NOT INCLUDED), and T cell proliferation
| Induction | Costimulation blockade | Steroids | Anti-inflammatory agent | T cell proliferation (%) | Follow-up (Days) | |||
|---|---|---|---|---|---|---|---|---|
| Group | ATG | Pre | Post | |||||
| Heart | B55 | Belatacept + Anti-CD40 | YES | None | NA | NA | 99 | |
| B57 | 15.3 | 2.3 | 130 | |||||
| B179 | Belatacept + Anti-CD40 | Tocilizumab | 18.6 | 9.3 | 124 | |||
| B180 | 16.2 | 5.1 | 118 | |||||
| Artery Patch | B129 | Belatacept + Anti-CD40 | NO | None | 20.8 | 1.1 | 2–3 months | |
| B92 | NA | NA | 2–3 months | |||||
| B54 | Belatacept + Anti-CD40 | CVF | 5.1 | 3.4 | 2 month | |||
| B59 | 11 | 1 | 2 month | |||||
| B95 | Belatacept + Anti-CD40 | Tocilizumab | NA | NA | 2–3 months | |||
| B126 | Belatacept only | 18.3 | 25 | 2–3 months | ||||
| B63 | Belatacept + Anti-CD40 | AAT | 17 | 11.8 | 2–3 months | |||
| B65 | Anti-CD40 only | 16.4 | 21.1 | 2–3 months | ||||
NA = not done
All recipients received grafts from GTKO/CD46 genetically engineered pigs with or without additional human complement (e.g CD55) or coagulation (Thrombomodulin or Endothelial protein C receptor) regulatory proteins.
Measurement of cytokines, chemokines, C-RP, D-Dimers, and fibrinogen
Serum pro-inflammatory cytokines/chemokines (IFNγ, TNFα, IL-1β, IL-17, IL-6, IL-8, monocyte chemotactic protein-1 (MCP-1), sCD40L) were measured by Luminex (Biolegend, San Diego, CA). Fibrinogen, D-Dimers, and C-RP were measured by the Central Laboratory of Presbyterian Hospital, UPMC.
CFSE-mixed lymphocyte reaction (MLR)
Carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR)-labelled recipient baboon peripheral blood mononuclear cells (PBMC, 2×106 cells/mL) were cocultured with irradiated (2,800 cGy) donor pig PBMC (2×106 cells/mL) for 6 days, at a ratio of 1:1. After coculture, cells were stained for CD3e (clone/SP34-2) (BD Pharmingen, Franklin Lakes, NJ). Flow cytometry was performed using LSR II flow (BD Bioscience, San Jose, CA). The percentage of T cell proliferation was measured by FlowJo software (Tree Star, Ashland, OR).
TF expression mRNA (Quantitative reverse transcription polymerase chain Reaction [RT-PCR])
RT-PCR was carried out as previously described (8). Briefly, total RNA pellets were suspended in RNase-free water, followed by treatment with RNeasy Mini Kit (QIAGEN, Hilden, Germany). RNA (<2μg) from each sample was used for reverse transcription with High Capacity DNA Reverse Transcription Kit (ThermoFisher Scientific, Foster, CA). Polymerase chain reaction (PCR) mixture was prepared using SYBR Green PCR Master Mix (Toyobo, Osaka, Japan). The primers used were as follows:
Baboon TF: 5-TGCTTTTACACAGCAGACACAGAGT-3 (forward) and 5-AAGACCCGTGCCAAGTACGT-3 (reverse).
Baboon β-actin: 5-TGGAAGAATGCGGCTCATATT-3 (forward) and 5 TACTATCCAATCCCTAGAAAGAACATG-3 (reverse).
Pig TF: 5-TTTACCAACTCGCCCCCCTTC-3 (forward) and 5-AATGTGCCGTTCACCCTGACTAA-3 (reverse).
Pig β-actin: 5-CTCGATCATGAAGTGCGACTG-3 (forward) and 5-GTGATCTCCTTCTGCATCCTGTC-3 (reverse). Thermal cycling conditions were 1min at 95°C, followed by 40cycles of 95°C for 15sec, and 60°C for 1min, using ABI 7500 System (Applied Biosystems).
RESULTS
T cell responses in xenograft recipients
In heart recipients, T cell responses were reduced after, compared to before transplantation (Table 1). In pig artery patch recipients, reduced T cell responses were observed, except in 2 recipients - B126 (belatacept only) and B65 (ant-CD40mAb only) where slight increases in T cell proliferation were observed.
IFN-γ, TNF-α, IL-1, and IL-17 levels are not elevated in immunosuppressed xenograft recipients
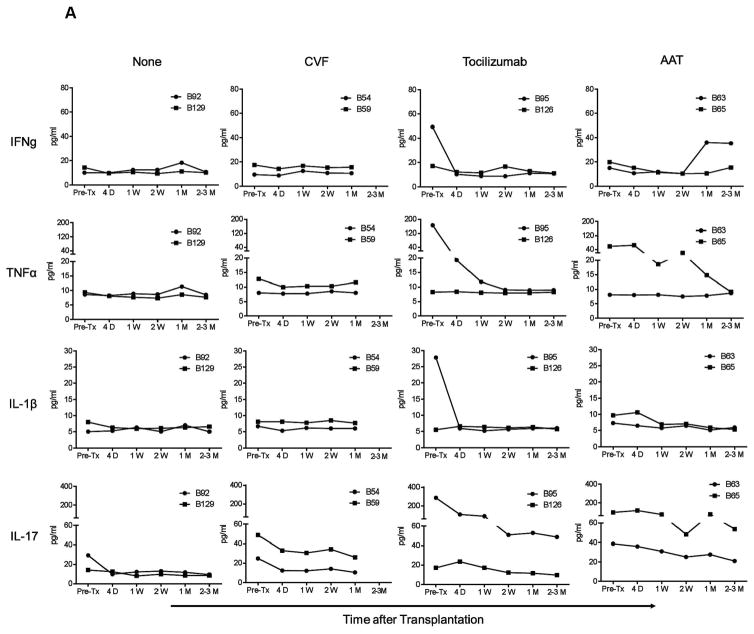
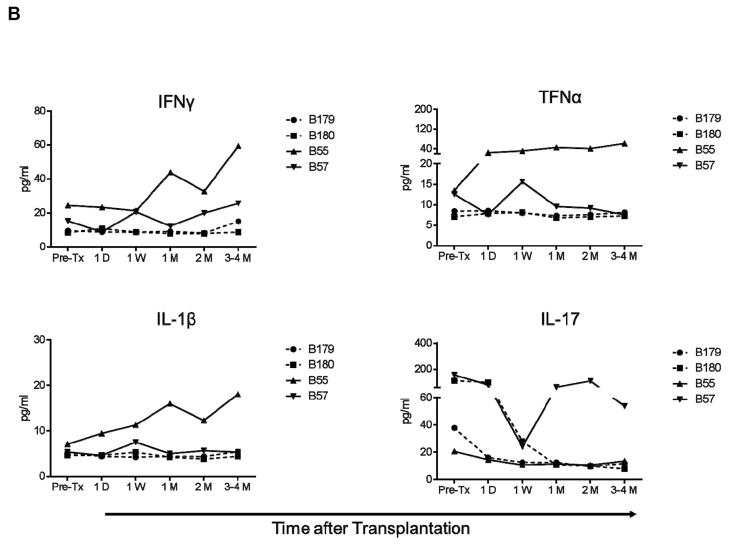
Despite the variability in pre-Tx levels, IFN-γ, TNF-α, IL-1, and IL-17 levels did not increase in any artery patch recipient baboon (Group 1), where they remained either comparable to or lower than pre-Tx, irrespective of the administration of anti-inflammatory agents (Figure 1A). Similarly, in heart recipients (Group 2), levels remained either comparable to or lower than pre-transplant (Figure 1B), except in one recipient (B55) where gradual increases in IFN-γ, TNF-α, and IL-1 were observed before termination of the experiment was required for cytomegalovirus (CMV) infection.
Figure 1. IFN-γ, TNF-α, IL-1, and IL-17 levels were not elevated after artery patch or heart xenotransplantation.
IFN-γ, TNF-α, IL-1, and IL-17 serum levels were measured before and after transplantation in pig artery patch (A) and heart (B) xenograft recipients. After pig artery patch transplantation, recipients received no anti-inflammatory agent (None), CVF, IL-6RA (tocilizumab), or AAT. After heart transplantation, 2 baboons received IL-6RA while 2 baboons did not. Serum samples were collected before transplantation (Pre-Tx), 4D (4 days), and 1W (1 week), 2W (2 weeks), 1M (1 month), and 2–3M or 3–4M (2–3 or 3–4 months) after transplantation. Levels were measured in pg/mL.
MCP-1, IL-6, IL-8, and sCD40L levels are elevated in immunosuppressed xenograft recipients
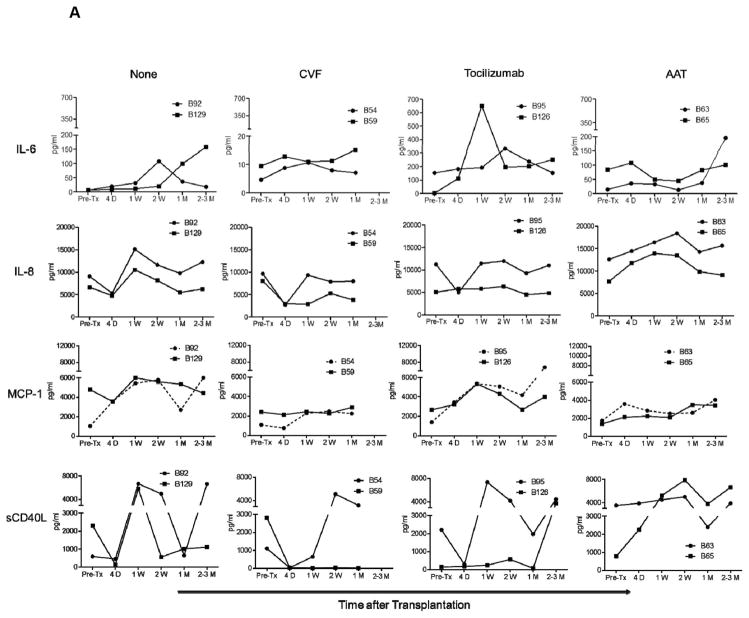
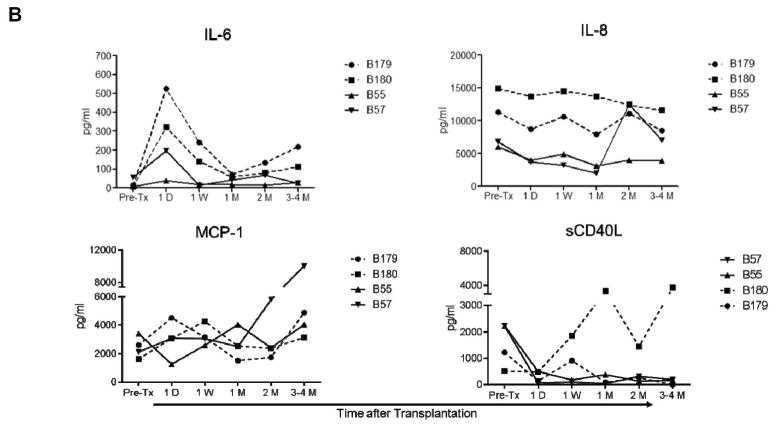
In Group 1, with no administration of anti-inflammatory agents, MCP-1, IL-6, and IL-8 were elevated (Figure 2A). After CVF administration, IL-6, IL-8, and MCP-1 remained lower than, or comparable to, pre-transplant levels. After IL-6RA, MCP-1 levels were also elevated, while IL-6 became transiently elevated. After AAT, IL-6 and IL-8 gradually increased after transplantation. sCD40L levels were sporadically elevated in Group 1 recipients, except in one recipient (B59; with CVF administration).
Figure 2. When no anti-inflammatory agent was administered, IL-6, IL-8, MCP-1, and sCD40L levels were increased after pig artery patch or heart transplantation.
IL-6, IL-8, MCP-1, and sCD40L levels were measured before and after transplantation in artery patch (A) and heart (B) xenograft recipients (treated as for Figure 1). Serum samples were collected before transplantation (Pre-Tx), and 4D (4 days), 1W (1 week), 2W (2 weeks), 1M (1 month), and 2–3M or 3–4M (2–3 or 3–4 months) after transplantation. Levels were measured in pg/mL.
In Group 2, IL-6 was transiently elevated after IL-6RA administration, while IL-8 remained comparable to before transplantation, except in one recipient (B57; with no IL-6RA administration), and MCP-1 gradually increased at one month in the same recipient baboon. sCD40L became elevated in only one baboon (B180; with IL-6RA administration).
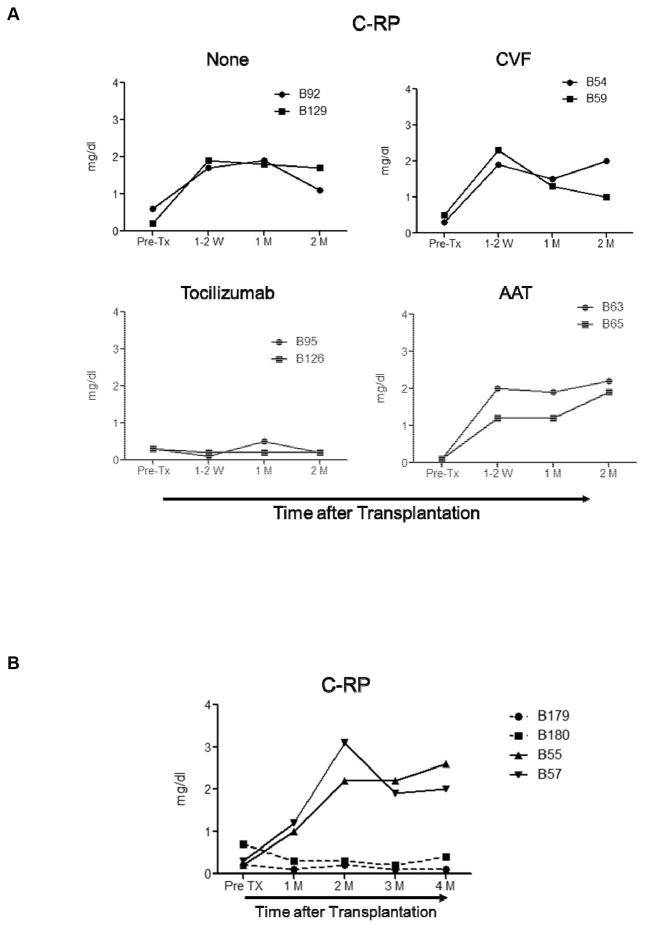
IL-6RA administration prevents increased C-RP levels
In Group 1, C-RP remained low only after IL-6RA administration (Figure 3A), but was elevated when no anti-inflammatory agent, CVF, or AAT had been administered. Similarly in Group 2, IL-6RA administration prevented a rise in C-RP (Figure 3B).
Figure 3. A rise in C-reactive protein was only prevented by IL-6RA administration.
C-RP levels were measured before and after transplantation in artery patch (A) and heart (B) xenograft recipients. Serum samples were collected before transplantation (Pre-Tx), and 1–2W (1–2 weeks), 1M (1 month), and 2M (2 months) after transplantation. C-RP levels were measured in mg/dL.
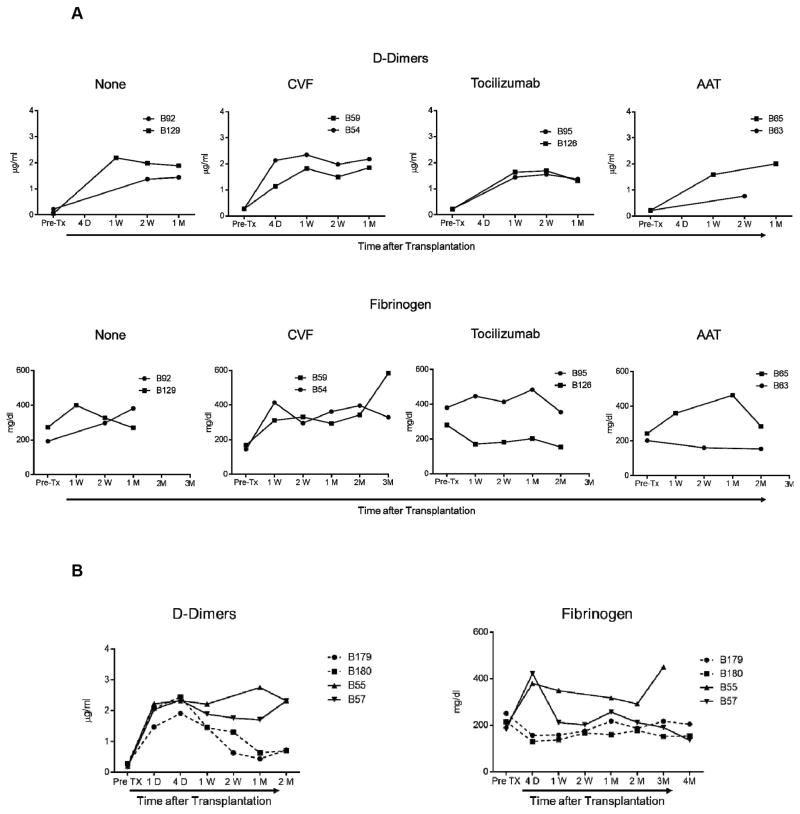
D-Dimers are elevated in all xenograft recipients
In Group 1, D-Dimers levels were elevated in all recipients (Figure 4A), irrespective of the administration of an anti-inflammatory agent. Also, in Group 2, D-Dimers became elevated in all recipients (Figure 4B), irrespective of the administration of IL-6RA.
Figure 4. D-Dimers became elevated in all xenograft recipients irrespective of the administration of anti-inflammatory agents, whereas fibrinogen became elevated in only some recipients.
D-Dimers and fibrinogen levels were measured before and after transplantation in artery patch (A) and heart (B) xenograft recipients. Plasma samples were collected before transplantation (Pre-Tx), and 4D (4 days), 1W (1 week), 1M (1 month), 2M (2 months), or 3M and 4M (3 and 4 months) (when available) after transplantation. D-Dimers were measured in μg/mL. Fibrinogen levels were measured in mg/dL.
Fibrinogen levels in xenograft recipients
In Group 1, fibrinogen levels were elevated when either no anti-inflammatory agent or CVF had been administered, and also increased in one recipient after AAT (B65) (Figure 4A). The increase was prevented by IL-6RA administration. In Group 2, higher levels of fibrinogen were prevented with IL-6RA administration after transplantation (Figure 4B).
Pig and baboon TF mRNA levels in heart xenografts
The heart xenografts from B57 (no IL-6 receptor antagonist), B179 and B180 (with IL-6 receptor antagonist) demonstrated higher levels of pig TF mRNA than two naive pig hearts (controls). Additionally, baboon TF mRNA levels were detectable in the heart xenografts, but not in the naive pig hearts (Figure 5).8 This suggests that IL-6 receptor blockade does not prevent upregulation of either pig or baboon TF in xenograft recipients.
Figure 5. Porcine and baboon TF mRNA levels in naive pig hearts and pig heart xenografts.
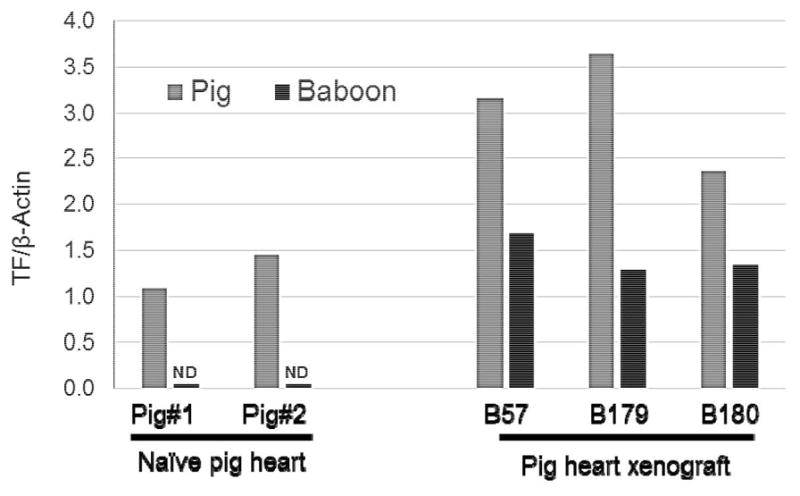
Porcine (gray) and baboon (black) TF mRNA levels were measured in naive pig hearts (left) and pig heart xenografts (right) at the time of euthanasia. Pig TF mRNA levels were elevated in heart xenografts obtained from B57 (no IL-6 receptor antagonist) and B179 and B180 (with IL-6 receptor antagonist), compared to naive pig hearts. Furthermore, baboon TF mRNA was detectable in the heart xenografts, but not in naive pig hearts. TF mRNA levels were normalized to those of β-actin.
DISCUSSION
In this small study we evaluated the therapeutic effect of several anti-inflammatory agents on selected cytokine and chemokine levels in baboons with genetically-engineered pig artery patch or heart xenografts. All recipients received anti-CD40mAb and/or belatacept-based immunosuppressive therapy. No significant increase in anti-pig T cell responses was observed (Table 1), except in 2 recipients (B126 and B65) that received belatacept and anti-CD40mAb, respectively). However, no marked differences in cytokine production were observed in these 2 recipients in comparison to others in their respective groups.
Despite inhibition of anti-pig T cell responses after transplantation (Table 1), no complete prevention of increased pro-inflammatory cytokines and chemokines was achieved after, when anti-inflammatory agents were not administered. These observations suggest that regulation of the inflammatory responses in xenograft recipients (SIXR) may require additional anti-inflammatory therapy, and may be essential for long-term xenograft survival, as previously reported in rodent models of allo-transplantation.1,19 The biological relevance of increased levels of these pro-inflammatory factors remains uncertain. It is yet to be determined whether the development of SIXR early or late after xenotransplantation is associated with an augmented innate immune response, xenograft injury, or enhanced dysregulation of coagulation.
The established interplay between the innate immune system and the coagulation system 3,4 may play a significant role in the development of dysregulation of coagulation, which is a characteristic feature of xenograft failure. The expression of tissue factor (CD142), a key player in the activation of the coagulation system, is upregulated on innate immune cells following cytokine stimulation 5,6 and in xenograft recipients.9,10 On the other hand, it has been shown that, fibrin and fibrin degradation products can induce expression of IL-6 20 and IL-8 21 by endothelial cells. These observations suggest potential mutual amplification of inflammation and coagulation in xenograft recipients and underscores the importance of the prevention of SIXR.7
In the current study, increased cytokine (IL-6) and/or chemokine (IL-8 and MCP-1) levels were not consistently regulated by the administration of individual anti-inflammatory agents. IL-6RA administration was associated with elevated IL-8 and MCP-1, while AAT administration was associated with elevated IL-8. However, the administration of corticosteroids in the Group 2 recipients appeared to be associated with lower levels of IL-6, IL-8, and MCP-1 compared to pre-transplantation. The sustained increase in D-Dimers in all recipients suggests that an inflammatory response persists despite suppression of anti-pig T cell responses and the administration of corticosteroids and/or anti-inflammatory agents. C-RP is a well-studied marker of inflammation, and the role of IL-6 in promoting C-RP production is well known. We have previously shown that serum C-RP levels were elevated in xenograft recipients when immunosuppression was administered, but did not increase when no therapy was administered 7. Notably, Administration of IL-6RA maintained low levels of C-RP.8 Our current observations confirm the therapeutic effect of IL-6RA on C-RP levels. However, IL-6RA administration did not prevent the upregulation of pig or baboon TF in organ xenografts in the current study.
Collectively, we show that elevated individual pro-inflammatory factors in xenograft recipients can be prevented by the administration of selected anti-inflammatory agents. However, the impact of anti-inflammatory agents on the prevention of SIXR and on xenograft survival has yet to be determined.
TABLE 2.
Anti-inflammatory therapy
| Agent | Dose | Duration |
|---|---|---|
| Cobra venom factor (CVF) (Complement Technology, Tyler, Texas) | 100 units/day i.v. | days −1, 0 |
| Tocilizumab (Genentech, South San Francisco, CA) | 10mg/kg i.v. | days −1, 7, 14, 21 in patch, days −1, 7, 14 and every 2 weeks in heart |
| α1-antitrypsin (AAT) (Aralast NP Baxter, Westlake Village, CA) | 60mg/kg i.v. | days 0, 4, 7, 11, 14 and 28 |
Acknowledgments
2C10R4 was kindly provided by Keith Reimann of the NIH NHP Resource Center, Boston, MA. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, #U19 AI090959, and # R21 A1074844, and # 1PO1 HL107152, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Baboon Research Resources, which is supported by the Office of the Director, NIH, under Award Number P40OD010431 and P40OD010988.
ABBREVIATIONS
- AAT
alpha 1-antitrypsin
- C-RP
C-reactive protein
- CVF
cobra venom factor
- IFN-γ
interferon-γ
- IL
interleukin
- IL-6RA
IL-6 receptor antagonist
- MCP-1
monocyte chemotactic protein-1
- sCD40L
soluble CD40 ligand
- SIXR
systemic inflammation in xenograft recipients
- TF
tissue factor
- TNFα,
tumor necrosis factor-α
Footnotes
Conflict of Interests
David Ayares is an employee of Revivicor, Inc. The other authors report no conflict of interest.
References
- 1.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459–471. doi: 10.1038/nri3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labarrere CA, Woods JR, Hardin JW, et al. Early inflammatory markers are independent predictors of cardiac allograft vasculopathy in heart-transplant recipients. PLoS One. 2014;9:e113260. doi: 10.1371/journal.pone.0113260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J Thromb Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strukova S. Blood coagulation-dependent inflammation. Coagulation-dependent inflammation and inflammation-dependent thrombosis. Front Biosci. 2006;11:59–80. doi: 10.2741/1780. [DOI] [PubMed] [Google Scholar]
- 5.Christenson RH, Defilippi CP, Kreutzer D. Biomarkers of ischemia in patients with known coronary artery disease: do interleukin-6 and tissue factor measurements during dobutamine stress echocardiography give additional insight? Circulation. 2005;112:3215–3217. doi: 10.1161/CIRCULATIONAHA.105.581918. [DOI] [PubMed] [Google Scholar]
- 6.Ikonomidis I, Athanassopoulos G, Lekakis J, et al. Myocardial ischemia induces interleukin-6 and tissue factor production in patients with coronary artery disease: a dobutamine stress echocardiography study. Circulation. 2005;112:3272–3279. doi: 10.1161/CIRCULATIONAHA.104.532259. [DOI] [PubMed] [Google Scholar]
- 7.Ezzelarab MB, Ekser B, Azimzadeh A, et al. Systemic inflammation in xenograft recipients precedes activation of coagulation. Xenotransplantation. 2015;22:32–47. doi: 10.1111/xen.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR) Xenotransplantation. 2015;22:399–405. doi: 10.1111/xen.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87:805–812. doi: 10.1097/TP.0b013e318199c34f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–316. doi: 10.1111/xen.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Taniguchi S, Neethling FA, et al. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation. 1997;64:1255–1261. doi: 10.1097/00007890-199711150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Koulmanda M, Sampathkumar RS, Bhasin M, et al. Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant. 2014;14:1543–1551. doi: 10.1111/ajt.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezzelarab MB, Ekser B, Echeverri G, et al. Costimulation blockade in pig artery patch xenotransplantation - a simple model to monitor the adaptive immune response in nonhuman primates. Xenotransplantation. 2012;19:221–232. doi: 10.1111/j.1399-3089.2012.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezzelarab MB, Ekser B, Isse K, et al. Increased soluble CD154 (CD40 ligand) levels in xenograft recipients correlate with the development of de novo anti-pig IgG antibodies. Transplantation. 2014;97:502–508. doi: 10.1097/TP.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 16.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22:211–220. doi: 10.1111/xen.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238:288–299. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein DR. Inflammation and transplantation tolerance. Semin Immunopathol. 2011;33:111–115. doi: 10.1007/s00281-011-0251-2. [DOI] [PubMed] [Google Scholar]
- 20.Wung BS, Hsu MC, Wu CC, Hsieh CW. Resveratrol suppresses IL-6-induced ICAM-1 gene expression in endothelial cells: effects on the inhibition of STAT3 phosphorylation. Life Sci. 2005;78:389–397. doi: 10.1016/j.lfs.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 21.Qi J, Goralnick S, Kreutzer DL. Fibrin regulation of interleukin-8 gene expression in human vascular endothelial cells. Blood. 1997;90:3595–3602. [PubMed] [Google Scholar]