Abstract
Methylmercury (MeHg) is known to biomagnify in marine food chains, resulting in higher concentrations in upper trophic level animals than their prey. To better understand how marine copepods, an important intermediate between phytoplankton and forage fish at the bottom of the food chain, assimilate and release MeHg, we performed a series of laboratory experiments using the gamma-emitting radiotracer 203Hg2+ and Me203Hg with the calanoid copepod Acartia tonsa. Assimilation efficiencies (AEs) of Hg2+ and MeHg ranged from 25 to 31% and 58 to 79%, respectively, depending on algal diets. The AEs were positively related to the fraction of mercury in the cytoplasm of the algal cells that comprised their diet. Efflux rates of Hg2+ (0.29/d) and MeHg (0.21/d) following aqueous uptake were similar, but efflux rates following dietary uptake were significantly lower for MeHg (0.11-0.22 /d) than Hg2+ (0.47-0.66 /d). The calculated trophic transfer factors (TTFs) in copepods were >1 for MeHg and consistently low (≤0.2) for Hg2+. We used the parameters measured in this study to (1) quantitatively model the relative importance of MeHg sources (water or diet) for copepods, and to (2) predict the overall MeHg concentrations in copepods in different marine environments. In general, MeHg uptake from diet accounted for most of the body burden in copepods (>50%). For an algal diet whose MeHg dry weight bioconcentration factor (BCF) is ≥106, over 90% of a copepod's MeHg body burden can be shown to derive from diet. Our model-predicted MeHg concentrations in the copepods were comparable to independent measurements for copepods in coastal and open-ocean regions, implying our measured parameters and model are applicable to natural waters.
Keywords: Bioaccumulation, Zooplankton, Assimilation efficiency, Efflux rate, Trophic transfer
Introduction
Phytoplankton concentrate many trace metals out of seawater [1] and serve as highly enriched sources of these metals for herbivorous animals, including zooplankton and bivalve mollusks [2-4]. Zooplankton are of particular interest as their sinking waste products and cast exoskeletons can vertically transport metals [5] and in so doing affect their oceanic residence times [4, 6]. Overall, zooplankton play an important role in the bioaccumulation of metals in marine food webs because they link phytoplankton, whose bioconcentration of metals is the single largest enrichment step in food chains, with fish and other metazoans; thus, trace metals that are assimilated into zooplankton could ultimately be transferred to higher trophic level organisms [4].
Although phytoplankton has been found greatly enriched in many essential and nonessential metals, most of the nonessential metals are not appreciably assimilated from phytoplankton food by zooplankton, let alone further biomagnifying in marine food chains [4]. Indeed, the concentrations of most metals in organisms at higher trophic levels typically decrease with increasing trophic level; the exception is methylmercury (MeHg) which is the only trace metal species known to substantially biomagnify in food chains. This biomagnification is due to its high assimilation efficiency in organisms at every trophic level and very slow release rates from animal tissues [7, 8]. As a consequence, MeHg is efficiently transferred from one trophic level to the next and is ultimately enriched in fish. It is known that MeHg accumulation in heterotrophic organisms is mainly from ingesting MeHg-containing food instead of direct uptake from water. Thus, food web structure could influence the MeHg transfer to higher trophic level organisms [9, 10]. Because seafood consumption is the primary route for human intake of MeHg [11] and such exposure may lead to adverse health consequences to exposed humans [12], there is interest in the food chain build-up of MeHg in marine ecosystems among public health agencies and the general public. While there have been comprehensive studies of the trophic transfer and cycling of many trace metals by zooplankton [4], MeHg has not been similarly studied for marine zooplankton, where few studies have examined the transfer of MeHg from phytoplankton to zooplankton.
Previous work has shown that a high assimilation efficiency (>90%) and low release rate (<5 %/d) of MeHg in a fresh water daphnid, Daphnia pulex feeding on a chlorophyte [13], led to a significant transfer of MeHg from phytoplankton to zooplankton. For marine organisms, Mason et al. [14] reported a high assimilation efficiency (>60%) of MeHg in marine copepods feeding on a diatom. This value was four times greater than Hg2+ and the fractions of MeHg and Hg2+ accumulated in algal cell cytoplasm were strongly correlated to the assimilation efficiencies in copepods. This correlation of cytoplasmic distribution in algal cells and AE in herbivorous copepods is consistent with findings for other elements [15]. Lawson and Mason [16] also found high assimilation efficiencies (>50%) of MeHg in marine copepods and amphipods feeding on diatoms. Mathews and Fisher [7], examining trophic transfer of MeHg in a simple estuarine food chain, reported a high assimilation efficiency of MeHg (>76%) for both zooplankton feeding on phytoplankton and small planktivorous fish feeding on zooplankton.
In this study, we conducted a series of laboratory experiments to evaluate assimilation efficiencies of both Hg2+ and MeHg in marine copepods that fed on three marine phytoplankton species belonging to different algal classes, and compared this with Hg2+ and MeHg uptake from the aqueous phase. We estimated Hg2+ and MeHg efflux rates from copepods and trophic transfer factors from phytoplankton to copepods. With those parameters, we used a simple model to estimate the relative importance of dietary and aqueous sources of MeHg in zooplankton and compared modeled MeHg concentrations in marine zooplankton with field data.
Materials and methods
Preparation of plankton
Three marine phytoplankton species were used to feed zooplankton, the diatom Thalassiosira pseudonana (clone 3H), chlorophyte Dunaliella tertiolecta (CCMP1320) and cryptophyte Rhodomonas salina (CCMP1319). These species, representing three different algal classes, have significantly different MeHg bioconcentration factors [17] as well as different cell wall characteristics and photosynthetic storage products, which may influence the trophic transfer of MeHg to copepod grazers. All species were held in clonal, unialgal cultures maintained axenically for generations at constant temperature (18°C) and 14 h:10 h light:dark cycle (200 μmol quanta/m2/s) with cool white fluorescent lamps. Routine cultures were maintained in sterile filtered (0.2 μm) surface seawater (35 psu) and enriched with f/2 nutrients [18].
The coastal calanoid copepod Acartia tonsa, was collected in early summer from Stony Brook Harbor, New York with a plankton net (160 μm mesh) and transferred into 0.2 μm filtered seawater. Immediately after collection, adult A. tonsa individuals were visually identified using a dissecting microscope and separated from other plankton. The separated copepods were maintained in a tank with filtered seawater under shimmer light and fed the algae R. salina and Isochrysis galbana every 12 h to allow them to acclimate to laboratory conditions for 2 d. Prior to experiments, the copepods were transferred to another tank with 0.2 μm filtered seawater without food for 12 h in order to evacuate their guts of ingested material. To put the amount of bioaccumulated Hg in copepods into context, we measured total background Hg concentrations in A.tonsa collected from Stony Brook Harbor. Total Hg, determined by analyzing freeze-dried copepods using a DMA-80 direct mercury analyzer (Milestone, Inc.), was found to be 30.2 ± 4.6 ng/g dry weight (n = 6, water content = 86 ± 1 %). The reported fraction of total Hg in the form MeHg in marine zooplankton generally ranges from 12 to 45% [19].
Hg Assimilation and depuration by copepods from food
The protocol of using radioisotopes to evaluate the accumulation and assimilation of elements in copepods has been well established [2, 15]. In this study, we used the gamma-emitting radioisotope, 203Hg, as a tracer to track the transfer of Hg (Hg2+ and MeHg) from phytoplankton to zooplankton. Short pulse-feeding experiments were performed to help avoid extensive excretion and recycling of Hg which would lead to ambiguous results.
Inorganic 203Hg2+ solution was obtained from Eckert and Ziegler Isotope Products (Valencia, CA) (specific activity: 5 Ci/g). It was further converted to methylmercury (Me203Hg) following methods described elsewhere [17, 20]. In brief, the 203Hg2+ solution was adjusted to pH 5 with acetate buffer and mixed with methylcobalamin (C63H91CoN13O14P). Allowing the reaction to proceed in the dark for 18 to 24 h, Me203Hg was formed and then extracted by dichloromethane (CH2Cl2) and re-dissolved in Milli-Q® water. The conversion yield (fraction of total 203Hg2+ recovered as Me203Hg) was 95 ± 3% (n = 6).
Algal cells for feeding experiments were cultured in f/2 medium without adding EDTA. Late-log phase cells were concentrated from water by either filtering onto polycarbonate membranes or centrifuging and resuspending into 0.2-μm sterile-filtered seawater. The algal cells were then radiolabeled with 203Hg2+ or Me203Hg (55-78 kBq/L, corresponding to concentrations 0.6-2.0 μg/L of Hg). The concentrations of Hg2+ and MeHg used in these experiments were higher by about 103 and 104 times, respectively, than those in natural waters. Because 203Hg2+ was prepared in 1 M hydrochloric acid, corresponding amounts of 1 M Suprapur NaOH were added immediately afterwards to maintain the seawater pH (~8.2). In order to have uniformly radiolabeled cells, the cultures were grown for at least 2 days (several cell divisions) to reach equilibrium. The analysis of the cellular distribution of the 203Hg (for both Hg2+ and MeHg) followed the protocol described in Reinfelder and Fisher [15].
Radiolabeled algal cells were concentrated and resuspended as described above and then added into transparent Teflon feeding bottles containing 100 ml filtered seawater with a cell density of 6 to 8 × 104 cells/ml, depending on algal species. Three replicates were used for each algal food treatment. In each bottle, 60 copepods fed on radiolabeled cells in the dark for 45 minutes. After feeding, the copepods were collected with a 160 μm nylon mesh, rinsed twice with 10 ml of unlabeled, filtered seawater and immediately transferred to 25 ml clean filtered seawater for radioanalysis (t = 0, assigned as the starting point of the depuration period). Fecal pellets egested by copepods during the feeding experiment were also collected with a 20 μm nylon filter, rinsed with filtered unlabeled seawater, and radioassayed. The copepods were transferred back to the original Teflon bottles with clean filtered seawater to depurate their ingested radiolabeled food. They were maintained under the same conditions as the feeding experiments but with non-radiolabeled food of the same species. The radioactivity of copepods and their fecal pellets was determined periodically (t = 4, 12, 24, 36, 48, 60, 72 h) over the 3-d depuration period. The food was added and the seawater was replaced at each time point. The gentle handling throughout resulted in negligible mortality of copepods.
Hg2+ and MeHg may desorb from radiolabeled algal cell surfaces into the dissolved phase during the feeding experiment. Consequently, it was possible that the copepods may have acquired Hg from both water and food sources. Thus, a control treatment was used to assess the copepod uptake of 203Hg (for both Hg2+ and MeHg) that had desorbed from the algae during the same time period used for radioactive feeding (45 min). To accomplish this, 100 ml of filtered seawater was exposed to radiolabeled algal cells and was then passed through 0.2 μm membrane to remove all particles. Uptake of desorbed radioactive Hg by copepods from this seawater constituted the control treatment for the feeding experiments.
MeHg uptake from the dissolved phase
Two experiments were conducted to measure MeHg uptake by copepods from the dissolved phase, one in which uptake was evaluated over time, the other in which MeHg was added at varying concentrations. For the former, Me203Hg was added to 0.2 μm filtered seawater at 0.35 nM and equilibrated for 2 h. Copepods were then exposed to this radioactive seawater (60 individuals in a beaker containing 100 ml filtered seawater, total of 8 beakers). Periodically, at 2, 4, 8, 12 h, all 60 copepods were collected from each of the two replicates onto 20 μm nylon membranes and rinsed with filtered clean seawater in preparation for radioanalysis. To evaluate the uptake over a range of MeHg concentrations, Me203Hg was added to 0.2 μm filtered seawater at five different concentrations (20, 50, 100, 250, 500 ng/L) and equilibrated for 2 h. Each treatment had two replicates. 60 copepods were exposed to each MeHg for 4 h, after which they were collected onto 20 μm nylon membranes and rinsed with filtered clean seawater for radioanalysis.
203Hg measurements
Radioactivity of 203Hg ingested by copepods was determined noninvasively using a large-well NaI(Tl) gamma detector. Radioactivities of labeled phytoplankton and seawater were measured using an LKB Wallac 1282 Compugamma NaI(Tl) gamma detector equipped with a well detector. 203Hg activity was assessed at 279 keV. Intercalibration between the two gamma counters was performed, and all samples were counted with standards and decay-corrected. Propagated counting errors were <5%.
Results
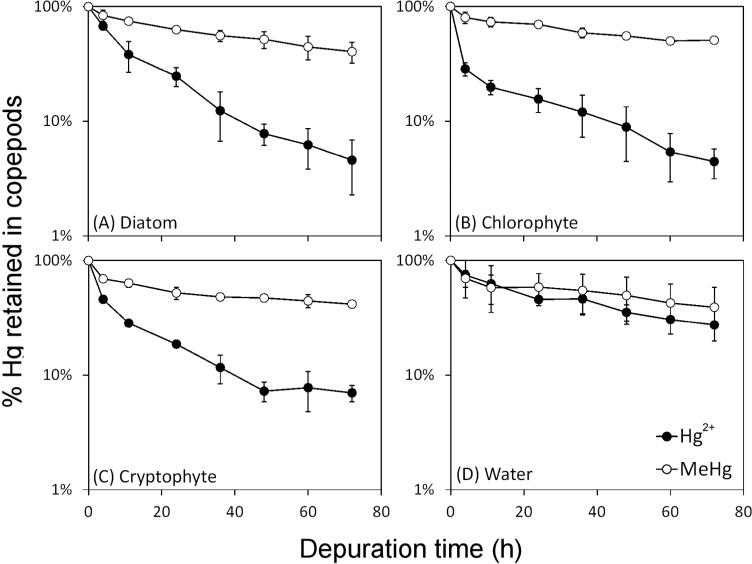
The copepods ingested radiolabeled cells during all feeding experiments. Figure 1 shows the percentage of 203Hg (203Hg2+ or Me203Hg) retained in them over different periods of depuration. All values shown are net values after subtracting radioactivity from control treatments; controls typically accounted for 5 to 15% of the total radioactivity acquired during feeding. The percentage of Hg was calculated as the radioactivity in the copepods divided by the radioactivity measured in the copepods at time zero of depuration (immediately after feeding). 203Hg in copepods decreased over time in all experiments during depuration. Depuration patterns typically displayed an initial, rapid loss of Hg from the copepods, followed by a more gradual decline after 24 h of depuration. The initial rapid loss reflects loss of unassimilated boluses of ingested algal food through defecation, whereas the slowly exchanging pool reflects loss of assimilated Hg driven by metabolic activity [2, 21]. Assimilation efficiencies (AEs) were calculated from the second stage of depuration (1-3 d), using the equation described by Wang and Fisher [2]:
| (1) |
where %A is the percentage of radioactivity retained in copepods at time t and k is the depuration rate constant during 1 to 3 d of depuration. A0 is the y-axis intercept of radioactivity retained in copepods and is defined as the AE of the ingested mercury. Calculated mercury AEs and efflux rate constants (ke's) are shown in Table 1. Copepods ingesting MeHg-labeled food had higher AEs (58-79%) than those ingesting Hg2+-labeled food (25-31%). AEs for both Hg2+ and MeHg among different algal species did not differ significantly. Efflux rate constants following uptake from the aqueous phase (kew) were similar among the two Hg species, 0.29 /d for Hg2+ and 0.21 /d for MeHg. Efflux rate constants (kef) following ingestion of Hg-labeled algal food were significantly lower for MeHg (0.11-0.22 /d) than for Hg2+ (0.47-0.66 /d). In both Hg2+ and MeHg feeding experiments, Hg loss in the form of fecal pellets during the depuration period accounted for 10-40% for Hg2+ and 1-13% for MeHg of total Hg released by copepods, suggesting that excretion of Hg into the dissolved phase was the primary route of Hg loss.
Figure 1.
Depuration curves of Hg2+ and MeHg in the copepod A. tonsa from all the experiments. Hg sources in (A) to (C) are different algal foods and (D) is the dissolved phase. Time zero represents the radioactivity measured immediately after feeding, which is assigned as 100%. In (A)-(C), radioactivity from controls (dissolved phase) were subtracted from radioactive measurements following feeding. Each point represents the average from three replicates and error bars denote 1 SD.
Table 1.
The calculated assimilation efficiencies (AEs, %) and efflux rate constants (ke, 1/d) of Hg2+ and MeHg in the copepod Acartia tonsa from different uptake routes.
| Uptake route | Hg2+ |
MeHg |
||
|---|---|---|---|---|
| AE (%) | ke (1/d) | AE (%) | ke (1/d) | |
| Diatom | 31 | 0.64 | 79 | 0.22 |
| Chlorophyte | 31 | 0.66 | 78 | 0.16 |
| Cryptophyte | 25 | 0.47 | 58 | 0.11 |
| Water | 0.29 | 0.21 | ||
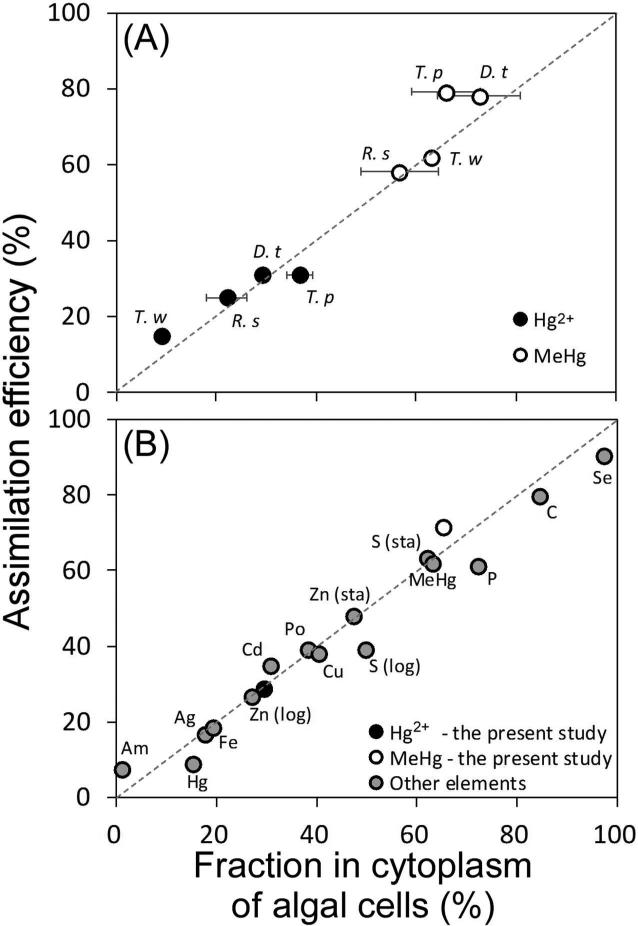
There was a strong relationship between AEs and cytoplasmic distribution of Hg in algal cells (r2 = 0.95); the slope of this regression line was about 1 and the y-intercept was about 0 (Figure 2A). The clear separation of MeHg from Hg2+ in AEs in A. tonsa reflects their different penetration into algal cytoplasm of the different algal species (Figure 2A).
Figure 2.
(A) Relationship between assimilation efficiencies of Hg in the copepod A. tonsa and cytoplasmic distributions of Hg in algal cells. The solid and open circles represent Hg2+ and MeHg, respectively. The dashed line is the 1:1 ratio line. T. p: Thalassiosira pseudonana, D. t: Dunaliella tertiolecta, R. s: Rhodomonas salina, T. w: Thalassiosira weissflogii; T. w data from Mason et al. [14]. The linear regression equation: y = 1.04x + 1.01, r2 = 0.95. (B) Assimilation efficiency of ingested elements in copepods as a function of the cytoplasmic distributions of those elements in the ingested algal cells. Data are average values from Reinfelder and Fisher [15], Hutchins et al. [38], Mason et al. [14], Chang and Reinfelder [39], Stewart and Fisher [24] and this study. “Sta” and “log” denote stationary phase and exponential growth phase of the phytoplankton cells, respectively, consumed by the copepods. The linear regression equation: y = 0.91x + 2.38, r2 = 0.97.
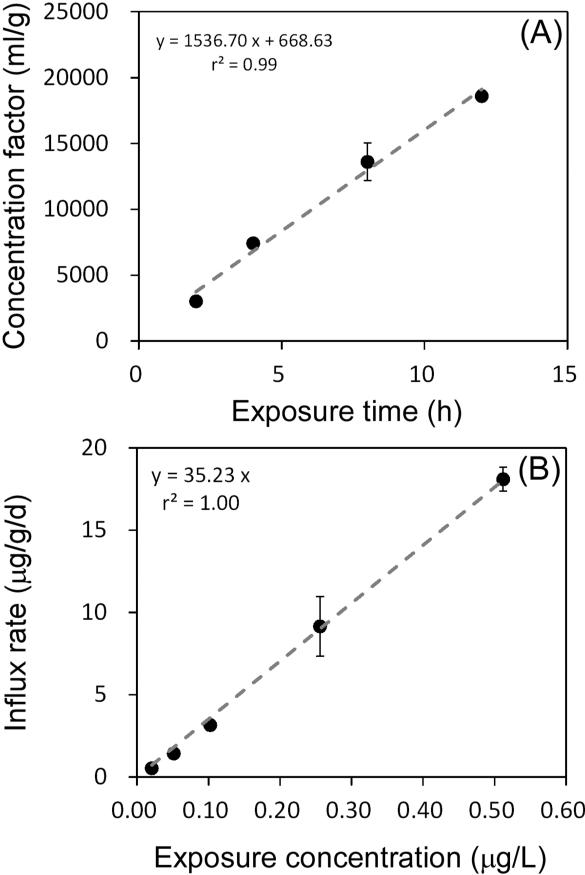
MeHg uptake from the dissolved phase by copepods increased linearly during the 12 h of exposure (Figure 3A), suggesting that MeHg uptake did not reach equilibrium within the first 12 h of exposure. The dry weight concentration factor ([Me203Hg Bq/g]copepod / [Me203Hg Bq/ml]dissolved phase) reached 3 × 104 at 12 h. Figure 3B shows the relationship between MeHg influx rates and dissolved MeHg concentrations after 4 h of exposure. The higher aqueous MeHg concentration led to a greater influx rate, suggesting the influx rate was directly dependent on the ambient MeHg concentration. The metal influx rate constant of 35 (L/g/d, dry weight basis) was obtained from the regression slope of Figure 3B.
Figure 3.
(A) The calculated concentration factors of MeHg in copepods exposed to dissolved MeHg as a function of exposure time. Concentration factors represent the [MeHg]copepods to [MeHg]water ratio for each treatment. (B) The influx rate of MeHg in A. tonsa from the aqueous phase as a function of ambient dissolved concentrations. Each point represents the average from two replicates and error bars denote 1 SD.
Discussion
Hg assimilation and depuration from ingested food
Calculated AEs of Hg2+ and MeHg in copepods were comparable to previous studies. Fisher et al. [22] found that 21% of Hg2+ was assimilated by a marine copepod, Anomalocera pattersoni, fed a prymnesiophyte diet, I. galbana. Mason et al. [14] reported that 15% of Hg2+ and 62% of MeHg were assimilated by marine copepods (A. tonsa, Temora longicornis, Centropages sp.) fed a diatom diet, Thalassiosira weissflogii. A similar experiment conducted by Lawson and Mason [16] using marine copepods (Eurytemora affinis) and amphipods (Hyallela azteca) fed T. weissflogii obtained an AE for MeHg in zooplankton of >50%. Williams et al. [23] reported AEs of Hg2+ and MeHg in the marine amphipod Leptocheirus plumulosus fed I. galbana to be 6% and 81%, respectively. Overall, these results suggest that the AEs of MeHg are much higher than the AEs of Hg2+, and that diet composition does not have a pronounced influence on the assimilation of Hg2+ and MeHg in marine copepods.
As shown in Figure 2A, the pattern of AEs and cellular distributions of mercury in algal diets agrees with other elements reported by Reinfelder and Fisher [15]. As noted above, Hg2+ and MeHg showed two distinct groups. MeHg penetrated into the cytoplasm of algal cells more than Hg2+ did, and AEs in zooplankton feeding on these algae followed correspondingly. Moreover, we further compared our Hg data with other elements taken from previous copepod studies (Figure 2B). Generally, there was a strong linear relationship between AE and cytoplasmic distribution of the elements (r2 = 0.97), with the slope close to 1 and the y-intercept close to 0. MeHg, which is biologically nonessential, has an AE that exceeds some essential metals (e.g., Zn, Cu and Fe) and is comparable to S and P (Figure 2B). Stewart and Fisher [24] reported that AEs of polonium in marine copepods varied significantly with different algal diets and ranged from 19 to 55%, which was also correlated to the percentage of Po in cytoplasm of algal cells. However, we did not observe significant differences in AEs and cytoplasmic distributions among the three algal diets for either Hg2+ or MeHg. The positive relationship of elemental distribution in algal cytoplasm with AEs in herbivorous animals probably reflects the degree to which the metal is released from ingested algal cells into the gut fluid of the copepod in a form that can cross the gut lining [15, 24]. Thus, metals bound to compounds in algal cytoplasm that are sought by the animal (such as proteins or amino acids) may more effectively cross the gut lining (become assimilated) than metals bound to cellular components (such as cell walls) that remain undissolved in the gut.
The efflux rate constants of Hg2+ (0.29 /d) and MeHg (0.21 /d) from the copepods following uptake from the aqueous phase (kew) were 1.5 to 3-fold higher than other elements such as Ag (0.17 /d), Cd (0.11 /d), Co (0.12 /d) and Zn (0.11 /d) [2] and an order of magnitude greater than Po (0.01-0.03 /d) [24]. For efflux rate constants following dietary uptake (kef), Hg2+ (0.47-0.66 /d) was much higher than MeHg (0.11-0.22 /d), as well as other elements like Ag (0.29 /d), Cd (0.30 /d), Co (0.28 /d), Se (0.16 /d), Zn (0.08 /d), and Po (0.01-0.07 /d) [2, 24]. Our results indicate that loss rates from copepods following uptake from food were generally greater than those obtained from water, consistent with findings of Wang and Fisher [2]. Whether this difference is attributable to different copepod tissue distributions of accumulated metal from diet vs. water remains to be determined.
Methylmercury uptake from the dissolved phase
The calculated concentration factor and influx rate of MeHg were closer to Ag (10.42 L/g/d) than other elements [2]. The study of the physiology of trace element uptake in crustaceans by Rainbow [25] noted that metals could enter these animals through either passive carrier-mediated transport or active transport through either ionic or protein channels. Like Ag and Cu, it is known that Hg (including MeHg) has a very strong affinity for sulfur and this may account for MeHg's very high concentration factors and influx rates from the dissolved phase.
Trophic transfer factors of Hg
Various models have been developed to assess and quantify bioaccumulation and biomagnification of elements and compounds in aquatic ecosystems. The combination of high assimilation efficiencies and low efflux rate constants of MeHg in copepods would result in potential biomagnification in food chains. Here we use a simple equation to evaluate the trophic transfer factor (TTF) for Hg2+ and MeHg [8]:
| (2) |
where AE is assimilation efficiency (%), IR is weight-specific ingestion rate (g/g/d, dry weight), kef is efflux rate constant from dietary uptake (1/d) and g is growth rate constant (1/d). For TTF values >1, biomagnification is expected. We used AE and kef values obtained from this study and literature values for growth rate (0.03 /d) for A. tonsa [26]. A range of ingestion rates (0.33-0.43 g/g/d) of marine copepods from previous studies [26-28] were taken to calculate TTF. Calculated TTFs are listed in Table 2. As expected, TTFs of Hg2+ were consistently low (≤0.2) and TTFs of MeHg were all >1, suggesting that marine copepods concentrate MeHg and act as an important intermediate between phytoplankton and forage fish to transfer MeHg in marine food chains.
Table 2.
The estimated trophic transfer factors (TTFs) of Hg2+ and MeHg in the copepod Acartia tonsa following uptake from different food types.
| Food type | AE (%) |
IR (g/g/d) |
kef (1/d) |
g (1/d) | TTF |
|||
|---|---|---|---|---|---|---|---|---|
| Hg2+ | MeHg | Hg2+ | MeHg | Hg2+ | MeHg | |||
| Diatom | 31 | 79 | 0.33-0.43 | 0.64 | 0.22 | 0.03 | 0.15-0.20 | 1.04-1.35 |
| Chlorophyte | 31 | 78 | 0.33-0.43 | 0.66 | 0.16 | 0.03 | 0.15-0.19 | 1.37-1.78 |
| Cryptophyte | 25 | 58 | 0.33-0.43 | 0.47 | 0.11 | 0.03 | 0.16-0.21 | 1.40-1.83 |
AE = assimilation efficiency; IR = ingestion rate; kef = efflux rate constant following uptake from food; g = growth rate.
Modeling of Hg uptake pathways in copepods
Metal accumulation in aquatic organisms can be depicted as a balance between metal uptake and loss from aqueous and dietary routes. We used a simple first-order equation to describe the relative contribution from each route and predict the overall final concentrations in zooplankton. At steady-state, when metal uptake is balanced by metal elimination and growth, the metal concentration in a given organism can be described by the following equations [8, 29]:
| (3) |
| (4) |
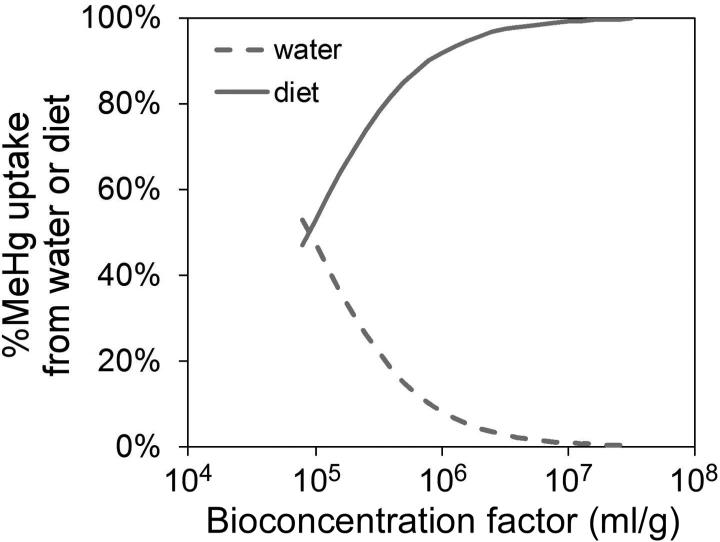
where Css is the metal concentration (μg/g dry weight) in an organism at steady-state, Css,w is the metal concentration (μg/g) obtained from the dissolved phase, Css,f is the metal concentration (μg/g) acquired from diet, ku is the metal uptake rate constant (L/g/d) from the dissolved phase, Cw and Cf are the metal concentration in the water (μg/L) and the prey (μg/g), respectively, kew and kef are the efflux rate constants (1/d) following aqueous and dietary uptake, respectively, AE is the metal assimilation efficiency (%), IR is the weight-specific ingestion rate (g/g/d, dry weight), and g is the growth rate constant (1/d). We applied equation 4 to understand MeHg accumulation in the copepods, and used average values of ku (35 L/g/d), kew (0.21 /d), kef (0.16 /d) and AE (72%) obtained from our experiments. Maximum IR (0.43 g/g/d, dry weight) and average g (0.03 /d) were taken from the previous studies mentioned above [26-28]. Css may vary due to variability in biologically mediated parameters such as AE and IR (which may vary due to food quality and/or concentration, and other physiological and/or environmental conditions) as well as variability in geochemical parameters (such as Cw and Cf which may vary regionally and temporally) in different aquatic environments. First, we used the reported bioconcentration factor (BCF, Cf-to-Cw ratio on a dry weight basis) of MeHg between water and phytoplankton to model the relative importance of aqueous and dietary uptake to marine copepods. The volume concentration factors of MeHg in diverse marine algae ranged from 104.2 to 106.8 [17], which was equivalent to BCFs of 104.9 to 107.5 (assuming mean cell volume-to-dry weight ratio of 5.0 [30]). Figure 4 shows the model predicted percentage of MeHg uptake from aqueous vs. dietary sources as a function of MeHg BCFs in algal food. Overall, diet is the major source (>50%) for MeHg accumulation in copepods. As the MeHg BCF increases, the MeHg uptake contributed from the dissolved phase decreases sharply. In the open ocean where MeHg BCFs in phytoplankton are typically greater than 106 [31, 32], our model suggests that the dietary route would be the predominant pathway (>90%) for copepods to acquire MeHg.
Figure 4.
The model prediction of percentage of total MeHg uptake in the copepod A. tonsa from dissolved or dietary sources as a function of MeHg bioconcentration factor (BCF) in algae, which is proportional to Cf (Eqn. 4). The assumed BCF ranged from 104.9 to 107.5 (see text, [17, 30]). The solid and dashed lines represent the contribution from diet and water, respectively. The parameters used in this calculation were the mean values from Table 1 and 2.
In addition, we incorporated field data into our model and compared the outcome with independently measured data. To date, the applicable field data we found were from five different marine environments (Table 3), including the NW Atlantic [33], the Central Pacific [31, 34], Long Island Sound [35], Groswater Bay, Canada [36] and the Gulf of Lions, France [37]. In Table 3, we present the field data and normalized them onto a dry weight basis. If the water content of biota samples was not clearly noted in the original paper, we assumed a water content of 95% for phytoplankton and 90% for zooplankton. We used a 200 μm cut-off to differentiate between phytoplankton (microseston) and zooplankton. The size range used in each study was also noted in Table 3. Although parameters used in our model referred specifically to the copepod A. tonsa, previous work found no pronounced differences for other metals among different calanoid copepod species [15]. Our model-predicted values of MeHg are comparable with those directly measured in bulk zooplankton (Table 3). Zooplankton collected in the Long Island Sound study were primarily composed of calanoid copepods (Acartia spp.) [35], and the predicted values closely match the measured values. In the Groswater Bay study, there was only one sample for microseston which may have biased the predicted value. The size range used for collecting microseston in the Gulf of Lions study was 80 to 200 μm, which did not comprehensively cover the size spectrum of marine nanophytoplankton and therefore may increase the uncertainty of the predicted value. Note that the ku value for our modeling was based on experiments using elevated Cw values of Hg2+ and MeHg. That the model prediction of Css in zooplankton matched independent field values (Table 3) suggests that this ku value is applicable to Hg concentrations in natural waters.
Table 3.
Comparison between measured MeHg concentrations in zooplankton collected from different regions and predicted MeHg concentrations from the model using lab-derived and field-collected parameters. Thus, Cw for these calculations equals the MeHg concentration reported for water at each site, Cf equals the MeHg concentration in microseston at each site, and other parameters for MeHg from equation 4 are means from values given in Table 1. Thus, kef = 0.16 (1/d), AE = 72 (%), IR = 0.43 (g/g/d dw), g = 0.03 (1/d), ku = 35 (L/g/d), kew = 0.21 (1/d). Model-predicted values for copepods are compared with independently measured MeHg concentrations in zooplankton at each site. Propagation of uncertainty (1 SD) was calculated if applicable.
| Study area | Water (ng/L) | Microseston (ng/g dw) | Zooplankton (ng/g dw) |
Reference | |
|---|---|---|---|---|---|
| measured | predicted | ||||
| NW Atlantic | 0.0066 ± 0.0020 | 2.8 ± 1.2 (0.2-200 μm) | 5.6 ± 5.6 (>200 μm) | 5.5 ± 2.0 | [31] |
| Central Pacific | 0.019 ± 0.023 | 11.7 ± 10.4 (0.2-200 μm) | 2 - 34 (>200 μm) | 21.5 ± 16.9 | [29, 32] |
| Long Island Sound | 0.03 | 5 (0.2-200 μm) | 11 (>200 μm) | 12 | [33] |
| Groswater Bay | 0.023 ± 0.012 | 0.16 (5-200 μm) | 7.0 (200-500 μm) | 3.6 | [34] |
| Gulf of Lions | 0.0045 ± 0.0026 | 1.4 ± 1.0 (80-200 μm) | 5.2 ± 3.9 (200-500 μm) | 2.9 ± 1.6 | [35] |
Overall, our model results suggested that it is possible to predict the concentration of MeHg in copepods given the concentration of MeHg in water and phytoplankton. Of course, it is easier to test model predictions in the field when there is a clear delineation of phytoplankton and zooplankton from other suspended particles in the same size ranges. Complications can arise when suspended abiotic particles (e.g., sediment particles) co-occur with the plankton. Recently, Lee and Fisher [17] reported that MeHg concentration factors in diverse marine phytoplankton cells are related to algal cell size. Therefore, the MeHg concentration in both phytoplankton and zooplankton can be calculated with only a few measured parameters. The parameters measured in this study along with the established model should be useful in refining the global biogeochemical models describing Hg cycling in the marine environment.
Acknowledgement
We appreciate many helpful comments from C. Hammerschmidt and two anonymous reviewers. Research reported in this publication was supported by NSF Award PLR 1260345, NIEHS Award P42ES007373, and NYSERDA Award 34357 to N. Fisher.
References
- 1.Fisher NS. On the reactivity of metals for marine phytoplankton. Limnol Oceanogr. 1986;31:443–449. [Google Scholar]
- 2.Wang W-X, Fisher NS. Accumulation of trace elements in a marine copepod. Limnol Oceanogr. 1998;43:273–283. [Google Scholar]
- 3.Wang W-X, Reinfelder JR, Lee B-G, Fisher NS. Assimilation and regeneration of trace elements by marine copepods. Limnol Oceanogr. 1996;41:70–81. [Google Scholar]
- 4.Fisher NS, Reinfelder JR. The trophic transfer of metals in marine systems. In: Tessier A, Turner DR, editors. Metal speciation and bioavailability in aquatic systems. John Wiley & Sons; Chichester, West Sussex, England: 1995. pp. 363–406. [Google Scholar]
- 5.Fowler SW, Knauer GA. Role of large particles in the transport of elements and organic compounds through the oceanic water column. Prog Oceanogr. 1986;16:147–194. [Google Scholar]
- 6.Fisher NS, Fowler SW. The role of biogenic debris in the vertical transport of transuranic wastes in the sea. In: O'Connor TP, Burt WV, Duedall IW, editors. Physicochemical processes and wastes in the ocean: Oceanic processes in marine pollution. Vol. 2. Krieger; Malabar, FL, U.S.A: 1987. pp. 197–207. [Google Scholar]
- 7.Mathews T, Fisher NS. Evaluating the trophic transfer of cadmium, polonium, and methylmercury in an estuarine food chain. Environ Toxicol Chem. 2008;27:1093–1101. doi: 10.1897/07-318.1. [DOI] [PubMed] [Google Scholar]
- 8.Reinfelder JR, Fisher NS, Luoma SN, Nichols JW, Wang WX. Trace element trophic transfer in aquatic organisms: A critique of the kinetic model approach. Sci Total Environ. 1998;219:117–135. doi: 10.1016/s0048-9697(98)00225-3. [DOI] [PubMed] [Google Scholar]
- 9.Morel FMM, Kraepiel AML, Amyot M. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 1998;29:543–566. [Google Scholar]
- 10.Cabana G, Rasmussen JB. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature. 1994;372:255–257. [Google Scholar]
- 11.Sunderland EM. Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environ Health Perspect. 2007;115:235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karimi R, Chen CY, Pickhardt PC, Fisher NS, Folt CL. Stoichiometric controls of mercury dilution by growth. PNAS. 2007;104:7477–7482. doi: 10.1073/pnas.0611261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason RP, Reinfelder JR, Morel FMM. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol. 1996;30:1835–1845. [Google Scholar]
- 15.Reinfelder JR, Fisher NS. The assimilation of elements ingested by marine copepods. Science. 1991;251:794–796. doi: 10.1126/science.251.4995.794. [DOI] [PubMed] [Google Scholar]
- 16.Lawson NM, Mason RP. Accumulation of mercury in estuarine food chains. Biogeochemistry. 1998;40:235–247. [Google Scholar]
- 17.Lee C-S, Fisher NS. Methylmercury uptake by diverse marine phytoplankton. Limnol Oceanogr. 2016;61:1626–1639. doi: 10.1002/lno.10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillard RRL, Ryther JH. Studies of marine planktonic diatoms I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 19.Mason RP, Choi AL, Fitzgerald WF, Hammerschmidt CR, Lamborg CH, Soerensen AL, Sunderland EM. Mercury biogeochemical cycling in the ocean and policy implications. Environ Res. 2012;119:101–117. doi: 10.1016/j.envres.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouleau C, Block M. Fast and high yield synthesis of radioactive CH3203Hg(II). Appl Organomet Chem. 1997;11:751–753. [Google Scholar]
- 21.Wang W-X, Fisher NS. Assimilation efficiencies of chemical contaminants in aquatic invertebrates: a synthesis. Environ Toxicol Chem. 1999;18:2034–2045. [Google Scholar]
- 22.Fisher NS, Nolan CV, Fowler SW. Assimilation of metals in marine copepods and its biogeochemical implications. Mar Ecol Prog Ser. 1991;71:37–43. [Google Scholar]
- 23.Williams JJ, Dutton J, Chen CY, Fisher NS. Metal (As, Cd, Hg, and CH3Hg) bioaccumulation from water and food by the benthic amphipod Leptocheirus plumulosus. Environ Toxicol Chem. 2010;29:1755–1761. doi: 10.1002/etc.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart GM, Fisher NS. Bioaccumulation of polonium-210 in marine copepods. Limnol Oceanogr. 2003;48:2011–2019. [Google Scholar]
- 25.Rainbow P. Ecophysiology of trace metal uptake in crustaceans. Estuar Coast Shelf Sci. 1997;44:169–176. [Google Scholar]
- 26.Mauchline J. The biology of calanoid copepods. Academic Press; 1998. [Google Scholar]
- 27.Lonsdale DJ, Cosper EM, Doall M. Effects of zooplankton grazing on phytoplankton size structure and biomass in the lower Hudson River estuary. Estuaries. 1996;19:874–889. [Google Scholar]
- 28.Berggreen U, Hansen B, Kiørboe T. Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol. 1988;99:341–352. [Google Scholar]
- 29.Thomann RV. Equilibrium model of fate of microcontaminants in diverse aquatic food chains. Can J Fish Aquat Sci. 1981;38:280–296. [Google Scholar]
- 30.Fisher NS, Bjerregaard P, Fowler SW. Interactions of marine plankton with transuranic elements. 1. Biokinetics of neptunium, plutonium, americium, and californium in phytoplankton. Limnol Oceanogr. 1983;28:432–447. [Google Scholar]
- 31.Gosnell KJ, Mason RP. Mercury and methylmercury incidence and bioaccumulation in plankton from the central Pacific Ocean. Mar Chem. 2015;177:772–780. [Google Scholar]
- 32.Hammerschmidt CR, Bowman KL. Vertical methylmercury distribution in the subtropical North Pacific Ocean. Mar Chem. 2012;132:77–82. [Google Scholar]
- 33.Hammerschmidt CR, Finiguerra MB, Weller RL, Fitzgerald WF. Methylmercury accumulation in plankton on the continental margin of the Northwest Atlantic Ocean. Environ Sci Technol. 2013;47:3671–3677. doi: 10.1021/es3048619. [DOI] [PubMed] [Google Scholar]
- 34.Munson KM, Lamborg CH, Swarr GJ, Saito MA. Mercury species concentrations and fluxes in the Central Tropical Pacific Ocean. Global Biogeochem Cy. 2015;29:656–676. [Google Scholar]
- 35.Hammerschmidt CR, Fitzgerald WF. Bioaccumulation and trophic transfer of methylmercury in Long Island Sound. Arch Environ Contam Toxicol. 2006;51:416–424. doi: 10.1007/s00244-005-0265-7. [DOI] [PubMed] [Google Scholar]
- 36.Schartup AT, Balcom PH, Soerensen AL, Gosnell KJ, Calder RS, Mason RP, Sunderland EM. Freshwater discharges drive high levels of methylmercury in Arctic marine biota. PNAS. 2015;112:11789–11794. doi: 10.1073/pnas.1505541112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cossa D, Harmelin-Vivien M, Mellon-Duval C, Loizeau V, Averty B, Crochet S, Chou L, Cadiou J-F. Influences of bioavailability, trophic position, and growth on methylmercury in hakes (Merluccius merluccius) from northwestern Mediterranean and northeastern Atlantic. Environ Sci Technol. 2012;46:4885–4893. doi: 10.1021/es204269w. [DOI] [PubMed] [Google Scholar]
- 38.Hutchins DA, Wang W-X, Fisher NS. Copepod grazing and the biogeochemical fate of diatom iron. Limnol Oceanogr. 1995;40:989–994. [Google Scholar]
- 39.Chang SI, Reinfelder JR. Bioaccumulation, subcellular distribution, and trophic transfer of copper in a coastal marine diatom. Environ Sci Technol. 2000;34:4931–4935. [Google Scholar]