Abstract
It has long been held that the parathyroid glands and parathyroid hormone evolved with the emergence of the tetrapods, reflecting a need for new controls on calcium homeostasis in terrestrial, rather than aquatic, environments. Developmentally, the parathyroid gland is derived from the pharyngeal pouch endoderm, and studies in mice have shown that its formation is under the control of a key regulatory gene, Gcm-2. We have used a phylogenetic analysis of Gcm-2 to probe the evolutionary origins of the parathyroid gland. We show that in chicks, as in mice, Gcm-2 is expressed in the pharyngeal pouches and the forming parathyroid gland. We find that Gcm-2 is present not only in tetrapods but also in teleosts and chondrichthyans, and that in these species, Gcm-2 is expressed within the pharyngeal pouches and internal gill buds that derive from them in zebrafish (Danio rerio), a teleost, and dogfish (Scyliorhinus canicula), a chondrichthyan. We further demonstrate that Gcm-2 is required for the formation of the internal gill buds in zebrafish. We also have identified parathyroid hormone 1/2-encoding genes in fish and show that these genes are expressed by the gills. We further show that the gills express the calcium-sensing receptor, which is used in tetrapods to monitor serum calcium levels. These results indicate that the tetrapod parathyroid gland and the gills of fish are evolutionarily related structures, and that the parathyroid likely came into being as a result of the transformation of the gills during tetrapod evolution.
Keywords: pharyngeal endoderm, vertebrate evolution, Gcm-2, gills
In tetrapods, the parathyroid glands play a pivotal role in regulating extracellular calcium homeostasis, which is important to many physiological processes such as muscle contraction, blood coagulation, and synaptic activity. These glands detect changes in the levels of calcium in blood by means of the calcium-sensing receptor (CasR), which in turn modulates the secretion of parathyroid hormone (PTH), which acts to release calcium from internal stores such as bone and modulates renal ion transport (1). Developmentally, the parathyroid glands arise from the endodermal pharyngeal pouches; in humans and chickens, from the third and fourth pouches, and in mice, from the third pouch only. Importantly, studies in mice have demonstrated that the transcription factor encoded by Gcm-2 is a key regulator of parathyroid gland development. The expression of this gene is restricted to the parathyroid glands, and if this gene is mutated, the parathyroid glands fail to form (2–4).
Fish, however, have been believed to lack parathyroid glands and PTH, and unlike tetrapods, the majority of calcium uptake in fish is from external sources. These differences are believed to reflect the fact that with the evolution of the tetrapods and the shift from an aquatic to a terrestrial environment, new controls for regulating calcium homeostasis had to be put in place, and thus the evolution of the parathyroid glands and PTH was a key event in facilitating this transition. This event freed the tetrapods from relying on calcium uptake from the water by giving them the ability to internally regulate their serum calcium levels.
Although the evolution of the parathyroid gland was a key event in the emergence of the tetrapods, there have been few studies on how this evolution was achieved. Here, we have exploited the rigid association between Gcm-2 and the parathyroid gland and conducted a phylogenetic analysis to gain insight into how this structure evolved. Our results demonstrate that the parathyroid gland of tetrapods and the gills of fish most likely share a common evolutionary origin; both express Gcm-2 and require this gene for their formation, and both express PTH and CasR. We thus suggest that the parathyroid gland came into being as the result of the transformation of the gills into the parathyroid glands of tetrapods.
Materials and Methods
DNA Cloning. Partial sequences of CasR, PTH, and PTH-related protein (PTHrP) in zebrafish and chicks were identified in expression sequence tag databases in the National Center for Biotechnology Information, the European Bioinformatics Institute, and the University of Delaware, Newark, and were isolated by RT-PCR from adult gills of zebrafish and thyroid and parathyroid in embryonic day (E) 11 chicken embryos, respectively. PTH genes in fugu were identified in the European Bioinformatics Institute database. The partial sequences of zebrafish Gcm-2 and chicken Gcm-1 were identified in the expressed sequence tag database in the National Center for Biotechnology Information and the University of Delaware. Partial sequences (278 bp) of Gcm-2 of chicken, Xenopus laevis, rainbow trout, Australian lungfish, and dogfish were amplified from total RNA from embryonic pharyngeal tissues by RT-PCR using degenerate primers. By using sequence-specific primers, each full-length cDNA was isolated by 5′ and 3′ RACE using the SMART RACE cDNA amplification kit (Clontech). All sequences have been deposited in the DNA Data Bank of Japan.
In Situ Hybridization and Histology. Whole-mount in situ hybridization for chicken and zebrafish embryos was performed as described in refs. 5 and 6. In situ hybridization for dogfish embryos was performed by using the same protocol as for chicken embryos, except that soaking in DMSO/methanol (1:1) solution was substituted for the proteinase K treatment. The stained chicken embryos were embedded in gelatin–albumin with 2.5% glutaraldehyde and sectioned at 50 μm with a Vibratome.
Morpholino-Modified Oligonucleotide (MO) Injection. Injection of zebrafish Gcm-2 antisense and control MOs was performed as described in ref. 7. MO antisense oligonucleotides (GeneTools, Philomath, OR) were designed against 25 bases around a splicing site at the end of the third exon-encoding ORF. This MO was designed as a splicing-blocking MO (8) to cause skipping of an exon encoding the DNA-binding domain of Gcm-2. All injections were performed on one-cell-stage embryos at concentrations between 2.5 and 5 μg/μl. The gene knockdown was confirmed by RT-PCR in eight individual injected embryos.
RT-PCR for Zebrafish PTH and CasR. PTH, CasR, and β-actin genes were amplified by the Onestep RT-PCR kit (Qiagen) using sets of sequence-specific primers from total RNA from whole gills and whole brain of a wild-type adult male zebrafish.
Results
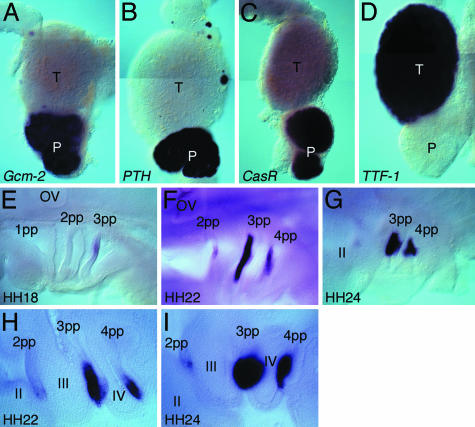
Gcm-2 Is Expressed in the Pharyngeal Pouches and Parathyroid Gland in a Nonmammalian Tetrapod. To date, Gcm-2 expression has been scrutinized in the mouse embryo only. We have therefore isolated the chicken (Gallus gallus) Gcm-2 gene and analyzed its expression pattern to determine whether it is similarly restricted to the pharyngeal pouches and the parathyroid gland in nonmammalian tetrapods. We find that, as in mice, this gene is expressed exclusively in both the parathyroid gland and the pharyngeal pouches from which it derives. Fig. 1 A–D shows the relative positions of the parathyroid and thyroid glands in an E11 embryo. The parathyroid gland is unambiguously identified through its expression of PTH (Fig. 1B) and CasR (Fig. 1C), and the thyroid through expression of TTF-1 (9) (Fig. 1D). We also find that, at earlier developmental stages, this gene is expressed in the pharyngeal pouches (Fig. 1 E–I) in the chick. Expression initiates in the third pouch, at stage 18 (Fig. 1E), and then occurs in the fourth pouch, and additionally in a small domain of the second pouch (Fig. 1F). It is clear when viewed in section that Gcm-2 expression is restricted to the pharyngeal endoderm (Fig. 1H). As development proceeds, expression is lost from the second pharyngeal pouch but becomes strongly up-regulated in the endodermal thickenings of the third and fourth pouches that are the primordia of the parathyroid glands in the chick (Fig. 1 G and I).
Fig. 1.
Expression of Gcm-2 in the parathyroid gland and the pharyngeal pouches in the chick. (A–D) Whole-mount in situ hybridization of chick E11 thyroid (T) and parathyroid (P) glands for the following probes: Gcm-2 (A), PTH (B), CasR (C), and TTF-1 (D). Gcm-2 can be seen to be expressed in two round masses, the parathyroid glands, which are adjacent to the thyroid, which expresses TTF-1. The parathyroid glands additionally express PTH and CasR. (E–I) Expression of Gcm-2 in chicken embryos, staged as described in ref. 16. In these micrographs, anterior is to the left and ventral is to the bottom. OV, otic vesicle; pp, pharyngeal pouch; II–IV, pharyngeal arches. Expression of Gcm-2 starts in the third pharyngeal pouch at stage 18 (E), and then, as development proceeds, expression is also evident in the fourth pharyngeal pouch and additionally weakly in the second pouch (F). At stage 24, expression in the third and fourth pouches is concentrated in a region dorsal of the pharyngeal pouches and is lost from the second pouch (G). In Vibratome sections of stage-22 embryos (H), it is clear that Gcm-2 expression is localized to the pharyngeal endoderm and that, by stage 24, the region of the pharyngeal endoderm expressing Gcm-2 has thickened and given rise to round masses that are the parathyroid gland rudiments of the third and fourth pouches (I).
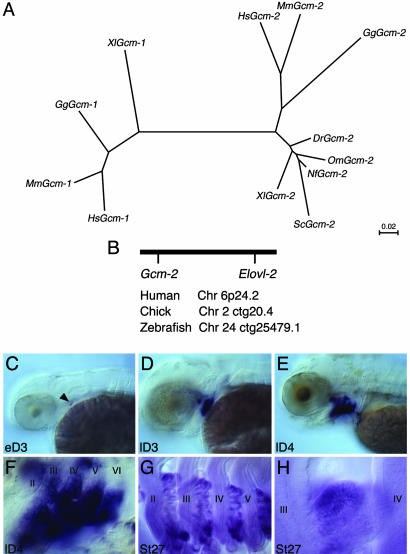
Gcm-2 Is Found Throughout the Gnathostomes. In mammals, two Gcm genes have been identified, Gcm-1 and Gcm-2 (2, 10). Importantly, it has been shown that, in the mice mutated for Gcm-2, PTH levels in serum are normal and the result of Gcm-1, which is expressed in the thymus, causing the ectopic production of PTH from cells in this organ (4). Given that Gcm-2 and, under certain circumstances, Gcm-1, can both elicit the production of PTH, we carried out an extensive phylogenetic analysis of the distribution of both genes to determine whether these genes evolved with the tetrapods. Surprisingly, we found that Gcm-2 is present not only in tetrapods (mammals Homo sapiens and Mus musculus, chicken, and Xenopus) but also in dipnoi fish (Australian lungfish, Neoceratodus forsteri), teleost fish (rainbow trout, Oncorhynchus mykiss; and zebrafish, Danio rerio), and from a chondrichthyan species (dogfish, Scyliorhinus canicula). Contrastingly, we have been able to identify Gcm-1 only in tetrapods. Searches of the fugu (Takifugu rubripes) and zebrafish genome fail to highlight the presence of a Gcm-1 gene. A clustal analysis of the Gcm sequences shows clearly that all of the Gcm-2 sequences that we have isolated group together phylogenetically, as do all of the Gcm-1 sequences (Fig. 2A). We also find from an analysis of the genomes sequences (www.ensembl.org) that Gcm-2 is invariably physically linked to another gene, ELOVL2, in humans (Chr6p24.2), chickens (Chr2ctg20.4), and zebrafish (Chr24ctg25479.1), further demonstrating that the fish Gcm-2 gene is the orthologue of the mammalian gene (Fig. 2B). Thus, Gcm-2 is widely distributed throughout the gnathostomes and is not restricted to species that possess a parathyroid gland.
Fig. 2.
Phylogenetic analysis of the distribution of Gcm-2 and its expression in teleost (zebrafish) and chondrichthyan (dogfish) species. (A) A phylogenetic tree of vertebrate Gcm genes based on clustal analysis. Dr, zebrafish (Danio rerio); Gg, chicken (Gallus gallus); Hs, human (Homo sapiens); Mm, mouse (Mus musculus); Nf, Australian lungfish (Neoceratodus forsteri); Om, rainbow trout (Oncorhynchus mykiss); Sc, dogfish (Scyliorhinus canicula); Xl, Xenopus (Xenopus laevis). Branch lengths are in units of number of amino acid substitutions per site. (B) Schematic depicting the conserved linkage between Gcm2 and Elovl2 in humans on chromosome 6p24.2, in chickens on chromosome 2 ctg20.4, and in zebrafish on chromosome 24 ctg25479.1. (C–F) Gcm-2 expression in zebrafish embryos. In these micrographs, anterior is to the left and ventral is to the bottom. Gcm-2 initiates expression in the second pharyngeal pouch in early 3-day-old larval fish (indicated by arrowhead in C). Subsequently, Gcm-2 is expressed sequentially in the more posterior pouches (D), and, by day 4, Gcm-2 is expressed in all of the pouches (E). It is also apparent by day 4 that Gcm-2 is expressed in the developing internal gill buds emerging from the pharyngeal pouches (F). (G and H) Gcm-2 expression in dogfish embryos. This gene is expressed in the internal gill buds protruding from the pharyngeal pouches in stage-27 dogfish embryos (17). The pharyngeal arches are numbered II–VI.
Gcm-2 Is Expressed in the Pharyngeal Pouches and Their Derivatives, the Internal Gill Buds, in Teleost and Chondrichthyan Species. We have characterized the expression profiles of Gcm-2 in zebrafish and dogfish and compared these with the chick. We find that, in all species, this gene is expressed exclusively in the pharyngeal pouch endoderm. In zebrafish larva, Gcm-2 expression initiates in the second pharyngeal pouch at early E3 (Fig. 2C) and over the next 24 h becomes established in all of the pharyngeal pouches (Fig. 2 D and E). On E4, it also is apparent that this gene is expressed in the buds of the internal gills that are derived from the pharyngeal pouches (11) (Fig. 2F). Similarly, in dogfish, Gcm-2 expression is found in the pharyngeal pouches and the internal gill buds that protrude from these structures (Fig. 2 G and H). Thus, the presence of Gcm-2 and its expression in the pharyngeal pouches and their elaborations was already in place before the emergence of osteichthyans and the subsequent split into actinopterygian and sarcopterygian lineages. These results indicate that the expression of Gcm-2 in the pharyngeal pouches and their derivatives, the internal gill buds and parathyroid gland, are an ancient conserved feature of all jawed vertebrates.
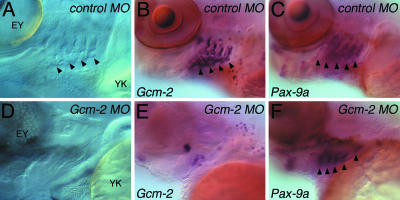
Gcm-2 Is Required for the Elaboration of the Internal Gill Buds from the Pharyngeal Pouches. To further dissect the function of Gcm-2 in fish, we have injected zebrafish with an antisense MO that causes exon-skipping and thus interferes with Gcm-2 function (8). In all embryos injected with the control MO (n = 49), gill buds formed as simply viewed with Nomarski optics (Fig. 3A) and also through Gcm-2 expression, which highlights these structures (Fig. 3B). Contrastingly, in 85% of the embryos that received an injection of the Gcm-2 antisense MO (n = 34), gills buds did not form. This absence of gill buds is evident both from morphology (Fig. 3D) and from the lack of Gcm-2-expressing tissues associated with each pharyngeal pouch (Fig. 3E). It is possible that the failure in the formation of the internal gill buds from the pharyngeal pouches could be due to early defects in the organization of the pouches themselves. To address this issue, we analyzed the expression profile of Pax-9a, which marks the pharyngeal pouches (12). In both control-injected embryos and embryos injected with the Gcm-2 antisense MO, the general patterning of the pharyngeal endoderm was unaffected, and Pax-9a expression clearly indicated the presence of the pharyngeal pouches (Fig. 3 C and F). Thus, in fish, Gcm-2 is specifically required for the formation of the internal gill buds from the pharyngeal pouches.
Fig. 3.
Gcm-2 is required for the elaboration of the internal gill buds from the pharyngeal pouches in zebrafish. Zebrafish embryos were injected at the one-cell stage with either control or antisense Gcm-2 MOs. The embryos were then analyzed at day 5 for the presence of internal gill buds. (A–C) Five-day-old zebrafish larva injected with control MO. (A) Nomarski view of the pharyngeal region of a day-5 embryo injected with the control MO. The internal gill buds protruding from the pharyngeal pouches are clearly evident (arrowheads). (B) Embryo injected with control MO hybridized for Gcm-2. Gcm-2-expressing internal gill buds can be clearly seen protruding from the pharyngeal pouches. (C) Embryo injected with control MO, showing normal pharyngeal pouch formation as judged by Pax-9a expression. Each pharyngeal pouch is indicated by an arrowhead. (D–F) Five-day-old zebrafish larva injected with Gcm-2 antisense MO. (D) Nomarski view of the pharyngeal region of a E5 embryo injected with the antisense Gcm-2 MO. There are no internal gill buds protruding from the pharyngeal pouches. (E) Embryo injected with the antisense Gcm-2 MO hybridized for Gcm-2. There are no Gcm-2-expressing internal gill buds protruding from the pharyngeal pouches. (F) Embryo injected with the antisense Gcm-2 MO, showing normal pharyngeal pouch formation as judged by Pax-9a expression. Each pharyngeal pouch is indicated by an arrowhead. EY, eye; YK, yolk. Anterior is to left and ventral is to the bottom.
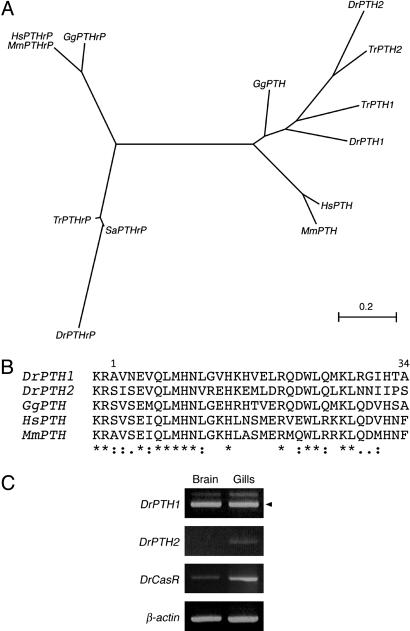
Teleosts Do Have PTH-Encoding Genes That Are Expressed in the Gills, as Is CasR. The fact that the gill buds of fish and the tetrapod parathyroid both require Gcm-2 function for their formation suggests that these structures are closely related. To further examine this relationship, we have taken advantage of the availability of the zebrafish and fugu genome sequences to search for a PTH-encoding gene, and we have identified two such genes in both species (Fig. 4 A and B). clustal analysis of the protein sequences encoded by these genes demonstrates that they group with the tetrapod PTH genes. These genes are distinct from the PTHrP genes from these fish, which group with the tetrapod PTHrP genes (Fig. 4A). It is further clear from a comparison of the peptide sequences of the zebrafish and amniote PTH genes that key residues within the N-terminal 34 aa that are required for biological activity are conserved (Fig. 4B). These results are in keeping with another recent study that similarly identified two PTH genes in zebrafish and fugu that are shown to be fully active (13, 14). We have further analyzed the tissues expression of the zebrafish PTH genes, and we find that these genes are expressed by the gills (Fig. 4C). Finally, we also have isolated CasR from zebrafish, and we demonstrate that this gene also is expressed by the gills (Fig. 4C).
Fig. 4.
PTH genes in zebrafish. (A) A phylogenetic tree of PTH and PTHrP genes in vertebrates. Dr, zebrafish (Danio rerio); Gg, chicken (Gallus gallus); Hs, human (Homo sapiens); Mm, mouse (Mus musculus); Sa, seabream (Sparus aurata); Tr, fugu (Takifugu rubripes). (B) Comparison of the partial peptide sequences of zebrafish PTH and amniote PTH. The N-terminal 34 aa of mature human PTH peptide are sufficient for the biological activity of PTH. This alignment includes the N-terminal amino acids (1–34) with 2 aa before the final proteolytic cleavage site. (C) Gills in adult zebrafish express both the PTH genes and CasR. PTH and CasR were amplified from adult brain and gills by RT-PCR. Arrowhead indicates cDNA product.
Discussion
In this study, we have analyzed the origin of the parathyroid gland. We find that Gcm-2, a gene that is expressed in the parathyroid gland and is absolutely required for its formation, is found not only in animals that possess a parathyroid gland, the tetrapods, but also in the jawed vertebrates. We also show that in amniotes and fish, Gcm-2 displays a conserved pattern of expression. In mouse, chicken, zebrafish, and dogfish, Gcm-2 is expressed in the pharyngeal pouches and, at later stages, in the structures that form from the pouches, the parathyroid gland in tetrapods and the internal gill buds in fish. We further demonstrate that Gcm-2 is required for the formation of the internal gill buds in zebrafish. Finally, we demonstrate that fish possess PTH-encoding genes that are expressed by the gills, which additionally express CasR.
The results presented here demonstrate that the parathyroid gland and the internal gill buds of fish are related structures and likely share a common evolutionary history. Both structures originate from the pharyngeal pouch endoderm, express Gcm-2, and require this gene function for their formation. Thus, Gcm-2 likely serves a conserved function across the gnathostomes with respect to the elaboration of pharyngeal pouches into internal gill buds and parathyroid. It is known that calcium uptake is a particularly important function of the gills (15), and we also show that fish do possess PTH genes, which have been shown by others to generate fully active peptides (13, 14), and that this gene is expressed in the gills. Thus, both the tetrapod parathyroid gland and the gills of fish contribute to the regulation of extracellular calcium levels. It is therefore reasonable to suggest that the parathyroid gland evolved as a result of the transformation of the gills into the parathyroid glands of tetrapods and the transition from an aquatic to a terrestrial environment. This interpretation also would explain the positioning of the parathyroid gland within the pharynx in the tetrapod body. Were the parathyroid gland to have emerged de novo with the evolution of the tetrapods, it could, as an endocrine organ, have been placed anywhere in the body and still exert its effect.
Acknowledgments
We thank Jean Joss (Macquarie University, New South Wales, Australia), Mike Jones (Institute for Cancer Research, London), Corinne Houart and Gareth Fraser (both of King's College London), and the London Aquarium for supplying material; Corinne Houart for helping to establish the MO injections; Imelda McGonnell for help with protocols; and Christine Ferguson, Jo Begbie, and Robyn Quinlan for critical comments on the manuscript. This work was supported by the Royal Society and grants from the Ministry of Education, Science, Sports, and Culture of Japan and the Japan Society for the Promotion of Science Research.
Author contributions: M.O. and A.G. performed research and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PTH, parathyroid hormone; PTHrP, PTH-related protein; CasR, calcium-sensing receptor; En, embryonic day n; MO, morpholino-modified oligonucleotide.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. AB175671 (Gcm-2, zebrafish), AB175672 (Gcm-2, chicken), AB175673 (Gcm-2, lungfish), AB175674 (Gcm-2, rainbow trout); partial sequence, AB175675 (Gcm-2, dogfish), AB175676 (Gcm-2, Xenopus), AB175677 (CasR, chicken); partial sequence, AB175678 (PTHrP, chicken), and AB175679 (PTH1, zebrafish)].
References
- 1.Brown, E. M. (2001) in The Parathyroids: Basic and Clinical Concepts (Academic, San Diego), pp. 167–181.
- 2.Kim, J., Jones, B. W., Zock, C., Chen, Z., Wang, H., Goodman, C. S. & Anderson, D. J. (1998) Proc. Natl. Acad. Sci. USA 95, 12364–12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon, J., Bennett, A. R., Blackburn, C. C. & Manley, N. R. (2001) Mech. Dev. 103, 141–143. [DOI] [PubMed] [Google Scholar]
- 4.Gunther, T., Chen, Z. F., Kim, J., Priemel, M., Rueger, J. M., Amling, M., Moseley, J. M., Martin, T. J., Anderson, D. J. & Karsenty, G. (2000) Nature 406, 199–203. [DOI] [PubMed] [Google Scholar]
- 5.Henrique, D., Adam, J., Myat, A., Chitnis, A., Lewis, J. & Ish-Horowicz, D. (1995) Nature 375, 787–790. [DOI] [PubMed] [Google Scholar]
- 6.Xu, Q., Holder, N., Patient, R. & Wilson, S. W. (1994) Development (Cambridge, U.K.) 120, 287–299. [DOI] [PubMed] [Google Scholar]
- 7.Houart, C., Caneparo, L., Heisenberg, C., Barth, K., Take-Uchi, M. & Wilson, S. (2002) Neuron 35, 255–265. [DOI] [PubMed] [Google Scholar]
- 8.Draper, B. W., Morcos, P. A. & Kimmel, C. B. (2001) Genesis 30, 154–156. [DOI] [PubMed] [Google Scholar]
- 9.Pera, E. M. & Kessel, M. (1997) Development (Cambridge, U.K.) 124, 4153–4162. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama, Y., Hosoya, T., Poole, A. M. & Hotta, Y. (1996) Proc. Natl. Acad. Sci. USA 93, 14912–14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kardong, K. V. (1998) Vertebrates: Comparative Anatomy, Function, and Evolution (Wm. C. Brown Publishers, Dubuque, IA), 3rd Ed.
- 12.Nornes, S., Mikkola, I., Krauss, S., Delghandi, M., Perander, M. & Johansen, T. (1996) J. Biol. Chem. 271, 26914–26923. [DOI] [PubMed] [Google Scholar]
- 13.Danks, J. A., Ho, P. M., Notini, A. J., Katsis, F., Hoffmann, P., Kemp, B. E., Martin, T. J. & Zajac, J. D. (2003) J. Bone Miner. Res. 18, 1326–1331. [DOI] [PubMed] [Google Scholar]
- 14.Gensure, R. C., Ponugoti, B., Gunes, Y., Papasani, M. R., Lanske, B., Bastepe, M., Rubin, D. A. & Juppner, H. (2004) Endocrinology 145, 1634–1639. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, W. S. (2002) J. Exp. Zool. 293, 264–283. [DOI] [PubMed] [Google Scholar]
- 16.Hamburger, V. & Hamilton, H. L. (1951) J. Morphol. 88, 49–92. [PubMed] [Google Scholar]
- 17.Ballard, W. W., Mellinger, J. & Lechenault, H. (1993) J. Exp. Zool. 267, 318–336. [Google Scholar]