To a pathologist, the concepts of staging and grading are a basic aspect of the histological evaluation of disease. The idea of disease stage encompasses extent: how far the disease has progressed from its inception to end stage. Staging disease has its most familiar application in the field of oncology, in which disease specific staging systems are used to mark a malignancy’s progress from early in-situ lesions to metastasis. Cancer stage has important applications for both therapy and prognosis. It is easily defined and understood, as it is based on discrete anatomic landmarks transgressed by the neoplasm. Grading, on the other hand, is a more nebulous concept. In oncology, grading generally refers to tumor differentiation, which broadly translates to a measure of tumor aggressiveness—the pace at which the disease progresses. More poorly differentiated tumors tend to grow faster and be more invasive and less responsive to systemic therapies. Histologically, tumor grade depends on architectural and cytological features and thus is less reproducible than tumor stage. While tumor stage always carries more prognostic weight than tumor grade, once stage is taken into consideration, grade may become important in prognosis and in therapeutic decision making.
The ideas of stage and grade are relatively recent concepts in chronic liver disease. In the 1980’s, disease classification in chronic hepatitis consisted of descriptive terms like chronic active hepatitis, chronic persistent hepatitis and chronic lobular hepatitis. Cirrhosis was noted if present, but lesser degrees of fibrosis might be ignored or diagnosed as “early cirrhosis”. Although the terms were defined, their relationship to each other was ill-defined and unsuited for evaluation of liver biopsies in therapeutic clinical trials for viral hepatitis. The Knodell Histology Activity Index attempted to side-step the issues raised by these descriptive terms by assigning numerical scores to represent different degrees of inflammation and fibrosis, with the sum of all of the scores providing a comprehensive estimate of liver injury(1). In the 1990’s, continued dissatisfaction with the descriptive terms led to proposals that chronic liver disease should be assessed on three axes: the etiology of the liver disease, the severity of the inflammation (activity or grade) and the degree of fibrosis (stage) (2). Like systems used in oncology, the stage of fibrosis was thought to be the best histological marker of disease progression while the severity of inflammation was reasonably thought to be the clearest marker of rate of progression. While fibrosis stage is always more important than inflammatory grade in prognostic models, longitudinal studies of fibrosis progression have supported the idea that the severity of inflammation is related to rate(3). This paradigm of classifying grade and stage in chronic hepatitis is sufficiently robust that it soon was translated into other chronic liver diseases like steatohepatitis(4) and primary biliary cholangitis(5).
There is an inherent danger to grading and staging, and that is to assume that a chronic liver disease can be completely characterized by its grade and stage. It is not that unusual to see a pathology report with a diagnostic line of “chronic hepatitis C, grade 2, stage 3”, accompanied by a footnote referencing the staging system and little description of other biopsy findings. Methods of noninvasive evaluation of liver disease emphasize fibrosis stage, arguing that other features may be safely ignored. This danger exists even within the more extensive histological scoring systems used for research. Of necessity, pathologists have to limit their evaluation to histological features that may be easily and reproducibly assessed. As an example, the character and degree of portal inflammation and ductular reaction may be important in disease progression in steatohepatitis(6, 7), but the former is not included in composite activity scores and the latter is not assessed at all in the major steatohepatitis scoring systems. Finally, all grading and staging systems used in chronic liver disease were developed based on histological changes in untreated individuals and so do not necessarily account for changes that occur after successful therapy. In particular, fibrosis regression is poorly assessed by existing staging systems, as demonstrated by the work of Wanless et al. in their descriptions of cirrhosis regression(8).
Into this gap, Sun et al. propose the “Beijing Classification” to help divide cases of chronic hepatitis B into those likely to show fibrosis regression from those at risk for continued progression, despite adequate viral suppression(9). The classification has elements of both grade and stage. Stage in that it is another way to assess fibrosis, but grade in that it tries to define the overall direction of fibrosis—either worsening or improvement. Using biopsies that show bridging fibrosis or cirrhosis, they defined three categories based on the quality of the fibrous scars. In biopsies categorized as predominantly progressive (P) most of the biopsy shows wide fibrotic bands with histological evidence of fibrogenesis (variable fiber staining on trichrome) and a moderate to marked infiltrate of inflammatory cells. In biopsies called predominantly regressive (R), the fibrotic bands are mainly thin and homogeneously dense, with little or no inflammation. A category of indeterminate (I) was included for biopsies that could not be clearly classified as either progressive or regressive. The method was robust, with an interobserver kappa statistic of 0.71. Conventional staging using the Ishak and Laennec fibrosis staging systems augmented the P-I-R fibrosis quality evaluation.
Using these criteria, the authors evaluated 71 pairs of pre and post-treatment biopsies from patients with chronic hepatitis B and advanced fibrosis. As expected, therapy induced apparent regression of Ishak fibrosis stage in 33 of 71 cases, and left it stable in 35 while only 3 showed apparent progression of stage. Necroinflammatory activity and non-invasive measures of liver injury and stiffness also improved across the cohort. Prior to therapy, most biopsies (58%) showed progressive fibrosis features while post-therapy, only 11% remained in this category. Interestingly, while the pre-treatment P-I-R classification correlated with inflammatory grade, aminotransferases and liver stiffness, these correlations disappeared after treatment, suggesting that the P-I-R classification was assessing an aspect of the liver biopsy not reflected in conventional measures. Of the 35 patients with apparently unchanged Ishak stage, 6 (17%) showed the progressive fibrosis phenotype on follow-up biopsy, while 2 of the 3 patients with apparent progression of fibrosis had the progressive phenotype. The third patient with increased stage on follow-up actually had the regressive fibrosis phenotype, suggesting that the apparent worsened stage was perhaps due to sampling. Post-treatment liver biopsy evaluation in chronic hepatitis B may therefore yield information about the potential for disease progression despite successful viral suppression. However, that judgment will need to wait for longer follow-up studies.
Liver biopsies remain a useful tool in particular clinical situations in which additional insight into the disease pathology can provide guidance for therapy or prognosis. The information content of the liver biopsy is greater that what can be conveyed using only the existing grading and staging systems and pathologists should be encouraged to explore aspects of liver pathology that can shed additional light on disease pathophysiology. Treatments may alter the natural history in unpredictable ways and we must be ready to adapt our evaluations accordingly. This study by Sun et al. offers one type of insight that goes beyond staging and grading to provide additional information. Existing biopsy sets from recent clinical trials could be used to confirm and extend these observations with critical follow-up data on post-treatment disease progression and hepatocellular carcinoma development.
Figure.
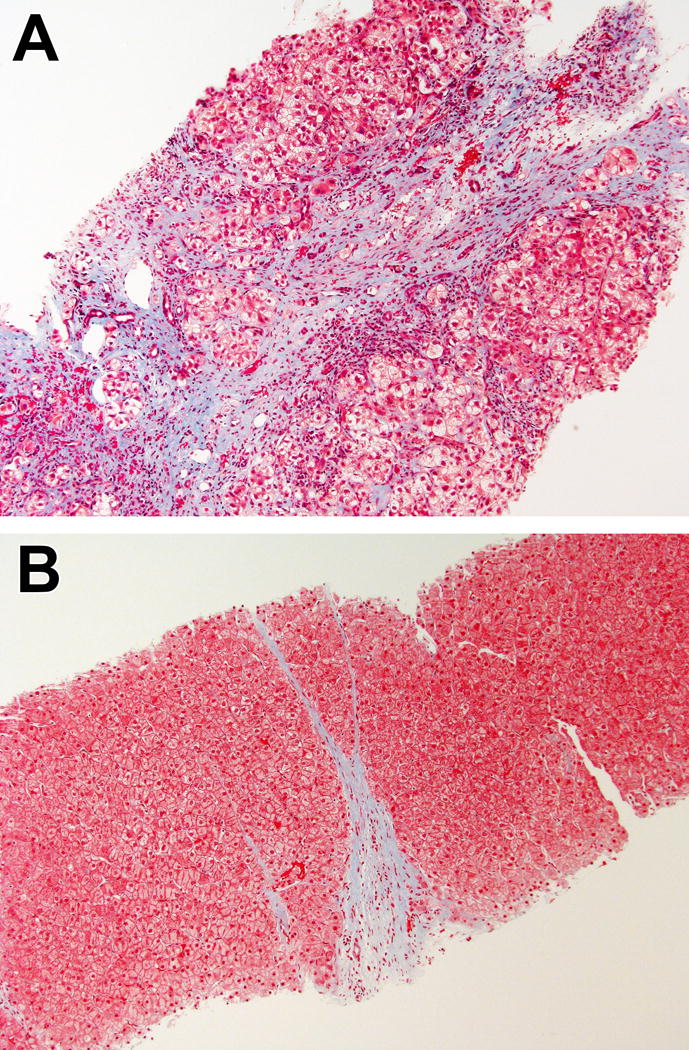
Examples of progressive (A) and regressive (B) fibrosis phenotypes. Both cases were staged as cirrhotic (Ishak fibrosis stage 6). In (A), the fibrous band is wide, with uneven staining and shows disruption of the adjacent parenchyma. In (B), the fibrous band is thin, with homogeneous staining and sharp borders. (Masson trichrome, 100× for both).
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- P-I-R
Progressive-Indeterminate-Regressive
Footnotes
Conflict of interest disclosure: The author states that has no financial or other conflicts of interest with respect to this work.
References
- 1.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 2.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 3.Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, Herion D, et al. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124:97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 4.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakanuma Y, Zen Y, Harada K, Sasaki M, Nonomura A, Uehara T, Sano K, et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int. 2010;60:167–174. doi: 10.1111/j.1440-1827.2009.02500.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, Neuschwander-Tetri BA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 8.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao H, Lu L, et al. New Classification of Liver Biopsy Assessment for Fibrosis in Chronic Hepatitis B Patients Before and After Treatment. Hepatology. 2016 doi: 10.1002/hep.29009. [DOI] [PubMed] [Google Scholar]


