Abstract
Background
Liver fibrosis is the most important predictor of mortality in nonalcoholic fatty liver disease (NAFLD). Quantitative risk of mortality by fibrosis stage has not been systematically evaluated. We aimed to quantify the fibrosis stage-specific risk of all-cause and liver-related mortality in NAFLD.
Methods
Through a systematic review and meta-analysis, we identified 5 adult NAFLD cohort studies reporting fibrosis stage specific mortality (0–4). Using fibrosis stage 0 as a reference population, fibrosis stage-specific mortality rate ratios (MRR) with 95% confidence intervals (CI), for all-cause and liver-related mortality, were estimated. The study is reported according to the PRISMA statement.
Results
1,495 NAFLD patients with 17,452 patient years of follow-up were included. Compared to NAFLD patients with no fibrosis (stage 0), NAFLD patients with fibrosis were at an increased risk for all-cause mortality and this risk increased with increase in the stage of fibrosis: stage 1, MRR, 1.58 (95% CI 1.19–2.11); stage 2, MRR, 2.52 (95% CI 1.85–3.42); stage 3, MRR, 3.48 (95% CI 2.51–4.83), and stage 4, MRR, 6.40 (95% CI 4.11–9.95). The results were more pronounced as the risk of liver-related mortality increased exponentially with increase in the stage of fibrosis: stage 1, MRR, 1.41 (95% CI 0.17–11.95); stage 2, MRR, 9.57 (95% CI 1.67–54.93); stage 3, MRR, 16.69 (95% CI 2.92–95.36); and stage 4, MRR, 42.30 (95% CI 3.51–510.34).
Limitations
Inability to adjust for co-morbid conditions or demographics known to impact fibrosis progression in NAFLD, and the inclusion of patients with simple steatosis and NASH without fibrosis in the reference comparison group.
Conclusion
The risk of liver-related mortality increases exponentially with increase in fibrosis stage. These data have important implications in assessing utility of each stage and benefits of regression of fibrosis from one stage to another.
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease in the United States, affecting nearly 100 million individuals.1 The development and progression of liver fibrosis are the most important predictors of disease outcomes in NAFLD.2–5 Accordingly, the early identification of the presence of fibrosis has become increasingly important.6–8 Although prior studies have shown an increased risk for mortality with advanced fibrosis, there has been a divergence in mortality estimates for early non-advanced fibrosis. This makes it difficult to understand the true risk of mortality with each fibrosis stage and the incremental risk with increasing fibrosis stage.
Younossi et al.9 and Ekstedt et al.10 observed that the presence of advanced fibrosis (stage 3–4) was associated with an increased risk for overall and liver-related mortality. Within these studies, the risk of mortality was numerically but not statistically higher in NAFLD patients with early non-advanced fibrosis (stage 1–2). In contrast, Angulo et al.11 observed that both advanced and non-advanced fibrosis were associated with an increased risk for mortality. Taken together, these studies reinforce the importance of advanced fibrosis in NAFLD, but they fail to adequately address the implications of early stage or non-advanced fibrosis. By pooling data across cohort studies we may be able to more accurately quantify the relative risk of all-cause and liver-related mortality with stage 1 and stage 2 fibrosis, which is of clinical importance when informing patients of their prognosis or health utility in the respective stage of disease,12, 13 and when assessing the overall impacts of improvements in stage of fibrosis.
In this meta-analysis we pooled published and unpublished fibrosis stage specific data from 5 multi-national NAFLD cohorts with over 17,000 patient years of follow-up, and aimed to systematically quantify the fibrosis stage-specific relative risk of all-cause mortality and liver-related mortality. We observed that all-cause and liver-related mortality increased exponentially with increasing fibrosis stage, and NAFLD patients were at an increased risk even at early stages of fibrosis. These data are of importance in clinical trials studying novel therapies aimed at reducing fibrosis progression and disease specific complications,14 and in clinical practice where a patient specific risk profile can be generated to help guide personalized treatment decisions.
METHODS
This systematic review was performed using an a priori established protocol (Supplementary Material), and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.15 (Supplementary Material)
Study Selection
Studies were included in this meta-analysis if they met the following criteria: (1) cohort study (retrospective or prospective); (2) adult NAFLD patients (≥ 18 years of age); (3) histologically confirmed diagnosis of NAFLD by at least 1 blinded liver pathologist based on the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) histologic scoring system; and (4) reported fibrosis stage-specific mortality rates (in person-years) or events.
Studies were excluded from this meta-analysis for the following reasons: (1) was not a cohort design (i.e., meta-analysis/review, cross-sectional, case-control); (2) participants did not have histologically confirmed diagnosis of NAFLD; (3) participants with other causes of liver disease were not excluded and/or NAFLD patient specific information was not available; or (4) fibrosis stage-specific mortality data was not available. If fibrosis stage-specific mortality data was not available then the study investigators contacted the primary authors to obtain unpublished data, which was provided for 4 of the 5 included studies.9, 10, 16, 17
Data Sources and Searches
The search strategy from a previously published systematic review on NAFLD was updated,2 with input from a medical librarian and study investigators, utilizing various databases from inception to November, 2016. Using controlled vocabulary supplemented with keywords, we searched for cohort studies of NAFLD patients that reported mortality. The databases included Medline, EMBASE, Scopus, Web of Science, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews. An example of the full electronic search strategy can be found in the Supplementary Material. Conference proceedings from Digestive Diseases Week, American College of Gastroenterology, European Association for the Study of the Liver, and the American Association for the Study of Liver Diseases annual meetings for 2013 to June 2016 were searched for unpublished studies. Titles and abstracts were reviewed independently by two study reviewers (PSD, JP), and studies were excluded if they did not address the research question of interest. Full-text of the remaining articles were reviewed to identify studies which met all inclusion criteria and were included in the quantitative synthesis. Supplementary Figure 1 details the study selection flowchart.
Data Abstraction
Data on study- and participant-related characteristics were abstracted by two authors (PSD, JP) independently. Discrepancies were resolved by consensus, referring back to the original article, in consultation with a third reviewer (SS). Data extracted from each study included the following: (1) study characteristics: location, primary author, time period of cohort observation, number of patients; (2) patient characteristics: age, gender, body mass index (BMI), co-morbid conditions (diabetes, cardiovascular disease, hypertension, metabolic syndrome); and (3) outcomes: fibrosis stage, overall mortality, liver related mortality, and person-years of follow-up.
Quality Assessment
The quality of included studies was assessed using a scale modified from the Newcastle-Ottawa scale for cohort studies.18 The original Newcastle-Ottawa scale is comprised of 8 items within 3 categories: selection of study groups, comparability of groups, ascertainment of exposure or outcome of interest. (Supplementary Material) In this modified version we accounted for the components assessed in the original scale and modified the questions to ascertain concepts and components relevant to NAFLD that may bias study results or comparability. This quality score consisted of 7 questions: (1) representative of the average NAFLD adult in the community (1 point for population-based studies, 0.5 points for multicenter studies, 0 points for a single-center study); (2) large cohort size (1 point if cohort size >200 patients with NAFLD, 0.5 points if cohort size between 100–200 NAFLD patients, 0 points if cohort size of <100 patients with NAFLD); (3) definite histological confirmation of NAFLD (1 point if confirmed by consensus of 2 expert pathologists, 0.5 points if reviewed by 1 expert pathologist, 0 points if reviewed only by community pathologist or not reported in study); (4) adequate follow-up of cohort for outcome to occur (1 point if mean or median follow-up of entire cohort >5 years, 0.5 points if cohort follow-up between 3–5 years, 0 points if follow-up of cohort <3 years); (5) clear information on duration of follow-up of patients by fibrosis stage (1 point if reported in total person-years, 0.5 point if reported as mean or median follow-up by fibrosis stage, 0 points if imputed from entire cohort); (6) attrition rate (1 point if >80% of cohort followed-up, 0.5 points if 60–80% cohort followed-up, 0 points if >40% lost to follow-up); (7) definite information on mortality (1 point if adequate information on rate of all-cause and liver-related mortality separately, 0.5 points if only information on all-cause mortality, without information on liver-related mortality). A score of ≥5, 3–4 and ≤2 was considered suggestive of high-, medium- and low-quality study.
Outcomes
The primary outcome of this study was to estimate the fibrosis stage-specific all-cause mortality rate (in relation to patients with stage 0 fibrosis) for NAFLD patients. A secondary outcome was to estimate the fibrosis stage-specific liver-related mortality rate for NAFLD patients. Liver-related mortality was defined per the study investigators.
Statistical Analysis
Crude mortality rates were calculated by dividing the total number of events by the total patient years of follow-up (PYF) for each fibrosis stage. Incremental risk of all-cause and liver-related mortality were then estimated. NAFLD patients with no fibrosis (stage 0) were used as a reference population. To account for variability in follow-up in different stages in cohort studies, mortality rate ratios (MRR) with 95% confidence intervals (CI), for all-cause and liver-related mortality, for each fibrosis stage (1–4) (vs. fibrosis stage 0) were estimated using fixed-effects meta-analysis using DerSimonian and Liard method. When mortality rate was zero for any stage, a correction of 0.05 was added in each column (number of deaths and person years follow-up) prior to statistical analysis.19 We assessed statistical heterogeneity using the inconsistency index (I2), with I2 values over 50% indicating substantial heterogeneity. Given the small number of studies identified in our analysis, statistical tests for assessing publications bias were not performed. Given the variability in definitions for liver-related mortality, the analysis for liver-related mortality was done using studies where only mortality was included in the definition of liver-related mortality. A sensitivity analysis was then performed by including the one study that included both liver-related events and liver-related mortality in the definition of liver-related mortality.11
RESULTS
A total of 844 studies were identified in our primary search, 5 of which were included in this meta-analysis.9–11, 16, 17 (Supplementary Figure 1) These 5 studies reported on 1,495 patients with 17,452 PYF. (Table 1, Supplementary Table 1) All 5 studies were cohort studies that utilized existing databases or cohorts of patients. No prospective cohort studies were performed specifically to assess for predictors and rates of mortality in NAFLD patients. The median of average age of patients was 49.3 years, and 53.5% were male. The prevalence of obesity within these cohorts was 40%, and ranged from 25.8% to 50% across cohorts. A similar prevalence and range of prevalence across cohorts for diabetes (34.7%, range 13.5% – 55.4%) and hypertension (42.1%, range 30.7% – 56.8%) were seen. The distribution of patients by fibrosis stage was: stage 0, n=570 (38.1%); stage 1, n=432 (28.9%); stage 2, n=203 (13.6%); stage 3, n=179 (12.0%); and stage 4, n=111 (7.4%). (Table 2)
Table 1.
Included Studies Reporting Mortality Associated with NAFLD Fibrosis
| Author, Publication Year | Location, Time Period | NAFLD sub-type, n | Men, n (%) | Age, mean/median years | Body Mass Index (BMI, kg/m2) | Comorbidities | Mean/Median Follow-up Time (years) | Study Quality* |
|---|---|---|---|---|---|---|---|---|
| Younossi9, 2011 Retrospective | USA | NAFL 78 NASH 132 |
79 (38%) | NAFLD - 48.1 NASH - 49.1 |
NAFL BMI: 34.9 ± 10.8 NASH BMI: 37.4 ± 10.3 |
Obese: 102 DM: 43 HLD: 47 |
Median 12.17 Max 28.5 |
HIGH |
| Sebastiani16, 2015 Retrospective | Canada, 2004–2013 | NASH 148 | 103 (70%) | 49.5 | 31.3 ± 5.4 | Obese: 74 DM: 49 HTN: 58 Met Synd: 42 |
Median 5 | HIGH |
| Ekstedt10, 2015 Prospective | Sweden, 1980–1993 | NAFLD 229 | 149 (65%) | 48.8 | 28.3 ± 3.7 | Obese: 59 DM: 31 HTN: 130 HLD: 78 |
Mean 26.4 ± 5.6 Range 6 – 33 |
HIGH |
| Angulo11, 2015 Retrospective | Multi-national, 1975–2005 | NAFL 335 NASH 284 |
232 (38%) | 49 | 30.7 | DM: 232 HTN: 190 |
Median 12.6 Range 0.3 – 35.1 |
HIGH |
| Leung17, 2016 Prospective | Hong Kong, 2006–2015 | NAFL 156 NASH 151 |
171 (56%) | 51 | 27.6 | DM: 170 HTN: 170 Met Synd: 186 |
Median 4.1 (IQR 11 – 82 months) | HIGH |
Based on the modified Newcastle-Ottawa scale for cohort studies. See Supplementary Table 1
NAFL: Non-alcoholic fatty liver; NASH: Non-alcoholic steatohepatitis; DM: Diabetes mellitus; HTN: hypertension; Met Synd: Metabolic syndrome; BMI: Body mass index; IQR: interquartile range
Table 2.
Clinical Outcomes According to Fibrosis Stage
| Author, Publication Year | Fibrosis Stage | Total No. of patients (n) | Person-years of follow-up | All-cause mortality (n) | Liver-related mortality (n) |
|---|---|---|---|---|---|
| Younossi9 2011* | Stage 0 | 55 | 663.9 | 10 | 0 |
| Stage 1 | 64 | 782.6 | 14 | 2 | |
| Stage 2 | 26 | 355.5 | 8 | 1 | |
| Stage 3 | 43 | 495.0 | 19 | 6 | |
| Stage 4 | 22 | 244.0 | 13 | 9 | |
| Sebastiani16 2015* | Stage 0 | 23 | 187 | 0 | 0 |
| Stage 1 | 53 | 422 | 0 | 0 | |
| Stage 2 | 22 | 157 | 1 | 0 | |
| Stage 3 | 28 | 194 | 3 | 0 | |
| Stage 4 | 22 | 102 | 5 | 3 | |
| Ekstedt10 2015* | Stage 0 | 95 | 2111 | 27 | 1 |
| Stage 1 | 67 | 1542 | 26 | 0 | |
| Stage 2 | 38 | 797 | 24 | 5 | |
| Stage 3 | 20 | 338 | 16 | 3 | |
| Stage 4 | 4 | 72 | 2 | 0 | |
| Angulo112015 | Stage 0 | 322 | 4,057.2 | 74 | 5 |
| Stage 1 | 141 | 1,776.6 | 42 | 4 | |
| Stage 2 | 85 | 1,071 | 36 | 6 | |
| Stage 3 | 53 | 667.8 | 27 | 7 | |
| Stage 4 | 18 | 226.8 | 14 | 4 | |
| Leung17 2016* | Stage 0 | 75 | 432.7 | 2 | 0 |
| Stage 1 | 107 | 395.0 | 2 | 0 | |
| Stage 2 | 32 | 91.7 | 0 | 0 | |
| Stage 3 | 35 | 110.0 | 0 | 0 | |
| Stage 4 | 45 | 140.8 | 2 | 1 |
Data obtained from primary study investigators
Fibrosis Stage Specific All-Cause Mortality
In the reference population (fibrosis stage 0), there were 113 deaths during 7,452 PYF equating to a crude all-cause mortality rate of 15.2 per 1,000 PYF. The crude all-cause mortality rate was higher according to fibrosis stage, with a crude rate of 17.1 for stage 1 fibrosis, 27.9 for stage 2 fibrosis, 36.0 for stage 3 fibrosis, and 45.8 per 1,000 PYF for stage 4 fibrosis. (Table 3) On meta-analysis, when compared to NAFLD patients with no fibrosis (stage 0), NAFLD patients with fibrosis had a higher MRR for all-cause mortality and this increased risk was seen even among those with stage 1 fibrosis (MRR 1.58, 95% CI 1.19 – 2.11). (Table 3, Figure 1 and 2) No heterogeneity (I2 = 0) was observed for the comparison of fibrosis stages 1–3 versus stage 0, and there was moderate heterogeneity (I2 = 52%) for the comparison of fibrosis stage 4 versus stage 0.
Table 3.
Pooled all-cause and liver-related mortality by fibrosis stage
| All-cause mortality | Liver-related mortality | |||
|---|---|---|---|---|
| Mortality rate (per 1,000 PYF) | Mortality Rate Ratio (95% CI) | Mortality rate (per 1,000 PYF) | Mortality Rate Ratio (95% CI) | |
| Stage 0 | 15.2 | Reference | 0.30 | Reference |
| Stage 1 | 17.1 | 1.58 (1.19 – 2.11) | 0.64 | 1.41 (0.17 – 11.95) |
| Stage 2 | 27.9 | 2.52 (1.85 – 3.42) | 4.28 | 9.57 (1.67 – 54.93) |
| Stage 3 | 36.0 | 3.48 (2.51 – 4.83) | 7.92 | 16.69 (2.92 – 95.36) |
| Stage 4 | 45.8 | 6.40 (4.11 – 9.95) | 23.3 | 42.30 (3.51 – 510.34) |
Statistically significant ratios are in bold
Figure 1. Incidence Rate of All-cause Mortality in NAFLD by Fibrosis Stage (vs. Stage 0 Fibrosis).
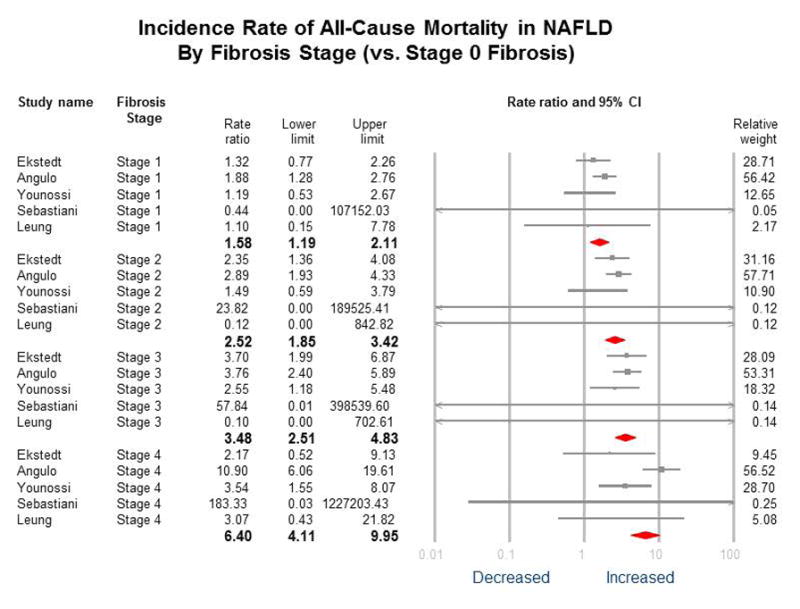
No heterogeneity for Fibrosis stages 1–3 vs. 0 (I2=0); for stage 4 vs. stage 0 = 52%; P-value for difference between groups=0.001, i.e. statistically different between groups
Figure 2. Fibrosis Stage Specific All-Cause Mortality Rate and Mortality Rate Ratio.
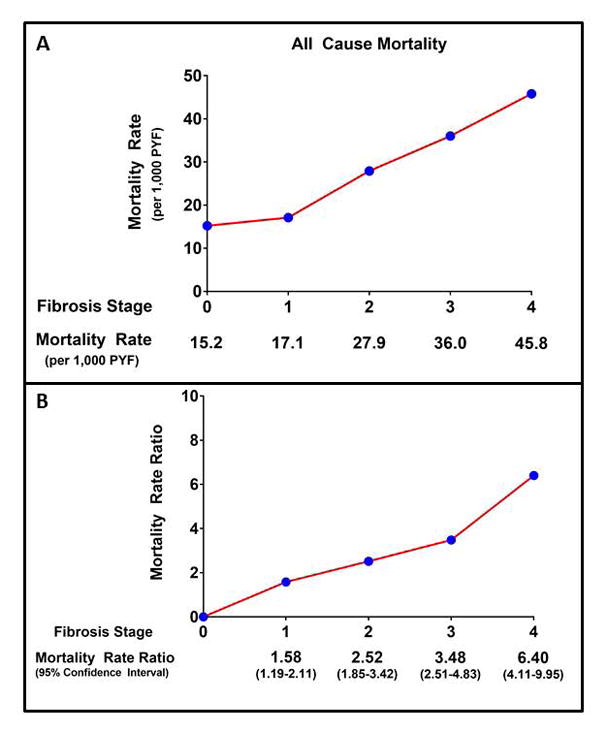
Panel A: Crude All-Cause Mortality Rate by Fibrosis Stage. Panel B: All-Cause Mortality Rate Ratio with 95% Confidence Intervals by Fibrosis Stage. PYF – patient years follow-up.
Fibrosis Stage Specific Liver-Related Mortality
In the reference population (fibrosis stage 0), there was 1 liver-related death during 3,395 PYF equating to a crude liver-related mortality rate of 0.30 per 1,000 PYF. The crude liver-related mortality rate was higher according to fibrosis stage, with a crude rate of 0.64 for stage 1 fibrosis, 4.28 for stage 2 fibrosis, 7.92 for stage 3 fibrosis, and 23.3 per 1,000 PYF for stage 4 fibrosis. (Table 3) On meta-analysis, when compared to NAFLD patients with no fibrosis (stage 0), NAFLD patients with fibrosis had an exponentially higher MRR for liver-related mortality. (Table 3, Figure 3 and 4) The increased risk for liver-related mortality was higher and statistically significant from stage 2 fibrosis (MRR 9.57, 95% CI 1.67 – 54.93). Liver-related MRR was numerically higher, but not statistically significant, in patients with stage 1 fibrosis (MRR 1.41, 95% CI 0.17 – 11.95). These estimates were largely unchanged after including the study by Angulo et al. (stage 1 fibrosis MRR 1.70, 95% CI 0.56 – 5.21; stage 2 fibrosis MRR 5.75, 95% CI 2.15 – 15.35). In patients with stage 2 fibrosis, liver-related mortality accounted for approximately 18% of all-cause mortality. In patients with stage 3 and 4 fibrosis, liver-related mortality accounted for approximately 24% and 59% of all-cause mortality, respectively. No heterogeneity (I2 = 0%) was observed for the comparison of all fibrosis stages (1–4) versus stage 0. (Figure 3)
Figure 3. Incidence Rate of Liver-related Mortality in NAFLD by Fibrosis Stage (vs. Stage 0 Fibrosis).
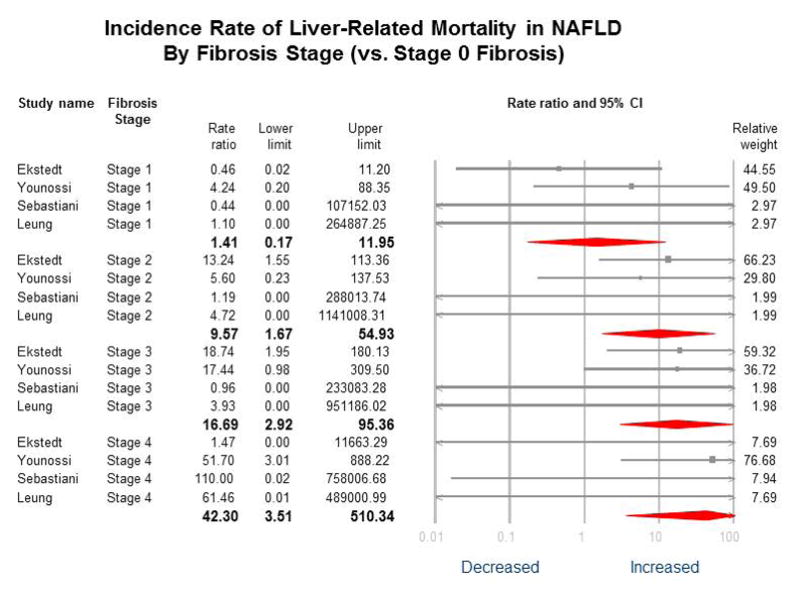
No heterogeneity for Fibrosis stages 1–4 vs. 0 (I2=0); P-value for difference between groups p=0.02, i.e. statistically different between groups. After including Angulo et al.11 which included liver-related events, instead of only liver-related mortality, estimates were similar: stage 1, MRR, 1.70 (95% CI 0.56–5.21); stage 2, MRR, 5.75 (95% CI 2.15–15.35); stage 3, MRR, 10.43 (95% CI 4.0–27.19); and stage 4, MRR, 18.12 (95% CI 5.67–57.97).
Figure 4. Fibrosis Stage Specific Liver-Related Mortality Rate and Mortality Rate Ratio.
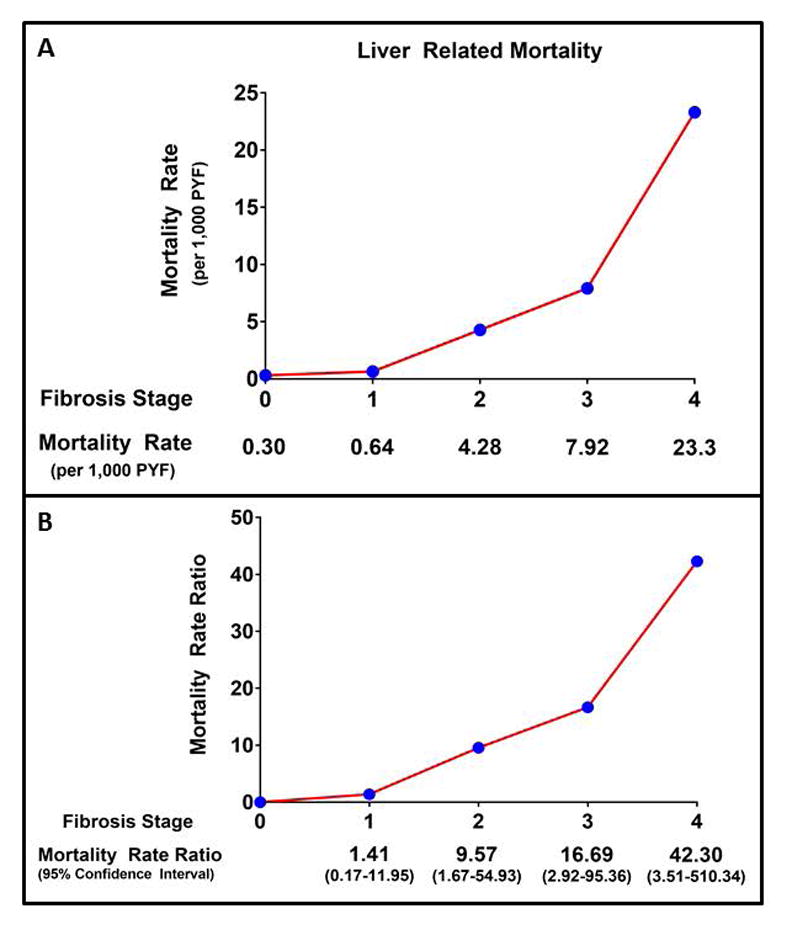
Panel A: Crude Liver-Related Mortality Rate by Fibrosis Stage. Panel B: Liver-Related Mortality Rate Ratio with 95% Confidence Intervals by Fibrosis Stage. PYF – patient years follow-up.
DISCUSSION
Main findings
Utilizing fibrosis stage-specific all-cause and liver-related mortality data from five cohort studies including 1,495 patients with 17,452 patient-years of follow-up, we have made three key observations that provide novel quantitative data on the risk of mortality in NAFLD. First, the risk of all-cause mortality is higher with increasing fibrosis stage, and even NAFLD patients with stage 1 fibrosis are at increased risk of mortality. Second, the risk of liver-related mortality increases on an exponential scale rather than on a linear scale with increase in the fibrosis stage. The risk of liver-related death is statistically higher only after progression to stage 2 fibrosis or higher. Finally, the quantitative risk of liver-related mortality was 1.22 for stage 1 fibrosis, 4.85 for stage 2 fibrosis, 8.86 for stage 3 fibrosis, and 21.6 per 1,000 PYF for stage 4 fibrosis. These data have important implications for understanding health utility of patients in the respective stage of disease and economic impact of benefits of improvement in the stage of fibrosis in the setting of a treatment modality. These data suggest that benefits of regression of fibrosis even by one stage may be more profound in patients with cirrhosis (stage 4) or in patients with bridging fibrosis (stage 3), than in earlier stage of fibrosis.
In context with published literature
Younossi et al.9 and Ekstedt et al.10 observed that NAFLD patients with advanced fibrosis (stage 3–4) were at an increased risk for all-cause mortality, but this increased risk was not seen among those with early stage fibrosis (stage 1–2). In contrast, Angulo et al.11 observed that NAFLD patients with stage 1 (HR 1.82, 95% CI 1.18 – 2.81) and stage 2 (HR 1.91, 95% CI 1.20 – 3.03) fibrosis were both at an increased risk for all-cause mortality. Furthermore, they observed that the risk for all-cause mortality was similar in patients with stage 2 and stage 3 fibrosis (HR 1.90, 95% CI 1.16 – 3.12), and an incremental increase in risk was only seen among NAFLD patients with stage 4 fibrosis (HR 6.35, 95% CI 3.35 – 12.04). This variation in estimates of risk across studies may be due to variations in study populations or follow-up. In our pooled meta-analysis, after accounting for variability in follow-up, our findings are consistent with those of Angulo et al.11 and we observed that NAFLD patients with stage 1 fibrosis were at an increased risk for all-cause mortality. We expand on prior literature with the finding that the risk of all-cause mortality is higher by fibrosis stage, and this increase in risk is seen with each stage of fibrosis progression. These pooled estimates help summarize in a quantitative manner the risk of all-cause mortality stratified by the stage of fibrosis in NAFLD.
Prior studies have also observed that the risk of liver-related mortality specifically, is increased with more advanced fibrosis.9, 11 The confidence in estimates for liver-related mortality by fibrosis stage, however, have varied considerably across studies. Younossi et al.9 estimated a five-fold increased risk with stage 4 fibrosis (HR 5.62, 95% CI 1.92 – 6.46) while Angulo et al.11 estimated a 47-fold increased risk with stage 4 fibrosis (HR 47.46, 95% CI 11.94 – 188.61). Furthermore, prior literature has suggested that the risk of liver-related mortality is only present after fibrosis progression to stage 2, and this risk is exponentially higher when transitioning from stage 3 to 4.11 In our pooled analysis, we similarly observed that the increased risk for liver-related mortality was only seen after progression to stage 2 fibrosis. Though the risk was numerically higher even in patients with stage 1 fibrosis, the number of events were small and did not reach statistical significance. We expand on prior literature by providing estimates for fibrosis stage-specific liver-related mortality, and by observing that the increased risk for liver-related mortality with increasing fibrosis stage is exponential at each stage of fibrosis progression. This is of particular importance when designing therapeutic trials for the prevention of fibrosis and liver-related mortality.
Strengths and limitations
Although our study allows for a clinically meaningful expansion of prior literature, it is not without limitations. First, we were able to account for variability in follow-up, but we were not able to adjust for important co-morbid conditions (i.e. diabetes, cardiovascular disease), demographics (i.e. age), or sub-types (steatohepatitis versus fatty liver) known to impact fibrosis progression and mortality risk in NAFLD.20–22 This is of importance as our reference group included patients with simple steatosis and NASH, which could have influenced our baseline estimates and comparisons. Second, patient level data on the exact cause of death could not be obtained for all studies and therefore variations in definitions for liver-related mortality could potentially impact our estimates. We have attempted to overcome this by excluding studies that allowed for non-fatal events in the definition of liver-related mortality, and our estimates did not change significantly. We are unable to determine the relative contribution for specific liver-related etiologies (i.e. hepatocellular carcinoma or liver transplantation) to liver-related mortality.23 Finally, we were unable to accurately quantify the non-liver related mortality and the relative increase in key outcomes, such as cardiovascular related mortality, with increasing fibrosis stage. It can be assumed that given the increasing proportional accountability of liver-related mortality for all-cause mortality seen with increasing fibrosis stage, that other key outcomes such as cardiovascular related mortality are of more significance in early stage fibrosis, but further studies with individual patient data are needed to accurately quantify these risks and the other limitations identified.
Conclusion
In conclusion, NAFLD patients are at an increased risk for all-cause and liver-related mortality and this risk of mortality increases exponentially as the fibrosis stage increases from stage 0 to stage 4. NAFLD patients with stage 1 fibrosis are an increased risk for all-cause, but not liver-related mortality. These data suggest that benefits of regression of fibrosis even by one stage may be more profound in patients with cirrhosis (stage 4) or in patients with bridging fibrosis (stage 3), than in earlier stage of fibrosis. We have provided estimates for mortality rates which are of significance when engaging in the shared decision making process with patients, and when designing therapeutic intervention trials aimed at preventing fibrosis progression to prevent disease related complications and off-set the natural course of NAFLD.
Supplementary Material
1NAFLD diagnosis by ultrasound, ICD codes, laboratory markers, MRS or risk factors for NAFLD (obesity, metabolic syndrome, diabetes)
2Primary study did not exclude for HBV/HCV/HIV and/or significant alcohol consumption
Acknowledgments
Funding Support: RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award and grant K23-DK090303 and R01-DK106419-01. PSD is supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) training grant 5T32DK007202. Research reported in this publication was supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number P42ES010337. GS is supported by a Chercheur-Boursier career award from the Fonds de recherche du Québec – Santé (FRQ-S). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Conflict of interests: The authors report no conflict of interests.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 4.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. e1–9. doi: 10.1016/j.cgh.2014.04.014. quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 8.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–82. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 10.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97. e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stepanova M, De Avila L, Afendy M, et al. Direct and Indirect Economic Burden of Chronic Liver Disease in the United States. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 13.Tapper EB, Hunink MG, Afdhal NH, et al. Cost-Effectiveness Analysis: Risk Stratification of Nonalcoholic Fatty Liver Disease (NAFLD) by the Primary Care Physician Using the NAFLD Fibrosis Score. PLoS One. 2016;11:e0147237. doi: 10.1371/journal.pone.0147237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noureddin M, Anstee QM, Loomba R. Review article: emerging anti-fibrotic therapies in the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2016;43:1109–23. doi: 10.1111/apt.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA StatementThe PRISMA Statement. Annals of Internal Medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Sebastiani G, Alshaalan R, Wong P, et al. Prognostic Value of Non-Invasive Fibrosis and Steatosis Tools, Hepatic Venous Pressure Gradient (HVPG) and Histology in Nonalcoholic Steatohepatitis. PLoS One. 2015;10:e0128774. doi: 10.1371/journal.pone.0128774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung JC, Loong TC, Wei JL, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2016 doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Sutton AJ. Methods for meta-analysis in medical research. Chichester ; New York: J. Wiley; 2000. [Google Scholar]
- 20.Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Goossens N, Hoshida Y, Song WM, et al. Nonalcoholic Steatohepatitis Is Associated With Increased Mortality in Obese Patients Undergoing Bariatric Surgery. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertolotti M, Lonardo A, Mussi C, et al. Nonalcoholic fatty liver disease and aging: epidemiology to management. World J Gastroenterol. 2014;20:14185–204. doi: 10.3748/wjg.v20.i39.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827–38. doi: 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1NAFLD diagnosis by ultrasound, ICD codes, laboratory markers, MRS or risk factors for NAFLD (obesity, metabolic syndrome, diabetes)
2Primary study did not exclude for HBV/HCV/HIV and/or significant alcohol consumption


