Abstract
Objectives
To develop a risk model that can be used to identify PCI patients at higher risk of readmission who may benefit from additional resources at the time of discharge.
Background
A high proportion of patients undergoing PCI are readmitted within 30 days of discharge.
Methods
The sample comprised patients aged ≥65 years who underwent PCI at a CathPCI Registry®-participating hospital and could be linked with 100% Medicare fee-for-service claims between 01/2007-12/2009. The sample (n=388,078) was randomly divided into risk score development (n=193,899) and validation (n=194,179) cohorts. We did not count as readmissions those associated with staged revascularization procedures. Multivariable logistic regression models using stepwise selection models were estimated to identify variables independently associated with all-cause 30-day readmission.
Results
The mean 30-day readmission rates for the development (11.36%) and validation (11.35%) cohorts were similar. In total, 19 variables were significantly associated with risk of 30-day readmission (p<0.05), and model c-statistics were similar in the development (0.67) and validation (0.66) cohorts. The simple risk score based on 14 variables identified patients at high and low risk of readmission. Patients with a score of ≥13 (15.4% of sample) had more than an 18.5% risk of readmission, while patients with a score ≤6 (41.9% of sample) had less than an 8% risk of readmission.
Conclusion
Among PCI patients, risk of readmission can be estimated using clinical factors present at the time of the procedure. This risk score may guide clinical decision-making and resource allocation for PCI patients at the time of hospital discharge.
Keywords: Percutaneous coronary intervention, health care outcomes, risk stratification, Medicare, quality improvement
Introduction
Thirty-day readmission following percutaneous coronary intervention (PCI) is an important quality metric since PCI is one of the most common and costly procedures performed,(1) and patients who experience early readmission are at increased risk of subsequent adverse events and 30-day and 1-year mortality (2-7). Despite the frequent use of PCI and decreases in PCI-related procedural and vascular complications, nearly one in seven are readmitted to the hospital within 30 days of discharge (5,8-10). Patients undergoing PCI may be readmitted for a variety of reasons, including procedural complications, planned processes of care, or as a result of underlying cardiac disease and comorbid conditions (6-9,11-13).
In 2013, the Centers for Medicare and Medicaid Services (CMS) and the American College of Cardiology (ACC) instituted voluntary public reporting of a National Quality Forum endorsed all-cause 30-day risk-standardized PCI readmission rates (14). Moreover, PCI patients account for a significant proportion of those patients represented in the heart failure and acute myocardial infarction readmission measures that are publicly reported and provide financial incentives for hospitals to prevent excess readmissions. Thus, healthcare providers are now challenged to develop mechanisms to reduce readmissions for PCI patients. One tool to help facilitate efforts to reduce PCI readmission may be through the implementation of a simple bedside risk score that will stratify PCI patients at the time of discharge according to their risk for an early readmission. This may be an ideal target for intervention for patients undergoing PCI since the medical care and transition teams are often organized around the procedure as opposed to the condition. The integration of a risk score in the clinical setting may also present an opportunity to target interventions to the patients at highest risk of readmission and effectively guide clinical decision-making and hospital resource allocation to ensure that patients are prepared at the time of discharge. To date, no such nationally representative risk-stratification schema exists to classify high-risk patients for readmission following a PCI procedure. Therefore, we sought to use variables from the 30-day PCI readmission measure (15) to derive a retrospective, observational multivariable risk prediction model that can be used to identify PCI patients at higher risk of readmission who may benefit from additional resources at the time of discharge.
Materials & Methods
Data Source
The National Cardiovascular Data Registry (NCDR) CathPCI Registry is the largest cardiovascular data registry in the United States, and the recruitment and quality processes have been described in great detail elsewhere (16,17). In brief, the NCDR CathPCI Registry prospectively collects data on PCI patient characteristics, including patient demographics, comorbid conditions, cardiac status, coronary lesion details, intracoronary device utilization and adverse event rates. Approximately 85% of all institutions performing PCI procedures elect to participate in the NCDR CathPCI Registry (17). Hospitals agree to submit their data on a quarterly basis for all patients undergoing cardiac catheterization and PCI procedures. Patient records focus on acute episodes of care, from admission to discharge. Data are entered by hospital personnel, and the data are only included in the analytic file if hospitals achieve >95% completeness of specific data elements, an indicator of data quality.
Patient Population
The cohort of the present study includes patients who underwent PCI at a hospital that reported to the NCDR CathPCI Registry between January 2007 and December 2009, and who had been successfully linked with corresponding administrative data from Medicare fee-for-service. The administrative data was used to identify variables associated with the endpoint of 30-day readmission. Eligibility for and enrollment in Medicare was determined through the Medicare denominator file. The study was approved by the Human Investigations Committee at Yale University.
Outcomes
The outcome of interest for the risk score was readmission within 30 days. Readmission was defined as a subsequent hospital inpatient admission within 30 days of the discharge date of an admission in the index cohort or claim end date (for PCI procedures performed as an outpatient service). Staged revascularization procedures were excluded as they often represent a planned strategy for treating patients with multi-vessel coronary artery disease rather than a quality signal (15).
PCI Readmission Measure
In partnership with the ACC and CMS, we have previously developed a measure using registry and administrative data that can be used to calculate hospital-level all-cause 30-day PCI readmission rates (15). The PCI readmission measure, which utilizes the robust clinical data collected by the NCDR CathPCI Registry, is suitable for public reporting and was endorsed by the National Quality Forum. Accordingly, each of the candidate variables included in the risk score development were derived from this measure and the NCDR CathPCI data collection form. The readmission measure used logistic regression with stepwise selection (entry p<0.05; retention with p<0.01) for variable selection (n=29). This resulted in a final risk-adjusted readmission model that included 20 variables (15). We included those 20 final variables in the present study, encompassing admission patient characteristics, history and risk factors, indications for PCI procedure, diagnostic studies, and PCI status.
For the current study we applied the same exclusions as for the readmission measure: admitted to the hospital less than 65 years of age; same-day discharges (patients would not be captured in the Part A inpatient data); admissions to hospitals with a missing, invalid, or duplicate Medicare Provider Number (MPN), and admissions in which the patients had identical information regarding age, sex, admission date, discharge date, and hospital MRN; patients admitted to the hospital who died during the index hospitalization or were transferred to another acute care facility and did not receive a PCI; admissions for patients with incomplete administrative data for the period 12 months prior to the index admission date or for the 30 days following discharge from the index hospitalization; procedures that were not the first PCI in hospital stay; PCI admissions within 30 days of discharge from an index PCI admission; patients who underwent staged PCI; patients who left the hospital against medical advice; patients not enrolled in Medicare Part A (fee-for-service); and when the PCI was performed >10 days following admission to the hospital. We also excluded patients who were missing data on the type of PCI (elective, urgent, emergency, salvage).
Statistical Analysis
To construct the risk score we randomly divided the study sample into development and validation cohorts. We then used the readmission measure specification to estimate a multivariable ordinary logistic regression model in the development sample. After estimating the initial model, we re-estimated the model using the lowest risk category as the reference category for each risk factor, to ensure that all odds ratios (ORs) would be greater than 1.0. Using the results of this second model, risk points were assigned corresponding to each significant risk factor with OR at least 1.2 based on the magnitude of the odds ratio; each risk factor was assigned points equal to (OR-1.1)*10. This scoring approach was chosen to capture the relative magnitude of the effect of each risk factor while providing simplicity of interpretation. To assess the predictive performance of the model we calculated the c statistic. In order to validate the construction of the risk score, we applied the risk points from the development sample model to the patients in the validation sample according to their risk factors, and summarized the readmission rate for patients in both groups according to total risk score. All statistical analyses for this report were performed at the Yale-New Haven Health Services Corporation/Center for Outcomes Research and Evaluation using Stata Version 13 (StataCorp LP, College Station, TX).
Results
Sample Characteristics
During the study period between January 1, 2007, and December 31, 2009, data from patients who underwent a PCI procedure at 1,043 CathPCI hospitals were available for analysis. After applying exclusion criteria, 388,078 patients were included in the overall sample. Patients were randomly selected to develop two approximately equally sized cohorts, namely the development cohort (n=193,899), and the validation cohort (n=194,179). The mean 30-day unplanned readmission rates for the development (11.35%; n=22,008) and validation (11.36%; n=22,059) cohorts were similar.
The clinical and demographic characteristics, and angiographic features of those patients in the development and validation cohorts are presented in Table I. The characteristics of patients in the two cohorts were similar, as were the characteristics of PCI patients in the derivation and validation cohorts who were readmitted within 30 days. In brief, the mean patient age in the sample was 72.5 years, there were more men than women, diabetes was prevalent in 33.5% of the population, and 14.5% and 38.3% of patients had a past history of congestive heart failure, and previous PCI procedure(s), respectively.
Table I. Baseline Characteristics of Patients in the Development and Validation Cohorts.
| Risk Factor | Development Cohort | Validation Cohort | ||
|---|---|---|---|---|
| Frequency | Readmitted* | Frequency | Readmitted* | |
| n(%) | n(%) | n(%) | n(%) | |
| Age | ||||
| Mean (SD) | 7.5 (0.7) | 7.5 (0.7) | ||
| 65-70 | 60,726 (31.3) | 5,554 (9.1) | 60,892 (31.4) | 5,564 (9.1) |
| 71-75 | 47,596 (24.5) | 4,927 (10.4) | 47,529 (24.5) | 4,981 (10.5) |
| 76-80 | 42,359 (21.8) | 5,030 (11.9) | 42,354 (21.8) | 4,962 (11.7) |
| 81-85 | 29,331 (15.1) | 4,210 (14.4) | 29,441 (15.2) | 4,235 (14.4) |
| 86+ | 13,887 (7.2) | 2,315 (16.7) | 13,963 (7.2) | 2,302 (16.5) |
| Female | ||||
| No | 114,557 (59.1) | 11,357 (9.9) | 114,293 (58.9) | 11,269 (9.9) |
| Yes | 79,342 (40.9) | 10,679 (13.5) | 79,886 (41.1) | 10,775 (13.5) |
| BMI | ||||
| Mean (SD) | 26.9 (3.3) | 26.9 (3.3) | ||
| ≤ 25 | 52,304 (27.0) | 7,134 (13.6) | 52,335 (27.0) | 7,121 (13.6) |
| ≤ 27.5 | 40,073 (20.7) | 4,326 (10.8) | 40,530 (20.9) | 4,359 (10.8) |
| > 27.5 | 101,522 (52.4) | 10,576 (10.4) | 101,314 (52.2) | 10,564 (10.4) |
| History of heart failure | ||||
| No | 165,733 (85.5) | 16,703 (10.1) | 166,031 (85.5) | 16,823 (10.1) |
| Yes | 28,166 (14.5) | 5,333 (18.9) | 28,148 (14.5) | 5,221 (18.5) |
| Previous valvular surgery | ||||
| No | 190,423 (98.2) | 21,496 (11.3) | 190,641 (98.2) | 21,489 (11.3) |
| Yes | 3,476 (1.8) | 540 (15.5) | 3,538 (1.8) | 555 (15.7) |
| Cerebrovascular disease | ||||
| No | 161,808 (83.4) | 17,350 (10.7) | 162,321 (83.6) | 17,253 (10.6) |
| Yes | 32,091 (16.6) | 4,686 (14.6) | 31,858 (16.4) | 4,791 (15.0) |
| Peripheral vascular disease | ||||
| No | 163,057 (84.1) | 17,269 (10.6) | 163,241 (84.1) | 17,407 (10.7) |
| Yes | 30,842 (15.9) | 4,767 (15.5) | 30,938 (15.9) | 4,637 (15.0) |
| Chronic lung disease | ||||
| No | 157,416 (81.2) | 16,045 (10.2) | 157,210 (81.0) | 16,146 (10.3) |
| Yes | 36,483 (18.8) | 5,991 (16.4) | 36,969 (19.0) | 5,898 (16.0) |
| Diabetes | ||||
| None | 128,941 (66.5) | 13,513 (10.5) | 128,896 (66.4) | 13,556 (10.5) |
| Non-insulin diabetes | 43,897 (22.6) | 5,015 (11.4) | 43,989 (22.7) | 5,007 (11.4) |
| Insulin diabetes | 21,061 (10.9) | 3,508 (16.7) | 21,294 (11.0) | 3,481 (16.3) |
| GFR | ||||
| Not measured | 8,772 (4.5) | 860 (9.8) | 8,750 (4.5) | 918 (10.5) |
| GFR < 30 | 8,401 (4.3) | 2,100 (25.0) | 8,528 (4.4) | 2,049 (24.0) |
| 30 ≤ GFR <60 | 68,431 (35.3) | 8,968 (13.1) | 68,684 (35.4) | 8,867 (12.9) |
| 60 ≤ GFR < 90 | 89,670 (46.2) | 8,282 (9.2) | 89,943 (46.3) | 8,368 (9.3) |
| 90 ≤ GFR | 18,625 (9.6) | 1,826 (9.8) | 18,274 (9.4) | 1,842 (10.1) |
| Renal failure - dialysis | ||||
| No | 189,997 (98.0) | 20,942 (11.0) | 190,143 (97.9) | 20,964 (11.0) |
| Yes | 3,902 (2.0) | 1,094 (28.0) | 4,036 (2.1) | 1,080 (26.8) |
| Hypertension | ||||
| No | 30,301 (15.6) | 2,939 (9.7) | 30,437 (15.7) | 2,999 (9.9) |
| Yes | 163,598 (84.4) | 19,097 (11.7) | 163,742 (84.3) | 19,045 (11.6) |
| History of tobacco use | ||||
| No | 170,318 (87.8) | 19,180 (11.3) | 170,609 (87.9) | 19,138 (11.2) |
| Yes | 23,581 (12.2) | 2,856 (12.1) | 23,570 (12.1) | 2,906 (12.3) |
| Previous PCI | ||||
| No | 119,613 (61.7) | 14,033 (11.7) | 120,021 (61.8) | 14,137 (11.8) |
| Yes | 74,286 (38.3) | 8,003 (10.8) | 74,158 (38.2) | 7,907 (10.7) |
| Current heart failure | ||||
| No | 169,335 (87.3) | 17,179 (10.1) | 169,709 (87.4) | 17,195 (10.1) |
| Yes | 24,564 (12.7) | 4,857 (19.8) | 24,470 (12.6) | 4,849 (19.8) |
| Symptoms on admission | ||||
| No MI | 138,393 (71.4) | 14,033 (10.1) | 138,550 (71.4) | 13,918 (10.0) |
| MI within 24 hrs | 44,490 (22.9) | 6,261 (14.1) | 44,805 (23.1) | 6,402 (14.3) |
| MI after 24 hrs | 11,016 (5.7) | 1,742 (15.8) | 10,824 (5.6) | 1,724 (15.9) |
| EF percentage | ||||
| Not measured | 54,945 (28.3) | 6,714 (12.2) | 55,680 (28.7) | 6,792 (12.2) |
| EF < 30 | 8,014 (4.1) | 1,589 (19.8) | 8,058 (4.1) | 1,555 (19.3) |
| 30 ≤ EF < 45 | 23,587 (12.2) | 3,406 (14.4) | 23,579 (12.1) | 3,457 (14.7) |
| 45 ≤ EF | 107,353 (55.4) | 10,327 (9.6) | 106,862 (55.0) | 10,240 (9.6) |
| PCI status | ||||
| Elective | 94,097 (48.5) | 8,324 (8.8) | 94,398 (48.6) | 8,318 (8.8) |
| Urgent | 74,544 (38.4) | 10,104 (13.6) | 74,463 (38.3) | 10,121 (13.6) |
| Emergency | 24,969 (12.9) | 3,554 (14.2) | 25,034 (12.9) | 3,548 (14.2) |
| Salvage | 289 (0.1) | 54 (18.7) | 284 (0.1) | 57 (20.1) |
| Highest risk lesion | ||||
| Unknown | 81,297 (41.9) | 8,726 (10.7) | 81,349 (41.9) | 8,758 (10.8) |
| pRCA/mLAD/pCIRC | 73,992 (38.2) | 8,609 (11.6) | 74,329 (38.3) | 8,572 (11.5) |
| pLAD | 33,537 (17.3) | 4,018 (12.0) | 33,607 (17.3) | 3,994 (11.9) |
| Left Main | 5,073 (2.6) | 683 (13.5) | 4,894 (2.5) | 720 (14.7) |
| Max prePCI TIMI flow: none | ||||
| No | 175,628 (90.6) | 19,591 (11.2) | 175,990 (90.6) | 19,608 (11.1) |
| Yes | 18,271 (9.4) | 2,445 (13.4) | 18,189 (9.4) | 2,436 (13.4) |
Readmitted within 30-days of index discharge for any reason. Abbreviations: BMI, body mass index; MI, myocardial infarction; GFR, glomular filtration rate; PCI, percutaneous coronary intervention; EF, ejection fraction.
Predicting 30-Day Readmission
Table II presents the logistic regression model of the 20 candidate discharge variables that were tested to develop the risk score. In total, 19 variables were significantly associated with risk of 30-day readmission (p<0.05); history of tobacco use was the only variable that was not associated with increased risk of 30-day readmission (p=0.09). After rounding all odds ratios (OR) to the nearest tenth and scoring based on a statistically meaningful OR threshold of 1.2 or greater, 14 independent predictors of readmission were included in the risk score. These variables ranged from the smallest risk of being readmitted for acute myocardial infarction occurring over 24 hours prior to the PCI procedure (OR=1.16, 95% CI 1.10 to 1.23) to the highest associated risk for readmission (renal function GFR < 30 ml/min (OR=1.75, 95% CI 1.63 to 1.88). The model's discrimination, measured in terms of the c statistic, in both the development and validation cohorts was similar at 0.67 and 0.66, respectively.
Table II. Independent Risk Factors for 30-day Readmission.
| Risk Factor | OR | 95% CI | P-value | Wald P-value |
|---|---|---|---|---|
| Intercept | 0.04 | [0.03, 0.04] | <0.001 | |
| Age | <0.001 | |||
| 65-70 | Ref | |||
| 71-75 | 1.10 | [1.06, 1.15] | <0.001 | |
| 76-80 | 1.23 | [1.18, 1.29] | <0.001 | |
| 81-85 | 1.45 | [1.38, 1.51] | <0.001 | |
| 86+ | 1.59 | [1.51, 1.69] | <0.001 | |
| Female | ||||
| No | Ref | |||
| Yes | 1.27 | [1.23, 1.30] | <0.001 | |
| BMI | <0.001 | |||
| ≤ 25 | 1.21 | [1.17, 1.26] | <0.001 | |
| ≤ 27.5 | 1.06 | [1.02, 1.10] | 0.004 | |
| > 27.5 | Ref | |||
| History of heart failure | ||||
| No | Ref | |||
| Yes | 1.33 | [1.27, 1.38] | <0.001 | |
| Previous valvular surgery | ||||
| No | Ref | |||
| Yes | 1.19 | [1.08, 1.31] | <0.001 | |
| Cerebrovascular disease | ||||
| No | Ref | |||
| Yes | 1.14 | [1.10, 1.18] | <0.001 | |
| Peripheral vascular disease | ||||
| No | Ref | |||
| Yes | 1.23 | [1.18, 1.27] | <0.001 | |
| Chronic lung disease | ||||
| No | Ref | |||
| Yes | 1.50 | [1.45, 1.55] | <0.001 | |
| Diabetes | <0.001 | |||
| None | Ref | |||
| Non-insulin diabetes | 1.10 | [1.06, 1.14] | <0.001 | |
| Insulin diabetes | 1.40 | [1.34, 1.46] | <0.001 | |
| GFR | <0.001 | |||
| Not measured | 1.00 | [0.93, 1.08] | 0.974 | |
| GFR < 30 | 1.75 | [1.63, 1.88] | <0.001 | |
| 30 ≤ GFR <60 | 1.20 | [1.17, 1.25] | <0.001 | |
| 60 ≤ GFR <90 | Ref | |||
| 90 ≤ GFR | 1.08 | [1.02, 1.14] | 0.006 | |
| Renal failure - dialysis | ||||
| No | Ref | |||
| Yes | 1.56 | [1.42, 1.71] | <0.001 | |
| Hypertension | ||||
| No | Ref | |||
| Yes | 1.11 | [1.07, 1.16] | <0.001 | |
| History of tobacco use | ||||
| No | Ref | |||
| Yes | 1.04 | [0.99, 1.09] | 0.09 | |
| Previous PCI | ||||
| No | Ref | |||
| Yes | 0.91 | [0.88, 0.94] | <0.001 | |
| Current heart failure | ||||
| No | Ref | |||
| Yes | 1.33 | [1.28, 1.39] | <0.001 | |
| Symptoms on admission | <0.001 | |||
| No MI | Ref | |||
| MI within 24 hrs | 1.08 | [1.04, 1.13] | <0.001 | |
| MI after 24 hrs | 1.16 | [1.10, 1.23] | <0.001 | |
| EF percentage | <0.001 | |||
| Not measured | 1.18 | [1.14, 1.22] | <0.001 | |
| EF < 30 | 1.53 | [1.43, 1.63] | <0.001 | |
| 30 ≤ EF<45 | 1.23 | [1.17, 1.28] | <0.001 | |
| 45 ≤ EF | Ref | |||
| PCI status | <0.001 | |||
| Elective | Ref | |||
| Urgent | 1.39 | [1.35, 1.44] | <0.001 | |
| Emergency | 1.53 | [1.44, 1.62] | <0.001 | |
| Salvage | 1.64 | [1.21, 2.23] | 0.002 | |
| Highest risk lesion | <0.001 | |||
| Unknown | Ref | |||
| pRCA/mLAD/pCIRC | 1.07 | [1.04, 1.11] | <0.001 | |
| pLAD | 1.09 | [1.04, 1.13] | <0.001 | |
| Left Main | 1.08 | [0.99, 1.18] | 0.07 | |
| Max prePCI TIMI flow: none | ||||
| No | Ref | |||
| Yes | 1.09 | [1.03, 1.15] | 0.001 |
Abbreviations: BMI, body mass index; EF, ejection fraction; GFR, glomular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention.
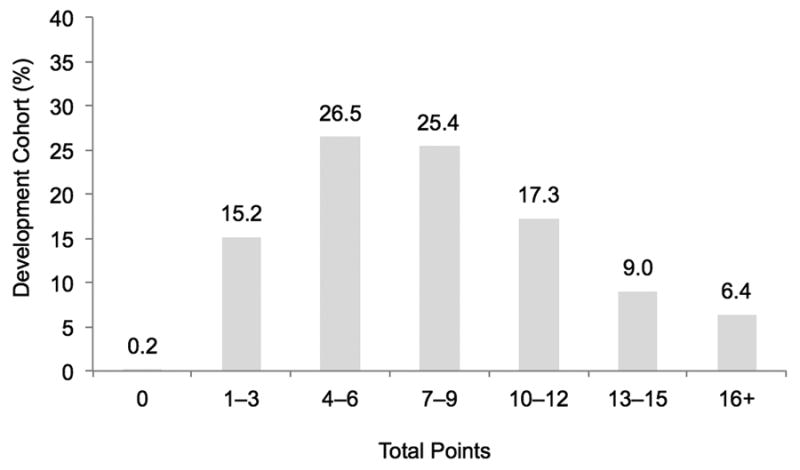
After applying the scoring algorithm and converting each risk factor to an integer score, points ranged from 1 to 6, with the former indicating the risk factors with smaller effect size (Table III). Total scores are calculated by summating the applicable points to estimate a patient's risk of being readmitted within 30-days of the index PCI procedure. Both the development and validation cohorts were similar with regard to their distribution of scores. Patients with a score of 6 or lower comprised almost 42% of the cohort and this group had less than an 8% risk of readmission (Figure 1). Meanwhile, patients with a score of 13 or higher comprised only 15% of the cohort but this group was at substantially higher risk for 30-day readmission (≥18.5% readmitted). Less than 1% (development cohort: 0.2%; n=440) of patients presented with no risk factors (score=0), while those patients presenting with 16 or more points had the highest predicted probability of readmission (25.2% readmitted; 6.4% of cohort) (Figure 2). Results were similar for the development cohort.
Table III. Risk Scores and Factors Associated with 30-day Readmission.
| Risk Factor | Rounded OR* | Points |
|---|---|---|
| Age | ||
| 65-70 | Ref | |
| 71-75 | 1.1 | |
| 76-80 | 1.2 | 1 |
| 81-85 | 1.4 | 3 |
| 86+ | 1.6 | 5 |
| Female | ||
| Yes | 1.3 | 2 |
| BMI | ||
| ≤ 25 | 1.2 | 1 |
| > 27.5 | Ref | |
| History of heart failure | ||
| Yes | 1.3 | 2 |
| Previous valvular surgery | ||
| Yes | 1.2 | 1 |
| Peripheral vascular disease | ||
| Yes | 1.2 | 1 |
| Chronic lung disease | ||
| Yes | 1.5 | 4 |
| Diabetes | ||
| None | Ref | |
| Insulin diabetes | 1.4 | 3 |
| GFR | ||
| GFR < 30 | 1.7 | 6 |
| 30 ≤ GFR < 60 | 1.2 | 1 |
| 60 ≤ GFR < 90 | Ref | |
| Renal failure - dialysis | ||
| Yes | 1.6 | 5 |
| Current heart failure | ||
| Yes | 1.3 | 2 |
| Symptoms on admission | ||
| No MI | Ref | |
| MI after 24 hrs | 1.2 | 1 |
| EF percentage | ||
| Not measured | 1.2 | 1 |
| EF < 30 | 1.5 | 4 |
| 30 ≤ EF < 45 | 1.2 | 1 |
| 45 ≤ EF | Ref | |
| PCI status | ||
| Elective | Ref | |
| Urgent | 1.4 | 3 |
| Emergency | 1.5 | 4 |
| Salvage | 1.6 | 5 |
p<0.001 for all variables with the exception of PCI Salvage (p=0.002). Abbreviations: BMI, body mass index; MI, myocardial infarction; GFR, glomular filtration rate; PCI, percutaneous coronary intervention; EF, ejection fraction.
Figure 1. Risk Score Distribution by Total Points.
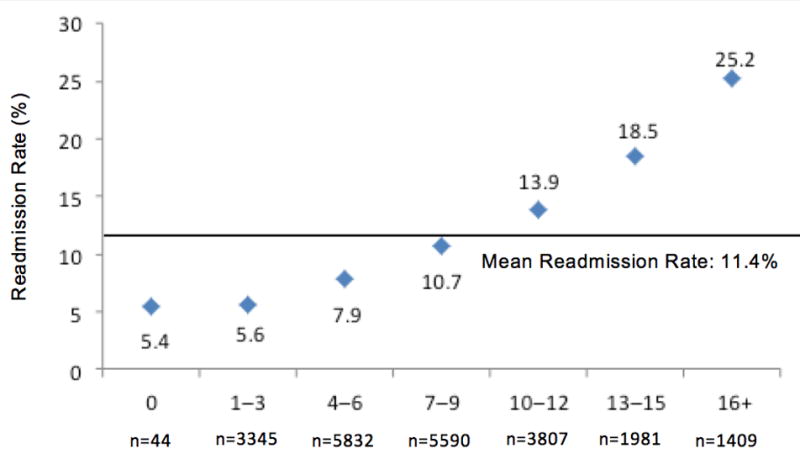
Figure 2. Distribution of Risk Score Points by Readmission Rate.
Discussion
Reducing and preventing PCI readmissions is a common goal for both hospitals and providers, yet we lack the tools necessary to target populations at highest risk of readmission. As a first step to assess a patient's risk of 30-day all-cause unplanned readmission following PCI, we have derived and validated a risk prediction tool using robust and clinically sensible data from 388,078 PCI patients obtained from the NCDR CathPCI Registry and Medicare databases. In our risk score, points are assigned for presence of each of 14 independent predictors of readmission including demographic, clinical, and angiographic features. The risk score can be used to identify those patients at the highest risk of readmission. For instance, less than one-fifth of patients had a score of 13 points or higher, yet the risk of 30-day unplanned readmission was over 18.5%. Meanwhile, those patients with a score of 6 or lower comprised over two-fifths of the cohort and had less than half of the risk of readmission (7.9%). The risk score can help to prioritize hospital resources to those patients at the highest risk of readmission.
While previous risk prediction algorithms have been derived and validated for patients' risk of mortality and major procedural complications both during and following PCI (18-33), few reports to date have specifically examined factors associated with 30-day readmission following PCI. Indeed, previous studies have found that 30-day readmission is associated with several sociodemographic (e.g., female sex, older age), clinical (e.g., diabetes mellitus, current congestive heart failure, acute myocardial infarction, peripheral arterial disease, previous PCI, complications related to the PCI, length of stay), and angiographic (e.g., depressed ejection fraction, higher number and fewer significant lesions treated, emergent or urgent PCI status) risk factors (5-8,11-13). In the present study, we have confirmed the associations of several of these risk factors in the Medicare population, as well as identified the important role of admission patient characteristics, history and risk factors, indications for PCI procedure, diagnostic studies, and PCI status in predicting readmission. Yet, only one study has translated a multivariable model that assessed predictors of 30-day readmission to a functional risk score (11). However, generalizability of that risk score is limited as the study sample only comprised one state (Massachusetts) and potentially planned revascularization procedures were included as an adverse event. Further, that study considered events that occurred after PCI and prior to discharge, whereas our risk score allows hospitals to calculate risk of readmission at the time of the procedure using readily available clinical characteristics. This is especially advantageous since PCI patient stays are generally short and the risk score can proactively identify patients at highest risk of readmission at the time of the procedure. This information could potentially be used to prioritize efforts to smooth the transition from the inpatient to the outpatient setting and allocate resources towards those who may benefit from more intensive follow-up care.
The utility of risk scores in interventional cardiology is well established. While some are easily calculated, such as the Thrombolysis in Myocardial Infarction (TIMI) risk score,(34) others are more complex and require the use of a calculator, such as the Yale-New Haven Hospital Center for Outcomes Research and Evaluation heart failure, pneumonia, and AMI readmission risk scores (35), or the Society for Cardiovascular Angiography and Interventions (SCAI) PCI Risk Calculator that uses pre-procedural patient information to estimate the post-intervention risks for mortality, acute kidney injury or transfusion (36). Due to the challenge of quickly estimating risk of 30-day readmission for PCI patients based on 14 variables, our risk score may be a candidate for future calculator app development. Nevertheless, the number of variables in our risk score is justified given the absence of any truly dominant risk factors associated with readmission, leading to superior risk stratification.
Our study is clinically meaningful because it provides a simple means of assessing a PCI patient's risk of 30-day readmission, and thus may be used to help guide clinical decision-making, resource allocation, and transitional care interventions to ensure that high-risk patients are prepared at the time of discharge. For instance, presented with a patient at high risk of readmission, a clinician could involve social work, schedule an earlier follow-up appointment, request for pharmacy to perform medication reconciliation, provide additional patient education, enroll the patient in a hospital-initiated transitional care program, or deploy home health care services. Two simple strategies identified in a recent study to be associated with low readmission rates following PCI included discharge with the date and time of a follow-up appointment already arranged, and regular meetings with cardiac rehabilitation to review the care of cardiac patients (37). However, since PCI is a high volume procedure, it is noted that hospitals may not be able to deploy all of these resources to all patients, so the true impact of the risk score is to target resources to patients at the highest risk of readmission. Correspondingly, risk of readmission is continuous with no obvious cut point, thus hospitals should choose a customized cut point for the allocation of additional resources based on the resources that are readily available.
Our study must be interpreted within the context of the following limitations. First, we used the NCDR CathPCI Registry to identify clinical factors present at the time of the procedure. Participation in the NCDR CathPCI Registry is voluntary and may not be representative of all PCI hospitals, thus findings should be extrapolated to other hospitals with caution. Nevertheless, our use of the NCDR CathPCI Registry is justified because it remains the largest and most generalizable source of clinical and angiographic features of PCI in the U.S. Second, our sample consisted of patients aged 65 years or older, thus potentially limiting the generalizability of the risk score to younger populations. However, it is worth noting that Medicare is the only U.S. data source with complete 30-day outcomes and that the Medicare population comprises the majority of PCI procedures (38-41). Further, we excluded Medicare Advantage beneficiaries which comprise up to 14% of patients aged 65 years or older (42). Third, we note a modest c statistic of 0.67 and 0.66 for the development and validation cohorts, respectively. Nonetheless, our models' discrimination is consistent with all readmission models used for public reporting (43), and other studies with a similar c statistic have been demonstrated to be clinically useful (e.g. TIMI risk score, c statistic: 0.65 (34)). Finally, our study only excluded planned revascularization procedures (e.g. CABG and PCI), consequently other planned non-revascularization procedures, such as ICD implantation, may have been included as an outcome. Post-procedural complications may also have been underreported. In light of our limitations, it is worth noting that this risk score should be replicated and validated in future prospective studies.
Conclusion
In summary, using data from the NCDR CathPCI Registry and the Centers for Medicare and Medicaid Services, we have derived and validated a prediction model that may be used to assess a patient's risk of all-cause 30-day readmission following PCI. This risk stratification tool is designed to identify those patients at the highest risk of readmission to potentially facilitate targeted interventions. The employment of this risk score in the clinical setting may translate to improved patient outcomes, proper quality assessment, and guided resource management, specifically for those patients at highest risk of readmission. Future research is needed to identify intervention strategies to reduce readmission rates (44), as well as identify the broader non-clinical factors that may be related to risk of 30-day readmission, such as access to and coordination of care, social support, and hospital culture and organizational behavior.
Acknowledgments
This study was funded by the National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH) grant #U012008713-05.
Footnotes
CathPCI Registry is an initiative of the American College of Cardiology with partnering support from The Society of Cardiovascular Angiography and Interventions.
Conflicts of Interest: KEM: None
JH: None
PF: None
JPC: Dr Curtis receives salary support under contract with the National Cardiovascular Data Registry to provide analytic services and with the Centers for Medicare and Medicaid Services to support development of quality measures. In addition, he holds equity interest in Medtronic.
Contributor Information
Karl E. Minges, Center for Outcomes Research and Evaluation, Yale School of Medicine, Yale-New Haven Hospital, New Haven, CT.
Jeph Herrin, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT; Senior Statistician, Health Research & Educational Trust, Chicago IL.
Paul N. Fiorilli, Cardiovascular Division, Hospital of the University of Pennsylvania, Philadelphia, PA.
Jeptha P. Curtis, Department of Internal Medicine, Yale University School of Medicine; Center for Outcomes Research and Evaluation, Yale School of Medicine, Yale-New Haven Hospital, New Haven, CT.
References
- 1.Medicare Payment Advisory Commission. Report to the Congress: promoting greater efficiency in Medicare. MedPAC; Washington, DC: 2007. [Google Scholar]
- 2.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare Fee-for-Service program. New Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Lin Z, Straube BM, Rapp MT, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2(5):407–13. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 4.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidan MT, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3(1):97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis JP, Schreiner G, Wang Y, Chen J, Spertus JA, Rumsfeld JS, Brindis RG, Krumholz HM. All-cause readmission and repeat revascularization after percutaneous coronary intervention in a cohort of medicare patients. J Am Coll Cardiol. 2009;54(10):903–7. doi: 10.1016/j.jacc.2009.04.076. [DOI] [PubMed] [Google Scholar]
- 6.Khawaja FJ, Shah ND, Lennon RJ, Slusser JP, Alkatib AA, Rihal CS, Gersh BJ, Montori VM, Holmes DR, Bell MR, et al. Factors associated with 30-day readmission rates after percutaneous coronary intervention. Arch Intern Med. 2012;172(2):112–7. doi: 10.1001/archinternmed.2011.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricciardi MJ, Selzer F, Marroquin OC, Holper EM, Venkitachalam L, Williams DO, Kelsey SF, Laskey WK. Incidence and predictors of 30-day hospital readmission rate following percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry) Am J Cardiol. 2012;110(10):1389–96. doi: 10.1016/j.amjcard.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannan EL, Zhong Y, Krumholz H, Walford G, Holmes DR, Jr, Stamato NJ, Jacobs AK, Venditti FJ, Sharma S, King SB., 3rd 30-day readmission for patients undergoing percutaneous coronary interventions in New York state. JACC Cardiovasc Interv. 2011;4(12):1335–42. doi: 10.1016/j.jcin.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Yeh RW, Rosenfield K, Zelevinsky K, Mauri L, Sakhuja R, Shivapour DM, Lovett A, Weiner BH, Jacobs AK, Normand SL. Sources of hospital variation in short-term readmission rates after percutaneous coronary intervention. Circ Cardiovasc Interv. 2012;5(2):227–36. doi: 10.1161/CIRCINTERVENTIONS.111.967638. [DOI] [PubMed] [Google Scholar]
- 10.Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, Chen AY, Klein LW, Masoudi FA, McKay C, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56(4):254–63. doi: 10.1016/j.jacc.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Wasfy JH, Rosenfield K, Zelevinsky K, Sakhuja R, Lovett A, Spertus JA, Wimmer NJ, Mauri L, Normand SLT, Yeh RW. A prediction model to identify patients at high risk for 30-day readmission after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. 2013 doi: 10.1161/CIRCOUTCOMES.111.000093. [DOI] [PubMed] [Google Scholar]
- 12.Wasfy JH, Strom JB, O'Brien C, Zai AH, Luttrell J, Kennedy KF, Spertus JA, Zelevinsky K, Normand SL, Mauri L, et al. Causes of short-term readmission after percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(1):97–103. doi: 10.1161/CIRCINTERVENTIONS.113.000988. [DOI] [PubMed] [Google Scholar]
- 13.Yost GW, Puher SL, Graham J, Scott TD, Skelding KA, Berger PB, Blankenship JC. Readmission in the 30 days after percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6(3):237–44. doi: 10.1016/j.jcin.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 14.National Quality Forum Press Release. NQF endorses patient outcome measures for high-impact conditions. 2010 Available: http://www.qualityforum.org/Publications/2014/11/NQF-Endorsed_Measures_for_Cardiovascular_Conditions.aspx.
- 15.Curtis JP, Drye EE, Duffy CO, Geary LL, Krumholz H, Partovian C, Wang Y. Hospital 30-day readmission following percutaneous coronary intervention measure, measure methodology. 2009;2013 Available https://www.ncdr.com/WebNCDR/docs/default-source/analytics-/pci-readmission-methodology-report_5-1-13.pdf?sfvrsn=2. [Google Scholar]
- 16.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37(8):2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 17.Dehmer GJ, Weaver D, Roe MT, Milford-Beland S, Fitzgerald S, Hermann A, Messenger J, Moussa I, Garratt K, Rumsfeld J, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 2011. J Am Coll Cardiol. 2012;60(20):2017–31. doi: 10.1016/j.jacc.2012.08.966. [DOI] [PubMed] [Google Scholar]
- 18.Lee CH, van Domburg RT, Hoye A, Lemos PA, Tanabe K, Smits PC, van der Giessen WJ, de Feyter P, Serruys PW. Predictors of survival after contemporary percutaneous coronary revascularization for acute myocardial infarction in the real world. J Invasive Cardiol. 2004;16(11):627–31. [PubMed] [Google Scholar]
- 19.Burjonroppa SC, Varosy PD, Rao SV, Ou FS, Roe M, Peterson E, Singh M, Shunk KA. Survival of patients undergoing rescue percutaneous coronary intervention development and validation of a predictive tool. JACC Cardiovasc Interv. 2011;4(1):42–50. doi: 10.1016/j.jcin.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Kimmel SE, Berlin JA, Strom BL, Laskey WK. Development and validation of simplified predictive index for major complications in contemporary percutaneous transluminal coronary angioplasty practice. The Registry Committee of the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol. 1995;26(4):931–8. doi: 10.1016/0735-1097(95)00294-4. [DOI] [PubMed] [Google Scholar]
- 21.Rihal CS, Grill DE, Bell MR, Berger PB, Garratt KN, Holmes DR., Jr Prediction of death after percutaneous coronary interventional procedures. Am Heart J. 2000;139(6):1032–8. doi: 10.1067/mhj.2000.105299. [DOI] [PubMed] [Google Scholar]
- 22.Singh M, Gersh BJ, Li S, Rumsfeld JS, Spertus JA, O'Brien SM, Suri RM, Peterson ED. Mayo Clinic Risk Score for percutaneous coronary intervention predicts in-hospital mortality in patients undergoing coronary artery bypass graft surgery. Circulation. 2008;117(3):356–62. doi: 10.1161/CIRCULATIONAHA.107.711523. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Hannan EL, Walford G, Ambrose JA, Holmes DR, Jr, King SB, 3rd, Clark LT, Katz S, Sharma S, Jones RH. A risk score to predict in-hospital mortality for percutaneous coronary interventions. J Am Coll Cardiol. 2006;47(3):654–60. doi: 10.1016/j.jacc.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 24.Addala S, Grines CL, Dixon SR, Stone GW, Boura JA, Ochoa AB, Pellizzon G, O'Neill WW, Kahn JK. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score) Am J Cardiol. 2004;93(5):629–32. doi: 10.1016/j.amjcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Moscucci M, Kline-Rogers E, Share D, O'Donnell M, Maxwell-Eward A, Meengs WL, Kraft P, DeFranco AC, Chambers JL, Patel K, et al. Simple bedside additive tool for prediction of in-hospital mortality after percutaneous coronary interventions. Circulation. 2001;104(3):263–8. doi: 10.1161/01.cir.104.3.263. [DOI] [PubMed] [Google Scholar]
- 26.Negassa A, Monrad ES, Bang JY, Srinivas VS. Tree-structured risk stratification of in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction: a report from the New York State percutaneous coronary intervention database. Am Heart J. 2007;154(2):322–9. doi: 10.1016/j.ahj.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connor GT, Malenka DJ, Quinton H, Robb JF, Kellett MA, Jr, Shubrooks S, Bradley WA, Hearne MJ, Watkins MW, Wennberg DE, et al. Multivariate prediction of in-hospital mortality after percutaneous coronary interventions in 1994-1996. Northern New England Cardiovascular Disease Study Group. J Am Coll Cardiol. 1999;34(3):681–91. doi: 10.1016/s0735-1097(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 28.Resnic FS, Ohno-Machado L, Selwyn A, Simon DI, Popma JJ. Simplified risk score models accurately predict the risk of major in-hospital complications following percutaneous coronary intervention. Am J Cardiol. 2001;88(1):5–9. doi: 10.1016/s0002-9149(01)01576-4. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhary S, Ivanov J, Mackie K, Seidelin PH, Dzavik V. The Toronto score for in-hospital mortality after percutaneous coronary interventions. Am Heart J. 2009;157(1):156–63. doi: 10.1016/j.ahj.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie TA, Malenka DJ, Olmstead EM, Piper WD, Langner C, Ross CS, O'Connor GT. Prediction of survival after coronary revascularization: modeling short-term, mid-term, and long-term survival. Ann Thorac Surg. 2009;87(2):463–72. doi: 10.1016/j.athoracsur.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Jin R, Grunkemeier GL. Validating the Clinical Outcomes Assessment Program risk model for percutaneous coronary intervention. Am Heart J. 2006;151(6):1276–80. doi: 10.1016/j.ahj.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Klein LW, Shaw RE, Krone RJ, Brindis RG, Anderson HV, Block PC, McKay CR, Hewitt K, Weintraub WS. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96(1):35–41. doi: 10.1016/j.amjcard.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 33.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55(18):1923–32. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284(7):835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 35.Yale-New Haven Hospital Center for Outcomes Research and Evaluation (CORE) CORE Readmission Risk Calculators iPhone App. 2015 Available: https://itunes.apple.com/us/app/core-readmission-risk-calculators/id507579947?mt=8.
- 36.The Society for Cardiovascular Angiography and Interventions (SCAI) PCI Risk Assessment Tool. 2015 Available: http://www.scai.org/PCIRiskAssessmentTools/default.aspx.
- 37.Minges KE, Herrin J, Desai N, Messenger J, Nallamothu B, Rumsfeld J, Elma MA, Chen P, Ting H, Curtis J. Associations of hospital strategies and 30-day risk-standardized readmission rate in percutaneous coronary intervention. J Am Coll Cardiol. 2016;67(13_S):2105. [Google Scholar]
- 38.Anderson HV, Shaw RE, Brindis RG, Hewitt K, Krone RJ, Block PC, McKay CR, Weintraub WS. A contemporary overview of percutaneous coronary interventions. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR) J Am Coll Cardiol. 2002;39(7):1096–103. doi: 10.1016/s0735-1097(02)01733-3. [DOI] [PubMed] [Google Scholar]
- 39.Gogo PB, Jr, Dauerman HL, Mulgund J, Ohman EM, Patel MR, Cohen DJ, Saucedo JF, Harrington RA, Gibler WB, Smith SC, Jr, et al. Changes in patterns of coronary revascularization strategies for patients with acute coronary syndromes (from the CRUSADE Quality Improvement Initiative) Am J Cardiol. 2007;99(9):1222–6. doi: 10.1016/j.amjcard.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 40.Roe MT, Chen AY, Cannon CP, Rao S, Rumsfeld J, Magid DJ, Brindis R, Klein LW, Gibler WB, Ohman EM, et al. Temporal Changes in the Use of Drug-Eluting Stents for Patients With Non–ST-Segment–Elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention From 2006 to 2008: Results From the Can Rapid risk stratification of Unstable angina patients Supress ADverse outcomes with Early implementation of the ACC/AHA guidelines (CRUSADE) and Acute Coronary Treatment and Intervention Outcomes Network–Get With The Guidelines (ACTION–GWTG) Registries. Circulation: Cardiovascular Quality and Outcomes. 2009;2(5):414–420. doi: 10.1161/CIRCOUTCOMES.109.850248. [DOI] [PubMed] [Google Scholar]
- 41.Dunlay SM, Rihal CS, Sundt TM, Gerber Y, Roger VL. Current trends in coronary revascularization. Curr Treat Options Cardiovasc Med. 2009;11(1):61–70. doi: 10.1007/s11936-009-0007-7. [DOI] [PubMed] [Google Scholar]
- 42.Friedman B, Jiang HJ, Russo CA. HCUP Statistical Brief #66. Agency for Healthcare Research and Quality; Rockville, MD: 2006. Medicare Hospital Stays: Comparisons between the Fee-for-Service Plan and Alternative Plans, 2006. [PubMed] [Google Scholar]
- 43.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–98. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minges KE, Curtis JP. Living in the Readmission Era. Circulation: Cardiovascular Interventions. 2014;7(1):9–10. doi: 10.1161/CIRCINTERVENTIONS.114.001174. [DOI] [PMC free article] [PubMed] [Google Scholar]


