Abstract
Background
Ibrutinib is an active therapy with acceptable safety profile for patients with CLL, including high-risk patients with del17p or with TP53 mutations. Ibrutinib is broadly indicated for the treatment of patients with chronic lymphocytic leukemia and specifically including those with 17p deletion. The optimal use of ibrutinib in combination with other agents, remains controversial.
Methods
We report the long term outcome [median follow up of 47 months (range 36-51 months)] of 40 patients with high risk CLL, treated on the first ibrutinib combination trial with rituximab (IR). The majority of patients (36/40) were previously treated.
Results
Median age was 65 years and 21 patients (52%) had 17p deletion. Median duration on treatment was 41 months (range 2-51 months) and median number of treatment cycles was 42 (range 2-49). Overall response rate (ORR) was 95% and 9 patients (23%) attained a complete remission (CR). 21 patients discontinued treatment, 10 due to disease progression, 9 for other causes, and 2 due to stem cell transplantation, the remaining 19 patients continue on ibrutinib. Median progression free survival (PFS) for all patients was 45 months, which was significantly shorter in the subgroup of patients with del17p (n=21, 32.3 months, p=0.02). Fourteen patients (35%) died, 5 from progressive disease, 5 from infections, and 4 from other causes. Median overall survival (OS) has not been reached.
Conclusions
IR combination therapy leads to durable remissions in high risk CLL; the possible benefit from the addition of rituximab is currently explored in a randomized trial.
Keywords: Ibrutinib, CLL, rituximab, chronic lymphocytic leukemia, deletion 17p
Introduction
Ibrutinib is a BTK (Bruton's tyrosine kinase) inhibitor with clinical activity in several B cell malignancies, including CLL, mantle cell lymphoma (MCL) and Waldenstrom's macroglobulinemia (WM).1 A series of clinical trials demonstrated a major improvement in progression free and overall survival in CLL patients treated with ibrutinib in the frontline2,3 and the relapse refractory4,5 disease setting. The advent of ibrutinib is especially significant for high-risk CLL patients, such as patients with 17p deletion, who generally do not achieve durable responses with chemo-immunotherapy. Three year follow up of a clinical trial with single agent ibrutinib in relapsed refractory and in treatment naïve patients (age ≥ 65 years) demonstrated durable responses and an acceptable safety profile with continued long-term therapy in the majority of patients6. However, with ibrutinib single-agent use, most patients, even after long-term therapy, achieve partial remissions, and complete remissions remain the exception. Therefore, ibrutinib combination therapy has been pursued in a number of clinical trials. We previously reported the results of a phase 2 trial of the combination of ibrutinib with rituximab (IR) in patients with high risk CLL7, which was the first trial to report ibrutinib combination therapy data. In this trial, we demonstrated that the addition of rituximab attenuated the redistribution lymphocytosis observed in studies with single agent ibrutinib in patients with CLL, which often results in PR with lymphocytosis and consequently patients achieved remissions within a shorter time. However, the long term impact of IR on survival, response, immunological parameters and the toxicities in patients with high risk CLL has not been characterized8. In this present report, we describe the longer-term follow up data of IR in patients with high risk CLL.
Patients and Methods
This single arm phase 2 study in high risk patients with CLL (n=40) treated with ibrutinib and rituximab (IR) was developed by the investigators in collaboration and supported by Pharmacyclics, Inc. and was approved by The University of Texas MD Anderson Cancer Center institutional review board. Informed consent was obtained in accordance with institutional guidelines and the Declaration of Helsinki. High-risk CLL patients included patients with the presence of a 17p deletion, TP53 mutation and/or 11q deletion and patients with short remission durations of <36 months after frontline treatment with chemoimmunotherapy (CIT). Details of the inclusion, exclusion criteria, study design, follow up and response assessment were previously reported7. Treatment consisted of ibrutinib (420 mg orally daily) combined with weekly rituximab (375 mg/m2) for weeks 1-4 (cycle 1), then monthly rituximab until cycle 6, followed by single-agent ibrutinib continuously. Patients remained on ibrutinib treatment until disease progression or toxicities or complications precluded further therapy. The primary end point was to assess the activity of ibrutinib and rituximab in high-risk CLL, measured as overall response rate (ORR) and progression-free survival (PFS). Statistical analyses were conducted using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California). Survival or times to progression functions was estimated using the Kaplan-Meier method. Toxicity was reported by the type, frequency and severity.
Results
Patients and treatment
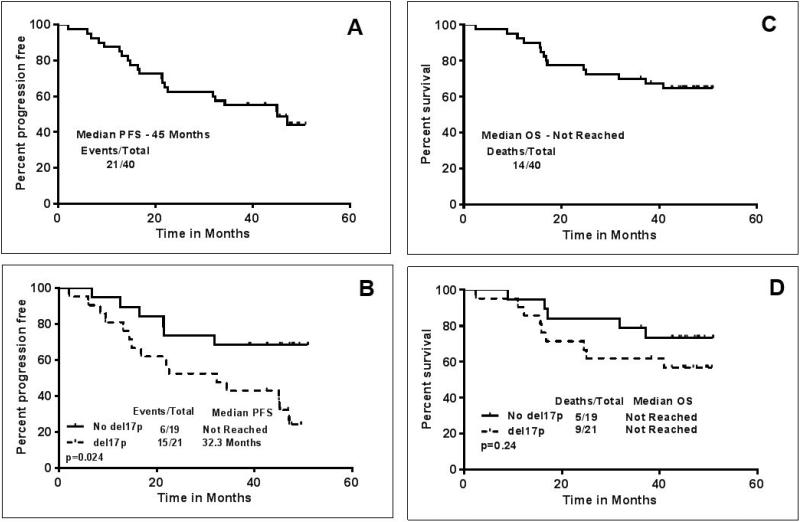
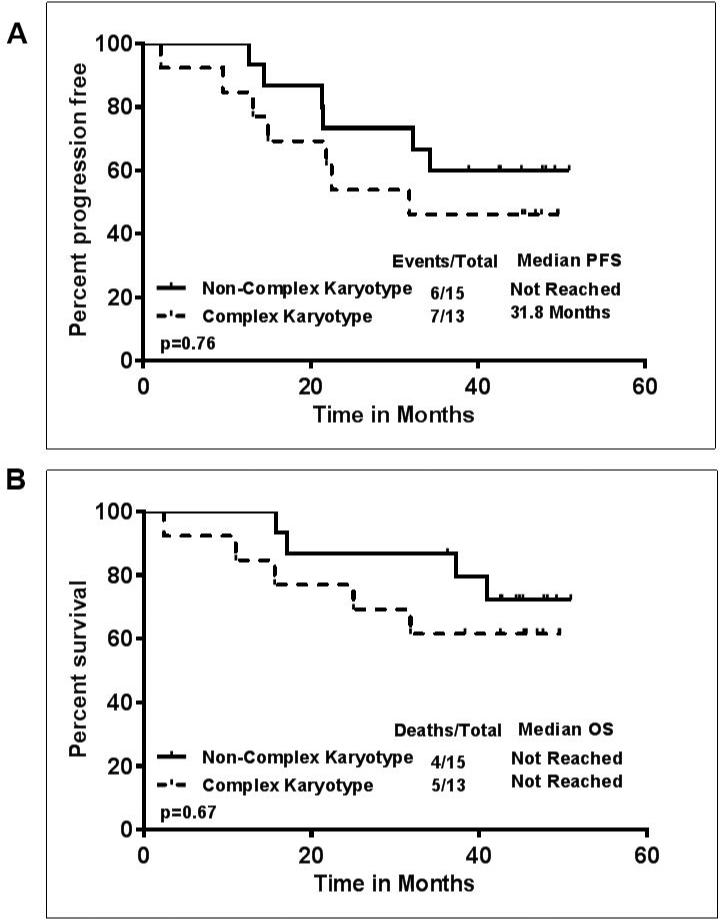
Previously untreated (n=4, all with del17p) and previously treated (n=36) patients were included in the study. The clinical characteristics of the patients are described in Table-1. Eleven patients (27%) were over the age of 70 years. 21 patients had del17p FISH (fluorescent in-situ hybridization) cytogenetic aberration, and 12 patients had del11q. Thirty-three patients had unmutated immunoglobulin heavy chain variable region genes (IGHV), one patient had mutated IGHV and 6 patients had inconclusive IGHV status. Median follow up of the patients was 47 months (range 36-51 months). At the time of last follow up, 19 (47%) patients remained on treatment (6 with del17p) and 21 (52%) had discontinued treatment, 10 due to disease progression, 9 due to other complications and 2 patients underwent stem cell transplantation in response. Causes of treatment discontinuation, death and clinical characteristics are detailed in Table-2. Of note, among the 4 previously untreated CLL patients with del17p, 2 patients are still continuing on study, and 2 discontinued treatment, one due to complicated ear infections and ear bleeding, and another due to significant arthralgias, enthesitis, and bruising. Among these 4 patients, 3 were alive and one died from disease progression approximately 2 months after discontinuation of ibrutinib. Among the 10 patients who discontinued due to disease progression, 2 patients developed Richter's transformation, both with del17p. Thirteen patients (46%) had complex karyotype, and disease progression was more frequent within this subgroup (in 7/13 or 54% of these patients). Overall, the median number of cycles received by the patients was 42. The majority of patients (38/40 or 95%) received ≥ 6 cycles of therapy. The median dose of ibrutinib was 420 mg daily. Median duration of ibrutinib therapy was 41 months (range 2 – 51 months). Among the 21 patients who came off therapy, the median survival after treatment discontinuation was 8 months. The best overall response rate (ORR) was 95%, with 28 patients (72%) achieving a partial remission, 9 (23%) a complete remission (CR), 2 patients did not respond, and 1 patient was not evaluable. Of the 9 patients with CR, 2 patients achieved a minimal residual disease (MRD) negative CR. The CR rate improved from 4 patients (10%) reported initially 7 to 9 patients (23%) with the longer follow-up, which is consistent with the single-agent ibrutinib experience and longer follow-up.6 Among the 21 patients with del17p, 18/21 responded (86%), 5 achieved a CR (24%) and 13 a PR (62%). Among the four patients who were previously untreated and had del17p, 3 (75%) achieved a CR (2 were MRD negative) and 1 patient a PR. The toxicities which were considered to be likely related to IR treatment are displayed in the supplemental Table-1. Overall, we noted that 16 patients (40%) developed clinically significant infections, requiring therapy, involving the upper respiratory tract and/or the lungs. Overall, 14 patients died, 5 while being on study, 3 due to complicated infections, 1 had a sudden death without antecedent illness, presumed cardiac, and 1 patient from metastatic non-small cell lung cancer. Figure 1 (A-D) shows the progression free (PFS) and overall survival (OS). Overall, the median PFS was 45 months and the median OS was not reached. The number of events and deaths were higher in patients with del17p, resulting in a significantly shorter PFS and shorter OS in patients with del17p (PFS, p=0.02; OS p=0.24)(Figure 1 B-D). The median PFS of patients with del17p was 32.3 months. Similarly, patients with complex karyotype abnormalities, which constitutes another high-risk subset of patients, had shorter PFS or OS (Figure-2 A-B), however these differences did not reach statistical significance. In a subset analysis, among the patients with del17p (n=21), the PFS and OS were not significantly different according to the presence (n=11) or absence of complex karyotype (n=4) (Supplemental figure-1). This could be due to smaller number of patients in each group and require large data set for the analysis.
Table 1.
Initial patient characteristics (n=40)
| Patients (N=40) | |
|---|---|
| Median age (range) | 65 (35 - 82) |
| Female | 14 (35 %) |
| Male | 26 (65 %) |
| Del (17p) * | 21 (52 %) |
| Del (11q) | 12 (30 %) |
| Del (13q) | 5 (12.5 %) |
| FISH negative | 2 (5 %) |
| Mutation status IGVH (immunoglobulin heavy chain variable region) | Unmutated (33) Not done (6) Mutated (1) |
| Median (range) | |
| Prior treatments | 2.0 (0 - 8) |
| Cycles completed | 42 (2 - 49) |
| Median follow-up time of alive patients (months) | 47 (36 - 51) |
| Hemoglobin (g/dL) | 11.7 (6.7 - 15.6) |
| Platelets (103/μL) | 91.5 (36 - 242) |
| White blood cell count (103/μL) | 22.4 (2.2 - 297.8) |
| Absolute lymphocytes (103/μL) | 19.9 (0.4 - 277) |
Overall, among the 40 patients, 21 were del17p by FISH, of which only 3 were del17p alone and the distribution of other FISH abnormalities in the remaining 18 patients was as follows: 1 with del11q, 1 with 11q and trisomy 12, 2 with trisomy 12, 6 with del11q and del13q and 8 with del13q. Similarly, there were 12 patients with del11q (without del17p), of which only 4 were del11q alone and 8 were del11q with del13q.
Table 2.
Summary of causes of discontinuation of ibrutinib with rituximab (IR) with patient characteristics (n=21)
| Patient Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Yrs) |
Gender | FISH | #IGHV Mutation Status |
Cause of discontinuation |
Type of complication |
Duration of Ibrutinib (Months) |
Salvage treatment |
Survival Status |
On study death (Y/N) |
Post IR survival (Months) |
Causes of death |
Disease Transformation (Y/N) |
| 65 | Male | Neg. | UM | Stem cell Transplant | None | 12.6 | Unknown | Dead | N | 4.5 | Disease progression | No |
| 63 | Female | 17p | UM | Stem cell Transplant | None | 14.9 | None | Alive | N | 23.4 | - | No |
| 62 | Male | 17p | UM | Complications | Ear infections | 16.8 | - | Dead | Y | 0.0 | Pulmonary complications | No |
| 67 | Male | 11q | UM | Complications | Unknown, found dead in his sleep | 16.4 | - | Dead | Y | 0.0 | Unknown | No |
| 61 | Male | 17p | UM | Complications | Pulmonary infections | 9.5 | - | Dead | Y | 1.5 | Pulmonary infections | No |
| 68 | Male | 11q | UM | Complications | Diarrhea, subdural hematoma | 21.5 | Restarted Ibrutinib | Alive | N | 14.8 | - | No |
| 72 | Male | 11q | UM | Complications | Progressive lung cancer | 31.8 | On Ibrutinib | Dead | Y | 0.0 | Lung cancer | No |
| 76 | Male | 17p | UM | Complications | Multifocal aspergillosis | 2.1 | - | Dead | Y | 0.2 | Pneumonia, brain abscess | No |
| 57 | Female | 17p | M | Complications | Mucositis | 6.0 | OFAR, Ibrutinib restarted | Dead | N | 18.5 | Sepsis | No |
| 82 | Male | 17p | UM | Complications | Comp-CHF, COPD | 8.4 | - | Dead | N | 3.9 | CHF, COPD | No |
| 61 | Female | 17p | UM | Complications | Toxicity-arthritis | 45 | Planning | Alive | N | 0 | - | No |
| 73 | Female | 17p | UM | Disease progression | - | 14.4 | Rituximab | Died | N | 1.4 | Resistant disease | No |
| 58 | Male | 17p | UM | Disease progression | - | 34.3 | Idelalisib+rituximab | Alive | N | 4.2 | - | No |
| 52 | Male | 13q | UM | Disease progression | - | 21.4 | ABT-199, HCVAD, Idelalisib | Died | N | 15.8 | Resistant disease with TP53 acquisition | No |
| 35 | Female | 11q | UM | Disease progression | - | 6.8 | - | Died | N | 2.1 | Pneumonia | No |
| 76 | Female | 17p | UM | Disease progression | - | 45.1 | Ibrutinib with BR | Alive | N | 0.0 | - | No |
| 65 | Female | 17p | UM | Disease progression | - | 13.1 | R-MP then IPI-145 | Died | N | 2.5 | Progressive disease | Yes |
| 73 | Female | 17p | UM | Disease progression | - | 32.3 | Expt. agents | Died | N | 8.6 | Infections | No |
| 73 | Female | 17p | UM | Disease progression | - | 21.9 | ABT-199 | Died | N | 3.1 | Progressive disease | Yes |
| 78 | Female | 17p | UM | Disease progression | - | 22.5 | Ofatumumab | Alive | N | 20.0 | - | No |
| 65 | Male | 17p | ND | Disease progression | - | 47.1 | Venetoclax | Alive | N | 2.9 | - | No |
Figure 1. Survival outcomes for patients treated with ibrutinib-rituximab (IR) - progression-free survival (PFS) and overall survival (OS) after a median follow up of 42 months - A).
Median PFS in all patients was 45 months B) PFS according to the presence or absence of deletion 17p; median survival not reached in patients without deletion 17p and median PFS 32 months in patients with deletion 17p (P=0.02) C) Median OS in all patients was not reached D) OS according to the presence or absence of deletion 17p; median survival not reached in both group of patients with/without deletion 17p (P=0.24).
Figure 2. Survival outcomes for patients treated with ibrutinib-rituximab (IR) – Complex vs Non-Complex karyotype A).
Median PFS in patients with Complex vs Non-Complex karyotype was 31.8 months vs not reached (P=0.76) B) OS according to the presence or absence of complex karyotype; median survival not reached in both group of patients (P=0.67).
Only one patient developed a secondary cancer after starting treatment, a squamous cell cancer of the lungs, diagnosed 2 years after starting IR therapy. Patterns of change in other disease associated parameters - absolute lymphocyte count (ALC), absolute neutrophil count (ANC), platelet count, serum beta2 microglobulin (β2M) levels are shown in Supplemental figure 2. Serum immunoglobulin levels (IgG, IgM, IgA) and absolute T cell subsets were analyzed and are summarized in the Supplement (Supplemental figures 3-4). There was no significant change in serum immunoglobulin levels over time, consistent with the ibrutinib single agent experience. The ALC levels decreased and stabilized and T cell subset numbers and β2M levels decreased after starting IR treatment and stabilized over time when followed for up to 36 months.
Discussion
In the current analysis, we present the long term follow up data of patients with high risk CLL treated with a combination of ibrutinib and rituximab (IR). The majority of patients (36/40) were previously treated. This study demonstrated that IR in high risk patients with CLL is well tolerated, active, and can induce durable remissions, including CR in patients with high-risk CLL. The improvement in long term progression-free survival in high risk patients with CLL with ibrutinib-based therapy is a significant advance in treatment of CLL. Recently, the 3 year follow up of single agent ibrutinib was reported6 and demonstrated that responses improve over time in treatment naïve and in relapsed refractory patients with CLL. In our study, with an extended follow up of over 3 years, we demonstrated that high-risk CLL patients treated with the IR combination have high response rates, which also further improve over time when compared to the original report.7 Median PFS and OS achieved after 3 years in our study (45 months and not reached) appear similar to single agent ibrutinib data in relapsed refractory patients, although cross trial comparisons are problematic. Nonetheless, since our study involved only CLL patients with high risk disease, the achievement of median PFS of 45 months in high risk CLL patients constitutes a major improvement over previously reported data in high risk CLL patients with more conventional treatments.9,10 Of note, in patients with del17p, the long term follow up data when using frontline chemo-immunotherapy with the FCR regimen showed a median PFS of 15 months11. The fact that 21 patients came off study (10 were due to disease progression) and 14 patients died (5 deaths due to CLL) emphasizes that, despite the major improvement in outcome with ibrutinib-based therapy in high-risk CLL, there remains an urgent need for further improvement in these patients, which may come from cellular therapy approaches (allogeneic SCT, CAR-T cell therapy) and/or BCL2 antagonist such as Venetoclax. Furthermore, this data set re-emphasizes that outcomes of CLL patients after they progress or develop disease transformation on ibrutinib-based treatment generally is poor (median survival 2.8 months).12 Interestingly, with longer follow-up serum immunoglobulin and T cell numbers remained stable with IR therapy.
In summary, our data shows that the combination of ibrutinib with rituximab is a potent treatment option for CLL patients with high-risk disease and can induce durable remissions in a majority of patients. However, data from the ongoing randomized study of IR vs ibrutinib single agent (Clinical trial Identifier-NCT02007044) are required to determine the benefit from the addition of rituximab to ibrutinib in the treatment of patients with CLL.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in this trial and their families, and the study investigators and coordinators at MD Anderson for sample and data collection.
Funding: Pharmacyclics, Inc., Leukemia & Lymphoma Society, NCI Grant P30 CA016672, and MD Anderson's Moon Shot Program in CLL.
Footnotes
Author Contributions
J.A.B. designed and supervised the trial and correlative studies, analyzed the data and wrote the paper with P.J., M.S.; M.J.K., A.F., Z.E., P. T., H.K., W.G.W., and S.O.B. contributed to the trial design, clinical patient management, sample collection, and clinical data analysis, and reviewed and approved the paper.
Conflict of interest
J.A.B. and S.O.B. received research funding from Pharmacyclics, Inc.; J.A.B. is a consultant for Janssen Pharmaceuticals, Inc.
P.T. has served on an advisory board and received an honorarium from Pharmacyclics
References
- 1.Ponader S, Burger JA. Bruton's tyrosine kinase: from X-linked agammaglobulinemia toward targeted therapy for B-cell malignancies. J Clin Oncol. 2014;32(17):1830–1839. doi: 10.1200/JCO.2013.53.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrd JC, Brown JR, O'Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15(10):1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robak T. Ibrutinib in chronic lymphocytic leukaemia: alone or in combination? Lancet Oncol. 2016;17(2):129–131. doi: 10.1016/S1470-2045(15)00519-7. [DOI] [PubMed] [Google Scholar]
- 9.Dreger P, Schetelig J, Andersen N, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124(26):3841–3849. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stilgenbauer S. Prognostic markers and standard management of chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2015;2015:368–377. doi: 10.1182/asheducation-2015.1.368. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208–215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 12.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.